Unusual Regularities of Propylene Carbonate Obtained by Propylene Oxide Carboxylation in the Presence of ZnBr2/Et4N+Br− System
Abstract
1. Introduction
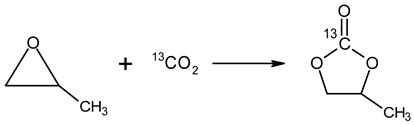
2. Materials and Methods
2.1. Chemicals
2.2. Catalytic Experiments
2.3. Product Analysis
2.4. Calculations
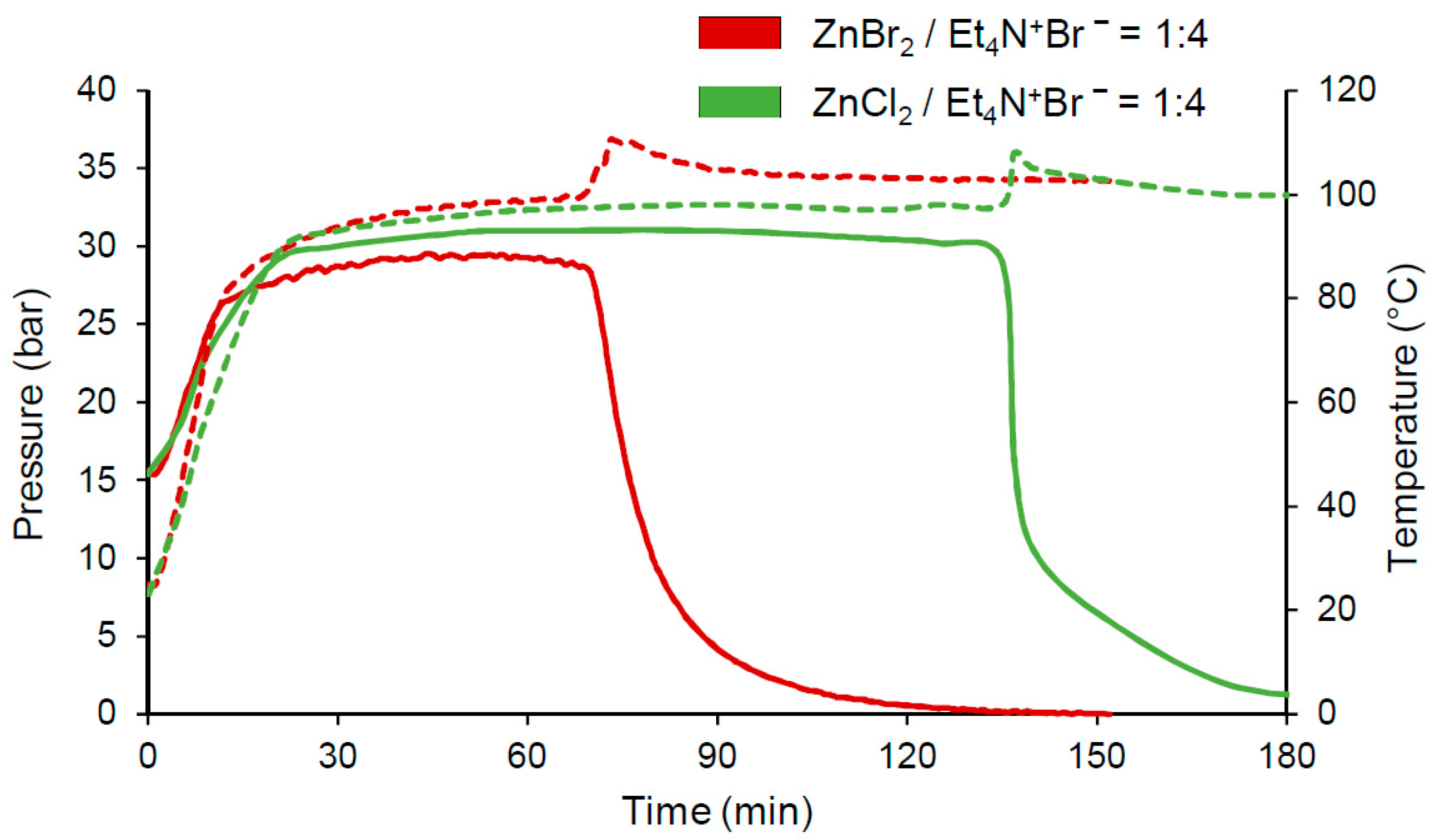
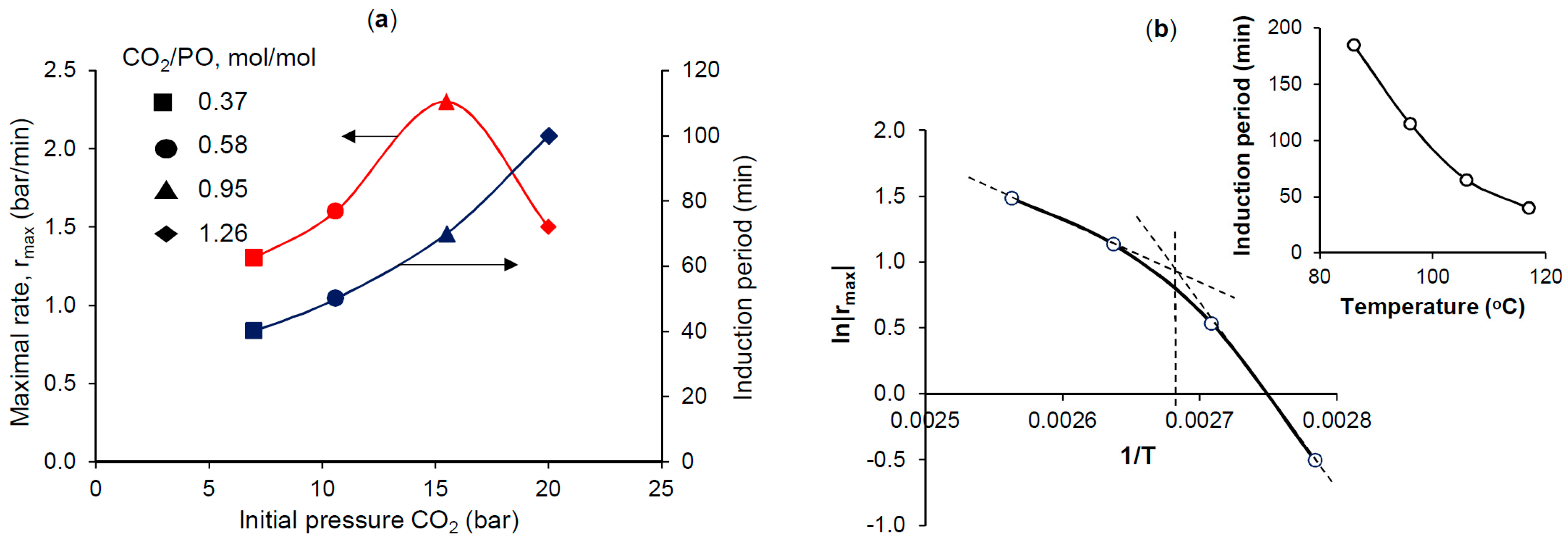
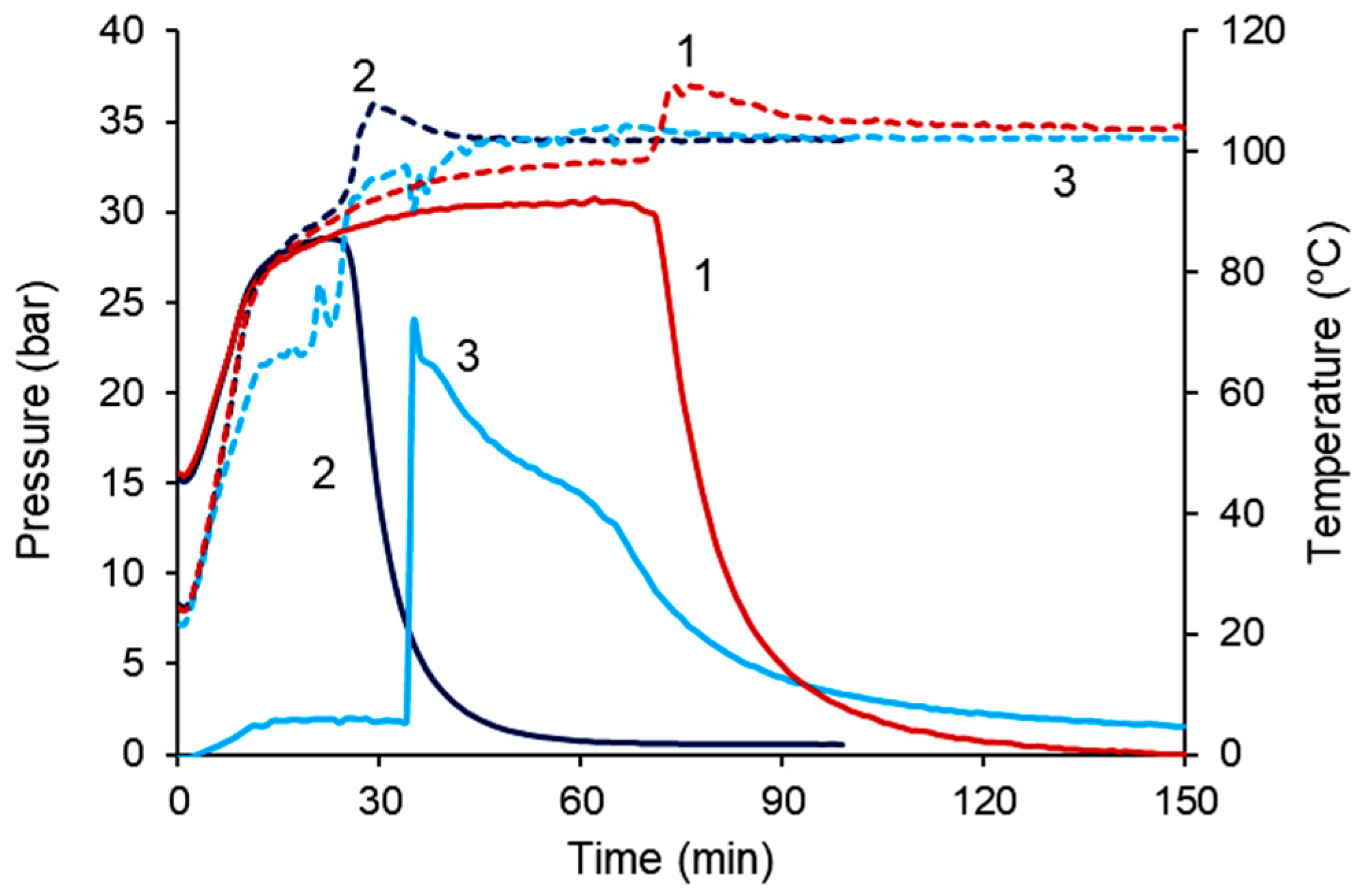
3. Results
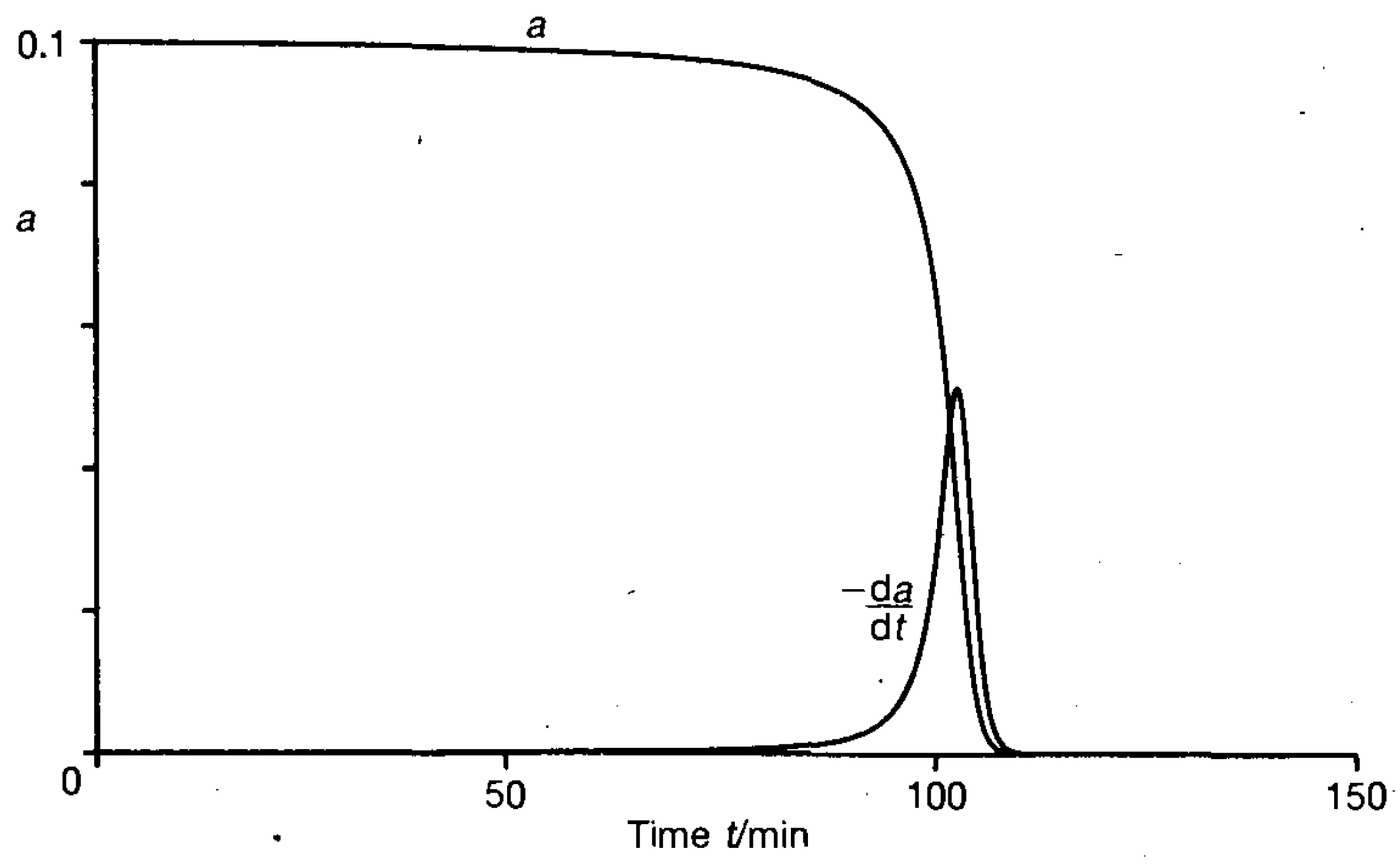
4. Discussion


5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
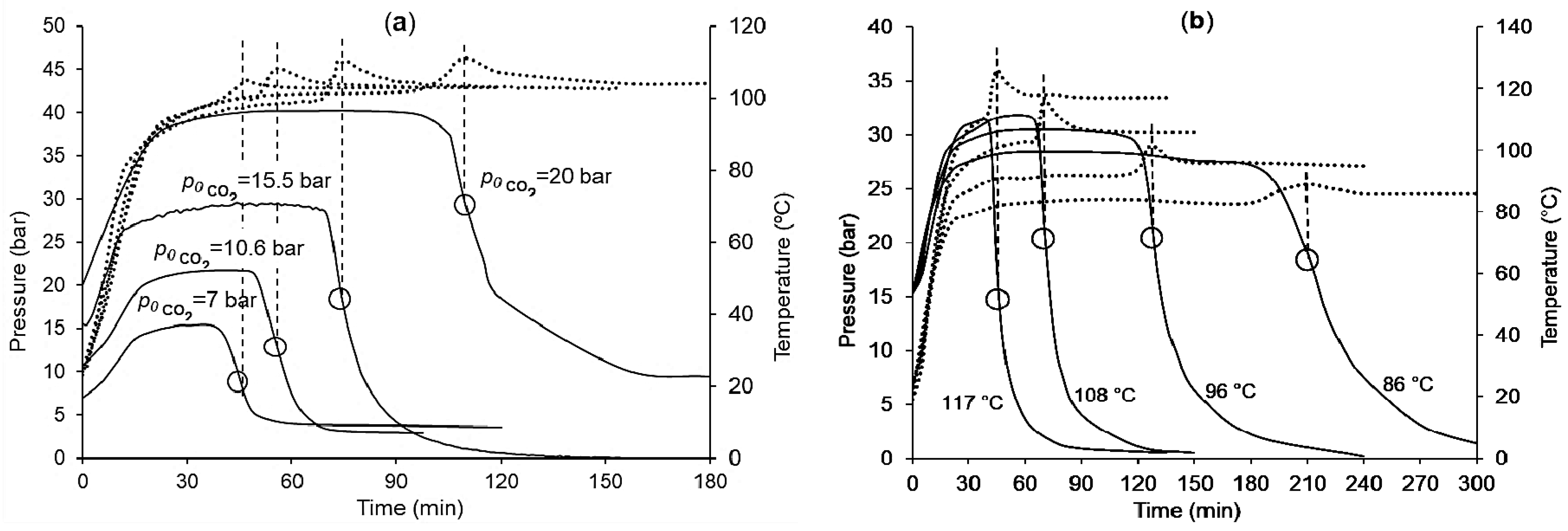
References
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Tamboli, A.H.; Kim, H. Ionic liquid as a catalyst for utilization of carbon dioxide to production of linear and cyclic carbonate. Fuel 2017, 200, 316–332. [Google Scholar] [CrossRef]
- Cheng, W.; Su, Q.; Wang, J.; Sun, J.; Ng, F.T.T. Ionic liquids: The synergistic catalytic effect in the synthesis of cyclic carbonates. Catalysts 2013, 3, 878–901. [Google Scholar] [CrossRef]
- Omae, I. Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord. Chem. Rev. 2012, 256, 1384–1405. [Google Scholar] [CrossRef]
- Plavnik, R.G.; Nevmerzhitskii, V.I.; Butorova, L.I.; Plavnik, T.E. Comparative evaluation mass spectrometry and infrared spectrometry during 13C-urea breath test for Helicobacter pylori. Klin. Med. 2015, 93, 42–45, (text in Russian, abstract and references in English). [Google Scholar]
- Elman, A.R.; Korneeva, G.A.; Noskov, Yu.G.; Khan, V.N.; Shishkina, E.Yu.; Negrimovskii, V.M.; Ponomarenko, E.P.; Kononov, L.O.; Bruk, L.G.; Oshanina, I.V.; Temkin, O.N.; et al. Sintez produktov, mechennykh izotopom 13C, dlya medicinskoi diagnostiki. Ross. Khim. Zhurnal 2013, 57, 3–24. [Google Scholar]
- Elman, A.R.; Ovsyannikova, L.V.; Davydov, I.E.; Kushnarev, D.I.; Gubanov, O.V.; Zyryanov, S.M.; Sidko, Yu.A. Method of Producing 13C-Urea. R.F. Patents RU2638837, 18 December 2017. [Google Scholar]
- Elman, A.R.; Davydov, I.E.; Stepanov, A.A. Synthesis of urea by ammonolysis of propylene carbonate. J. Chem. Chem. Eng. 2018, 12, 26–30. [Google Scholar] [CrossRef]
- Ryzhenkov, A.M. Propilenkarbonat. In Khimicheskaya Enciclopediya; Zefirov, N.S., Ed.; Bolshaya Rossiiskaya Enciclopediya: Moscow, Russia, 1995; Volume 4, p. 104. [Google Scholar]
- SYSSOFT. TableCurve 2D v5.01.05, Automated Curve Fitting & Equation Discovery; SYSTAT Software Inc.: San Jose, CA, USA, 2002; Available online: https://www.syssoft.ru/Systat (accessed on 4 May 2019).
- Maeda, C.; Taniguchi, T.; Ogawa, K.; Ema, T. Bifunctional catalysts based on m-phenylene-bridged porphyrin dimer and trimer platforms: Synthesis of cyclic carbonates from carbon dioxide and epoxides. Angew. Chem. Int. Ed. 2015, 54, 134–138. [Google Scholar] [CrossRef]
- Offermans, W.K.; Bizzarri, C.; Leitner, W.; Müller, T.E. Surprisingly facile CO2 insertion into cobalt alkoxide bonds: A theoretical investigation. Beilstein J. Org. Chem. 2015, 11, 1340–1351. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef] [PubMed]
- Shmid, R.; Sapunov, V.N. Neformal’naya Kinetika. V Poiskakh Putei Khimicheskikh Reaktsii (Nonformal kinetics. Nosing for Paths of Chemical Reactions; Mir: Moscow, Russia, 1985; p. 75, (English–Russian translation). [Google Scholar]
- Temkin, O.N. Homogeneous Catalysis with Metal Complexes: Kinetic Aspects and Mechanisms; John Wiley & Sons Ltd.: Chichester, UK, 2012; pp. 613, 617–619. [Google Scholar]
- Gray, P.; Scott, S.K. Chemical Oscillations and Instabilities. Non-linear Chemical Kinetics; Clarendon Press: Oxford, UK, 1990; p. 10. [Google Scholar]
- Rulev, Y.A. Novyye Kataliticheskiye Sistemy dlya Sinteza Ciklicheskih Karbonatov. Ph.D. Thesis, INEOS RAN, Moscow, Russia, 2017; p. 7. [Google Scholar]
- Rulev, Y.A.; Gugkaeva, Z.; Maleev, V.I.; North, M.; Belokon, Y.N. Robust bifunctional aluminium–salen catalysts for the preparation of cyclic carbonates from carbon dioxide and epoxides. Beilstein J. Org. Chem. 2015, 11, 1614–1623. [Google Scholar] [CrossRef]
- Hiraoka, M. Kraun-Soedineniya. Svoistva i Primeneniye; Mir: Moscow, Russia, 1986; p. 55. [Google Scholar]
- Luinstra, G.A.; Haas, G.R.; Molnar, F.; Bernhart, V.; Eberhardt, R.; Rieger, B. On the formation of aliphatic polycarbonates from epoxides with chromium(III) and aluminum(III) metal–salen complexes. Chem. Eur. J. 2005, 11, 6298–6314. [Google Scholar] [CrossRef] [PubMed]
- Longwitz, L.; Steinbauer, J.; Spannenberg, A.; Werner, T. Calcium-based catalytic system for the synthesis of bio-derived cyclic carbonates under mild conditions. ACS Catal. 2018, 8, 665–672. [Google Scholar] [CrossRef]
- Steinbauer, J.; Spannenberg, A.; Werner, T. An in situ formed Ca2+–crown ether complex and its use in CO2-fixation reactions with terminal and internal epoxides. Green Chem. 2017, 19, 3769–3779. [Google Scholar] [CrossRef]
- Desens, W.; Werner, T. Convergent activation concept for CO2 fixation in carbonates. Adv. Synth. Catal. 2016, 358, 622–630. [Google Scholar] [CrossRef]
- Kaneko, S.; Shirakawa, S. Potassium iodide−tetraethylene glycol complex as a practical catalyst for CO2 fixation reactions with epoxides under mild conditions. ACS Sustain. Chem. Eng. 2017, 5, 2836–2840. [Google Scholar] [CrossRef]
- Kim, H.-G.; Lim, C.-S.; Kim, D.-W.; Cho, D.-H.; Lee, D.-K.; Chung, J.S. Multifunctional alkanolamine as a catalyst for CO2 and propylene oxide cycloaddition. Mol. Catal. 2017, 438, 121–129. [Google Scholar] [CrossRef]
- Yuan, G.; Zhao, Y.; Wu, Y.; Li, R.; Chen, Y.; Xu, D.; Liu, Z. Cooperative effect from cation and anion of pyridine-containing anion-based ionic liquids for catalysing CO2 transformation at ambient conditions. Sci. China Chem. 2017, 60, 958–963. [Google Scholar] [CrossRef]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent developments in the synthesis of cyclic carbonates from epoxides and CO2. In Chemical Transformations of Carbon Dioxide; Springer: Cham, Switzerland, 2017; pp. 89–144. [Google Scholar]
- Blass, J.; Brunke, J.; Emmerich, F.; Przybylski, C.; Garamus, V.M.; Feoktystov, A.; Bennewitz, R.; Wenz, G.; Albrecht, M. Interactions between shape-persistent macromolecules as probed by AFM. Beilstein J. Org. Chem. 2017, 13, 938–951. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.J.; Lee, B.G.; Jung, O.S.; Jang, H.G.; Kang, S.O. Isolation of a pyridinium alkoxy ion bridged dimeric zinc complex for the coupling reactions of CO2 and epoxides. Angew. Chem. Int. Ed. 2000, 39, 4096–4098. [Google Scholar] [CrossRef]
- Elman, A.R.; Zharkov, S.A.; Ovsyannikova, L.V. Organic catalysis: Synthesis of propylene carbonate by the carboxylation of propylene oxide in the presence of phenols and fluorinated alcohols. Russ. J. Gen. Chem. 2018, 88, 1562–1567. [Google Scholar] [CrossRef]
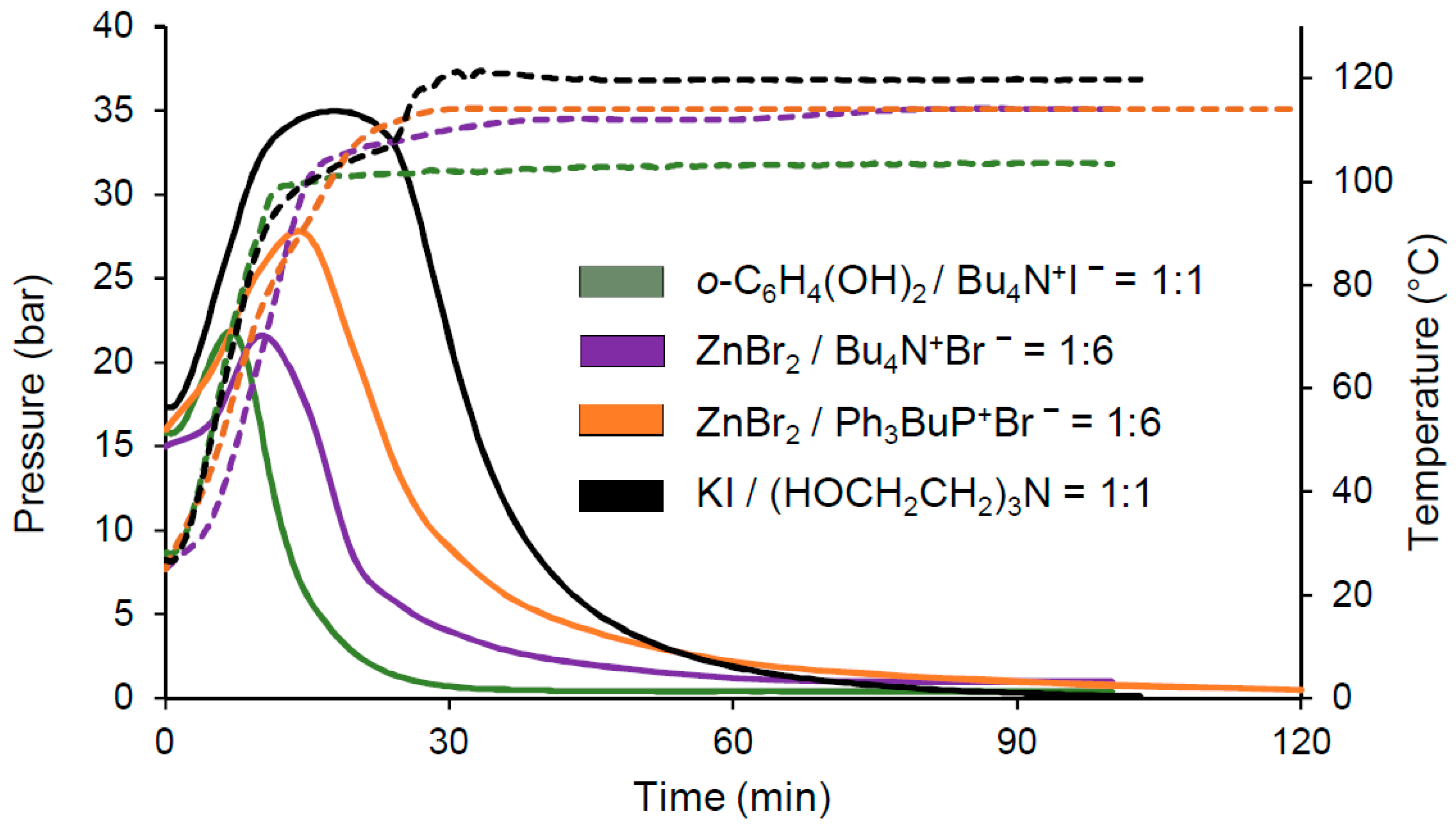
| Run | Catalyst (mol%) | Cocatalyst (mol%) | CO2/PO, mol/mol | p01, bar | T, °C | Time, min | Yield PC 2, % | TON | TOF 3, h−1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ZnBr2 (0.05) | Bu4N+Br− (0.30) | 0.74 | 12.0 | 114 | 80 | 97.0 | 1417 | 1214 |
| 2 | ZnBr2 (0.05) | Ph3BuP+Br− (0.28) | 0.83 | 13.7 | 114 | 120 | 98.8 | 3217 | 958 |
| 3 | o-C6H4(OH)2 (1.0) | Bu4N+I− (1.0) | 0.89 | 14.9 | 104 | 40 | 95.7 | 85 | 231 |
| 4 | KI (1.0) | (HOCH2CH2)3N (1.0) | 0.99 | 17.0 | 120 | 100 | 97.3 | 96 | 115 |
| 5 | ZnBr2 (0.05) | Et4N+Br− (0.20) | 0.95 | 15.6 | 103 | 150 | 93.8 | 1713 | 1285 |
| 6 | ZnBr2 (0.05) | Et4N+Br− (0.20) | 0.37 | 7.0 | 103 | 70 | 98.5 | 717 | 2152 |
| 7 | ZnBr2 (0.05) | Et4N+Br− (0.20) | 1.26 | 20.0 | 104 | 180 | 74.4 | 1839 | 1839 |
| 8 | ZnBr2 (0.05) | Et4N+Br− (0.20) | 0.97 | 15.9 | 86 | >300 4 | 94.7 | 1816 | 908 |
| 9 | ZnBr2 (0.025) | Et4N+Br− (0.20) | 0.99 | 16.7 | 102 | 180 | 93.1 | 3603 | 1802 |
| 10 | ZnBr2 (0.008) | Et4N+Br− (0.20) | 0.93 | 15.8 | 103 | 300 | 92.9 | 11,102 | 2961 |
| 11 | ZnCl2 (0.06) | Et4N+Br− (0.20) | 0.89 | 15.4 | 100 | 210 | 99.3 | 1547 | 1237 |
| 12 | ZnBr2 (0.05) | – | 0.93 | 15.2 | 102 | 360 | No prod. 5 | – | – |
| 13 | – | Et4N+Br− (0.20) | 0.93 | 15.3 | 104 | 360 | 14.5 | 69 | 11 |
| 14 | AlBr3 (0.07) | Et4N+Br− (0.20) | 0.91 | 15.4 | 103 | >300 4 | 88.3 | 1211 | 242 |
| 15 | AlCl3 × 6H2O (0.05) | Et4N+Br− (0.20) | 0.90 | 15.4 | 104 | >300 4 | 64.5 | 1129 | 226 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elman, A.R.; Zharkov, S.A.; Ovsyannikova, L.V. Unusual Regularities of Propylene Carbonate Obtained by Propylene Oxide Carboxylation in the Presence of ZnBr2/Et4N+Br− System. ChemEngineering 2019, 3, 46. https://doi.org/10.3390/chemengineering3020046
Elman AR, Zharkov SA, Ovsyannikova LV. Unusual Regularities of Propylene Carbonate Obtained by Propylene Oxide Carboxylation in the Presence of ZnBr2/Et4N+Br− System. ChemEngineering. 2019; 3(2):46. https://doi.org/10.3390/chemengineering3020046
Chicago/Turabian StyleElman, Alexander R., Sergei A. Zharkov, and Liudmila V. Ovsyannikova. 2019. "Unusual Regularities of Propylene Carbonate Obtained by Propylene Oxide Carboxylation in the Presence of ZnBr2/Et4N+Br− System" ChemEngineering 3, no. 2: 46. https://doi.org/10.3390/chemengineering3020046
APA StyleElman, A. R., Zharkov, S. A., & Ovsyannikova, L. V. (2019). Unusual Regularities of Propylene Carbonate Obtained by Propylene Oxide Carboxylation in the Presence of ZnBr2/Et4N+Br− System. ChemEngineering, 3(2), 46. https://doi.org/10.3390/chemengineering3020046






