Fracture Strength Evaluation of Agglomerates of Fatty Acid-Coated CaCO3 Nanoparticles by Nano-Indentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Structural Characterization
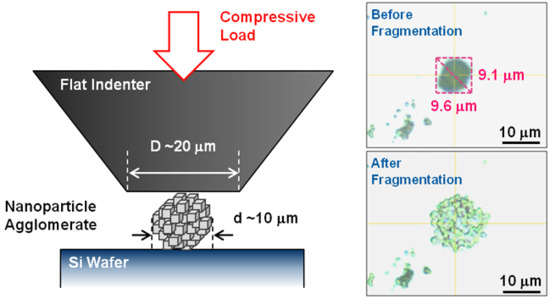
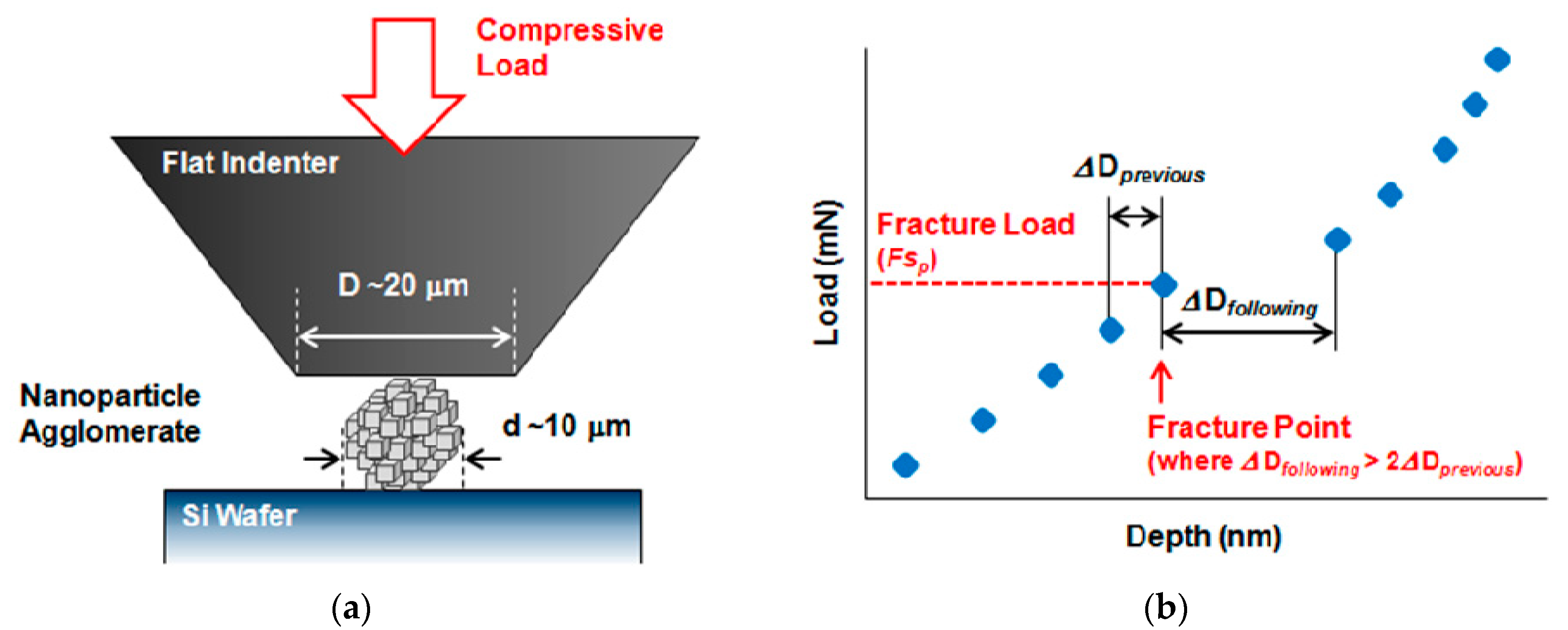
2.3. Nano-Indentation
3. Results and Discussion
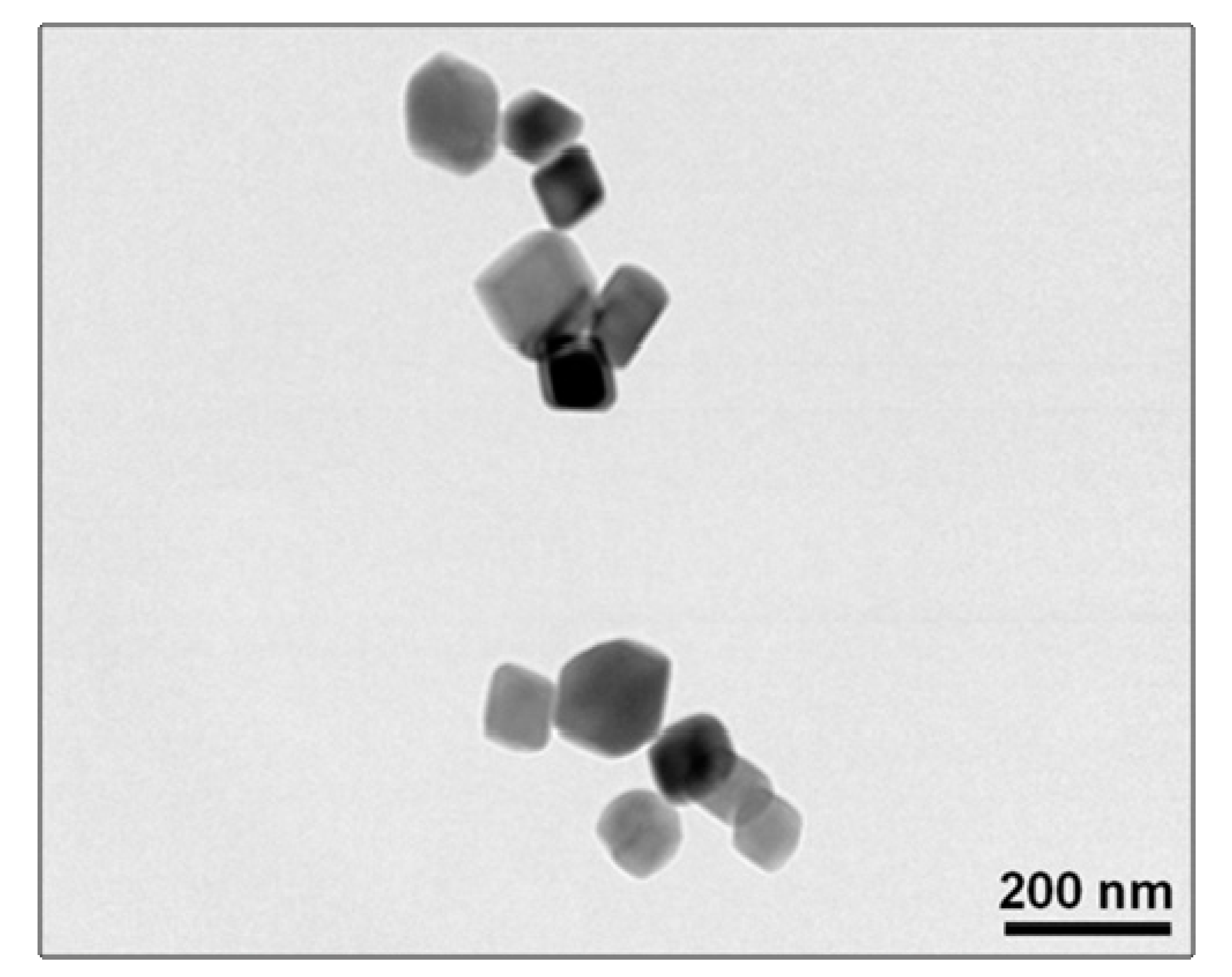
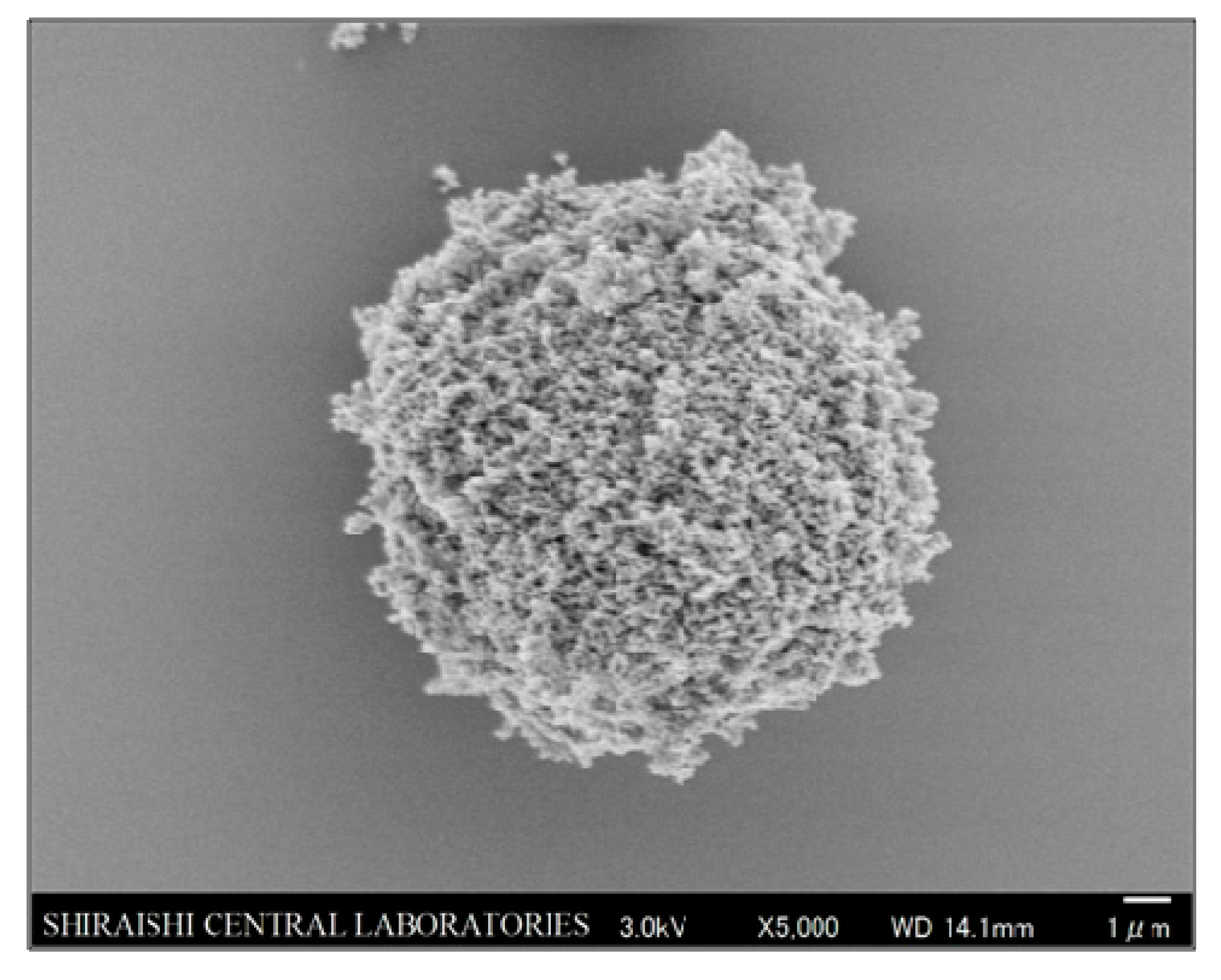
3.1. Primary Particle Structure
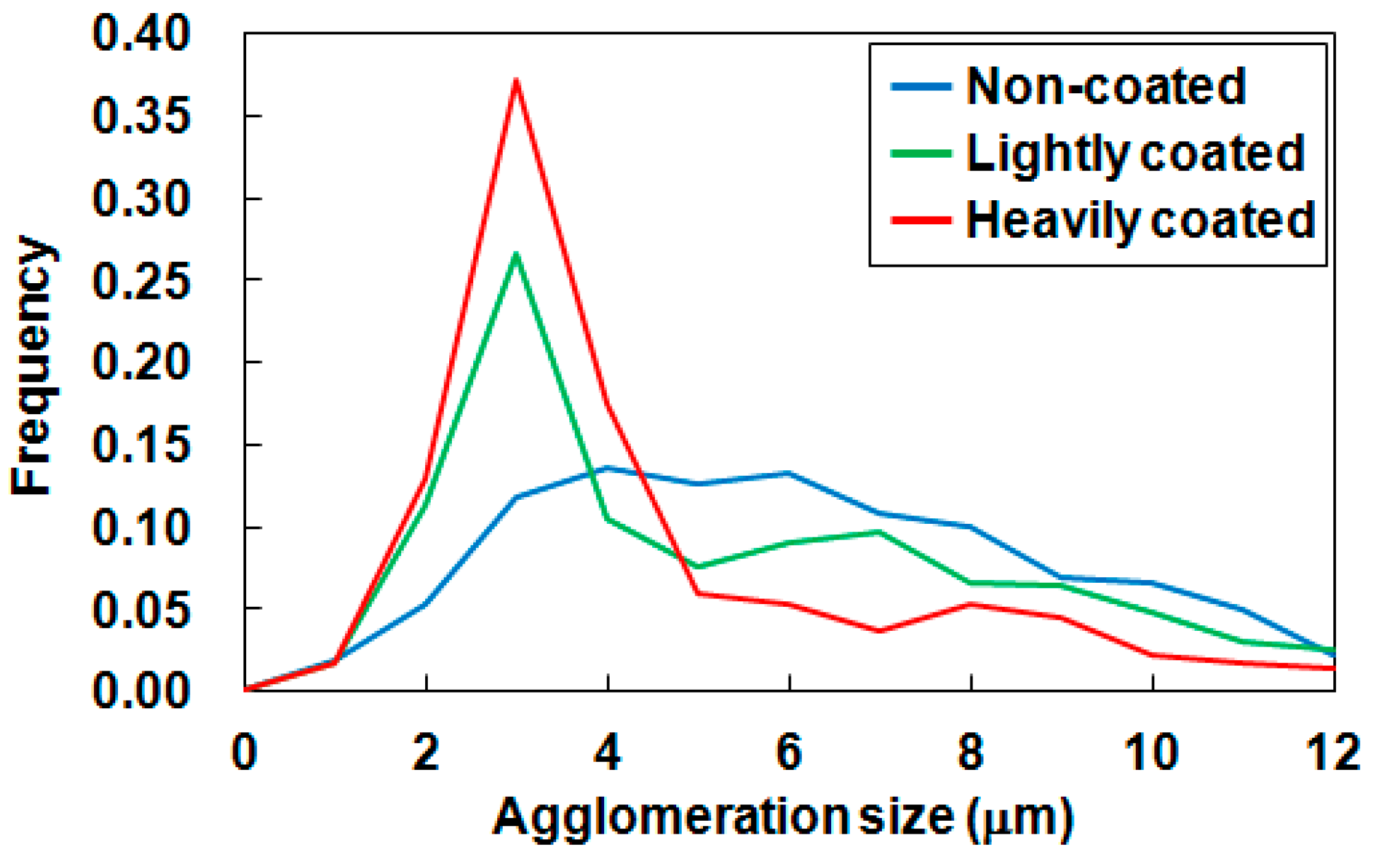
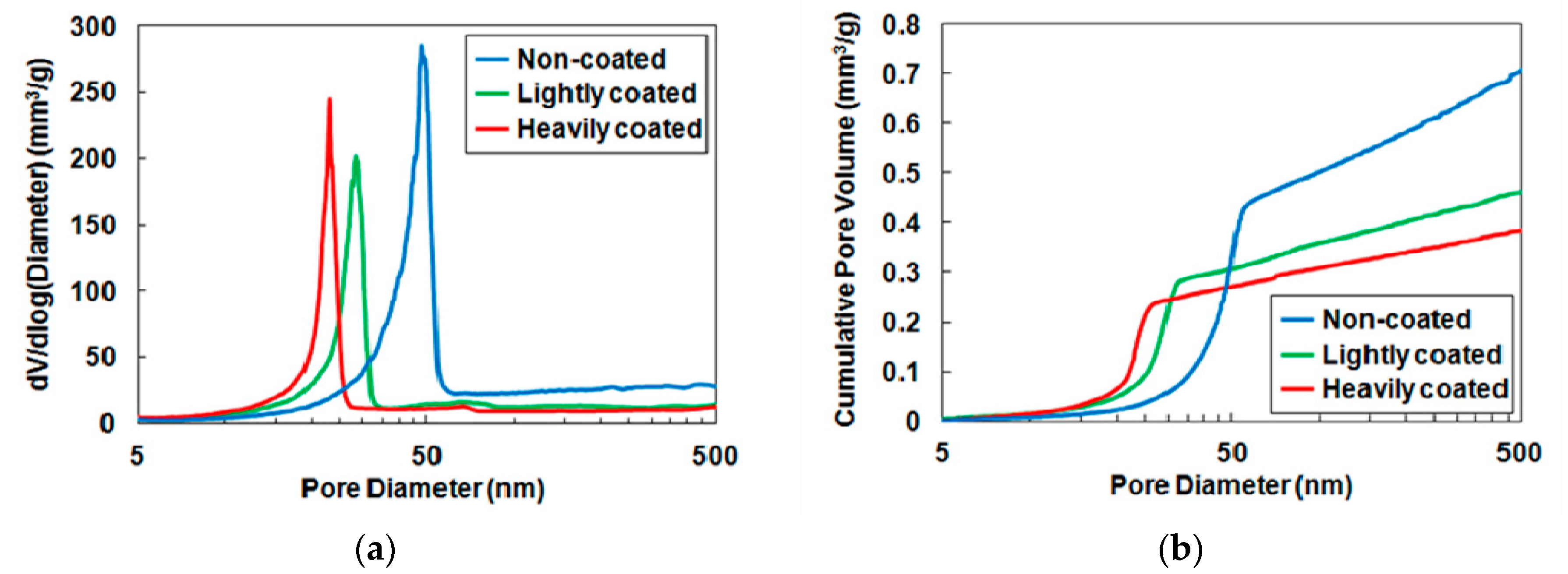
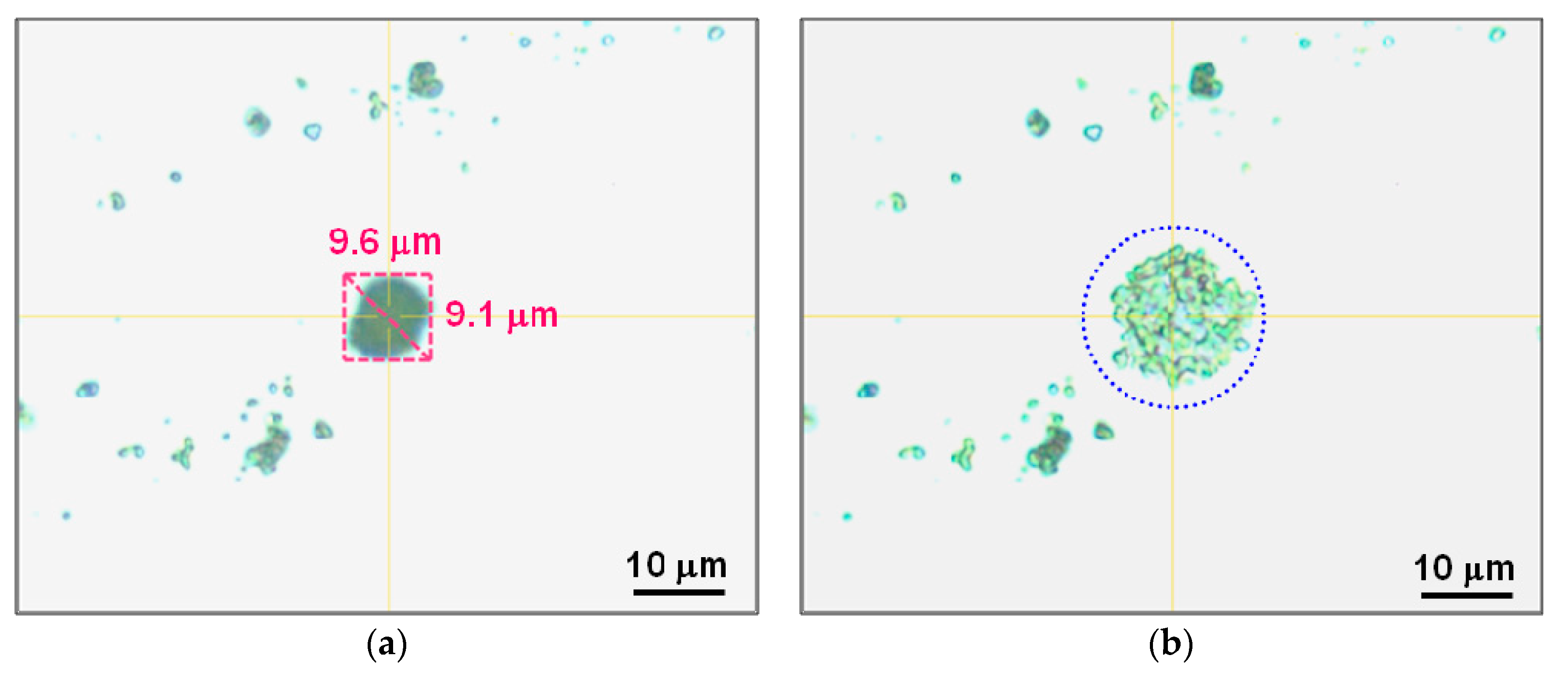
3.2. Agglomeration States
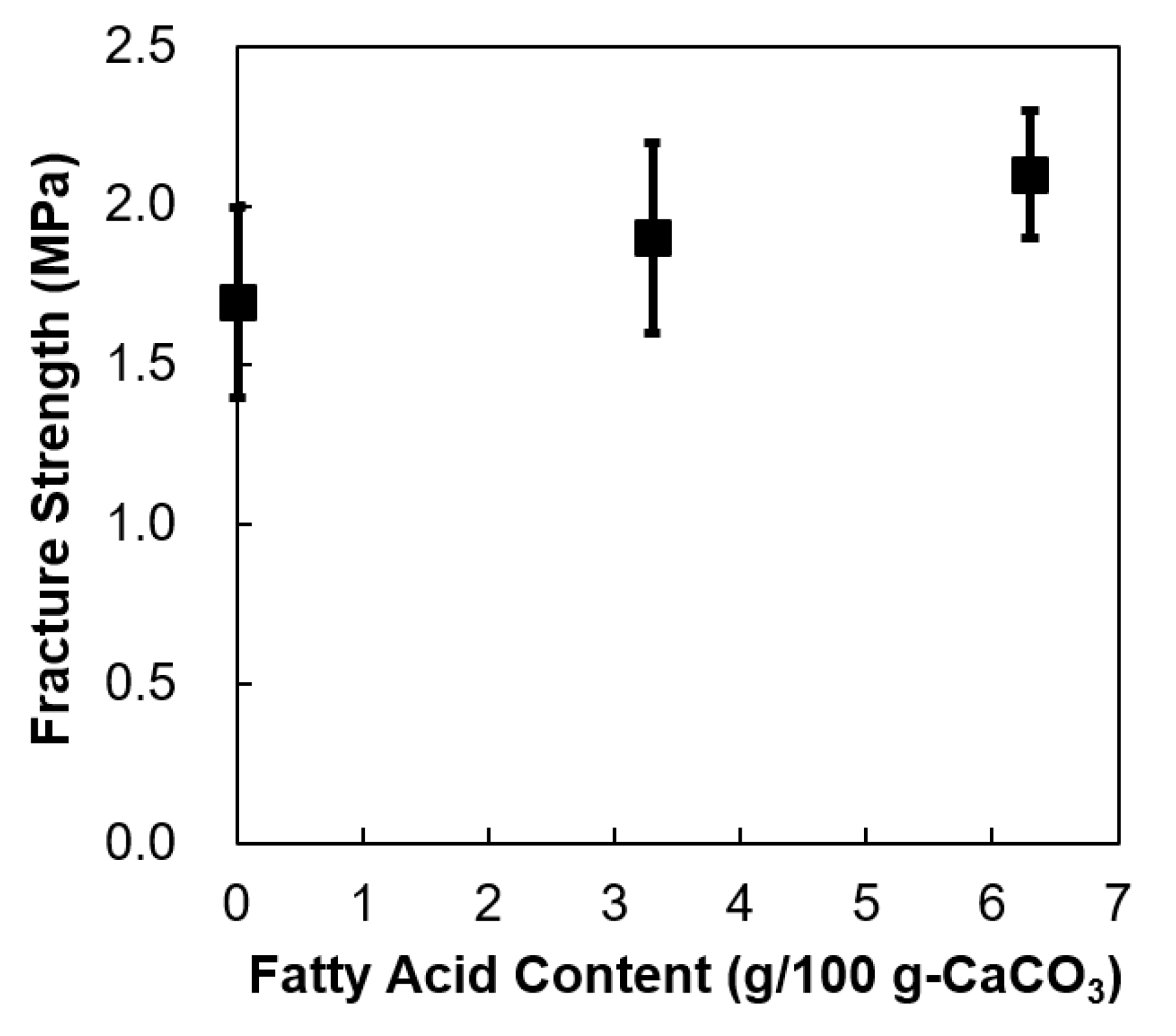
3.3. Nano-Indentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.C. (Ed.) Paper Chemistry, 2nd ed.; Springer: Heidelberg, Germany, 1996. [Google Scholar]
- Zhoua, S.; Wu, L.; Suna, J.; Shen, W. The change of the properties of acrylic-based polyurethane via addition of nano-silica. Prog. Org. Coat. 2002, 45, 33–42. [Google Scholar] [CrossRef]
- Hosokawa, M.; Nogi, K.; Naito, M.; Yokoyama, T. (Eds.) Nanoparticle Technology Handbook, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Pejchal, V.; Žagar, G.; Charvet, R.; Dénéréaz, C.; Mortensen, A. Compression testing spherical particles for strength: Theory of the meridian crack test and implementation for microscopic fused quartz. J. Mech. Phys. Solid. 2017, 99, 70–92. [Google Scholar] [CrossRef] [Green Version]
- Saha, R.; Nix, W.D. Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 2002, 50, 23–38. [Google Scholar] [CrossRef]
- Corcoran, S.G.; Colton, R.J.; Lilleodden, E.T.; Gerberich, W.W. Staircase yielding phenomenon of single-crystal Au. Phys. Rev. B 1997, 55, 16057–16060. [Google Scholar] [CrossRef]
- Gu, X.W. Mechanical Properties of Architected Nanomaterials Made from Organic–Inorganic Nanocrystals. JOM 2018, 70, 2205–2217. [Google Scholar] [CrossRef]
- Clarke, D.R.; Tandon, R. Factors affecting the fracture resistance of silicon nitride ceramics. Mater. Sci. Eng. A 1995, 195, 207–214. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Fiori, L.; Pelillo, E. Nano-indentation of polymeric surfaces. J. Phys. D Appl. Phys. 1998, 31, 2395–2405. [Google Scholar] [CrossRef]
- Raichman, Y.; Kazakevich, M.; Rabkin, E.; Tsur, Y. Inter-Nanoparticle Bonds in Agglomerates Studied by Nanoindentation. Adv. Mater. 2006, 18, 2028–2030. [Google Scholar] [CrossRef]
- Schilde, C.; Westphal, B.; Kwade, A. Effect of the primary particle morphology on the micromechanical properties of nanostructured alumina agglomerates. J. Nanopart. Res. 2012, 14, 745. [Google Scholar] [CrossRef]
- Hiramatsu, Y.; Oka, Y.; Kiyama, H. Rapid Determination of the Tensile Strength of Rocks with Irregular Test Pieces. J. Min. Metall. Inst. Jpn. 1965, 81, 1024–1030. [Google Scholar] [Green Version]
- Hiramatsu, Y.; Oka, Y. Determination of the tensile strength of rock by a compression test of an irregular test piece. Int. J. Rock Mech. Min. Sci. 1966, 3, 89–90. [Google Scholar] [CrossRef]
- Shiraishi, T. Method of Manufacturing Monodispersed Carbonate of Alkali Earths. U.S. Patent No. 1,863,945, 21 June 1932. [Google Scholar]
- Takasaki, M.; Kezuka, Y.; Tajika, M.; Oaki, Y.; Imai, H. Evolution of calcite nanocrystals through oriented attachment and fragmentation: Multistep pathway involving bottom-up and break-down stages. ACS Omega 2017, 2, 8997–9001. [Google Scholar] [CrossRef]
- Kezuka, Y.; Kuma, Y.; Nakai, S.; Matsubara, K.; Tajika, M. Calcium carbonate chain-like nanoparticles: Synthesis, structural characterization, and dewaterability. Powder Technol. 2018, 335, 195–203. [Google Scholar] [CrossRef]
- Roskill Information Services Ltd. Ground and Precipitated Calcium Carbonate: Global Industry Markets and Outlook, 1st ed.; Roskill Information Services Ltd.: London, UK, 2012. [Google Scholar]
- Yoğurtcuoğlu, E.; Uçurum, M. Surface modification of calcite by wet-stirred ball milling and its properties. Powder Technol. 2011, 214, 47–53. [Google Scholar] [CrossRef]
- Mihajlović, S.R.; Vučinić, D.R.; Sekulić, Ž.T.; Milićević, S.Z.; Kolonja, B.M. Mechanism of stearic acid adsorption to calcite. Powder Technol. 2013, 245, 208–216. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Mikhail, R.S.; Brunauer, S. Surface area measurements by nitrogen and argon adsorption. J. Coll. Interf. Sci. 1975, 52, 572–577. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen. Nachr. Ges. Wiss. Göttingen 1918, 2, 96–100. [Google Scholar]
| Non-Coated | Lightly-Coated | Heavily-Coated | |
|---|---|---|---|
| Fatty acid content (g/100 g-CaCO3) | - | 3.3 | 6.3 |
| Crystallite size (nm) | 75 | 75 | 74 |
| BET-SSA (m2/g) | 18.7 | 16.5 | 16.7 |
| Agglomeration size (μm) | |||
| D10 | 2.3 | 1.8 | 1.8 |
| D50 | 5.3 | 4.0 | 3.0 |
| D90 | 9.6 | 9.2 | 8.2 |
| Most frequent pore diameter (nm) | 48 | 28 | 23 |
| Pore volume (mm3/g) | 0.45 | 0.28 | 0.25 |
| Fracture strength (Csp) (MPa) | 1.7 ± 0.3 | 1.9 ± 0.3 | 2.1 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kezuka, Y.; Tajika, M. Fracture Strength Evaluation of Agglomerates of Fatty Acid-Coated CaCO3 Nanoparticles by Nano-Indentation. ChemEngineering 2019, 3, 73. https://doi.org/10.3390/chemengineering3030073
Kezuka Y, Tajika M. Fracture Strength Evaluation of Agglomerates of Fatty Acid-Coated CaCO3 Nanoparticles by Nano-Indentation. ChemEngineering. 2019; 3(3):73. https://doi.org/10.3390/chemengineering3030073
Chicago/Turabian StyleKezuka, Yuki, and Masahiko Tajika. 2019. "Fracture Strength Evaluation of Agglomerates of Fatty Acid-Coated CaCO3 Nanoparticles by Nano-Indentation" ChemEngineering 3, no. 3: 73. https://doi.org/10.3390/chemengineering3030073