Ionic Liquid Hydrogel Composite Membranes (IL-HCMs)
Abstract
1. Introduction
2. Experimental Section
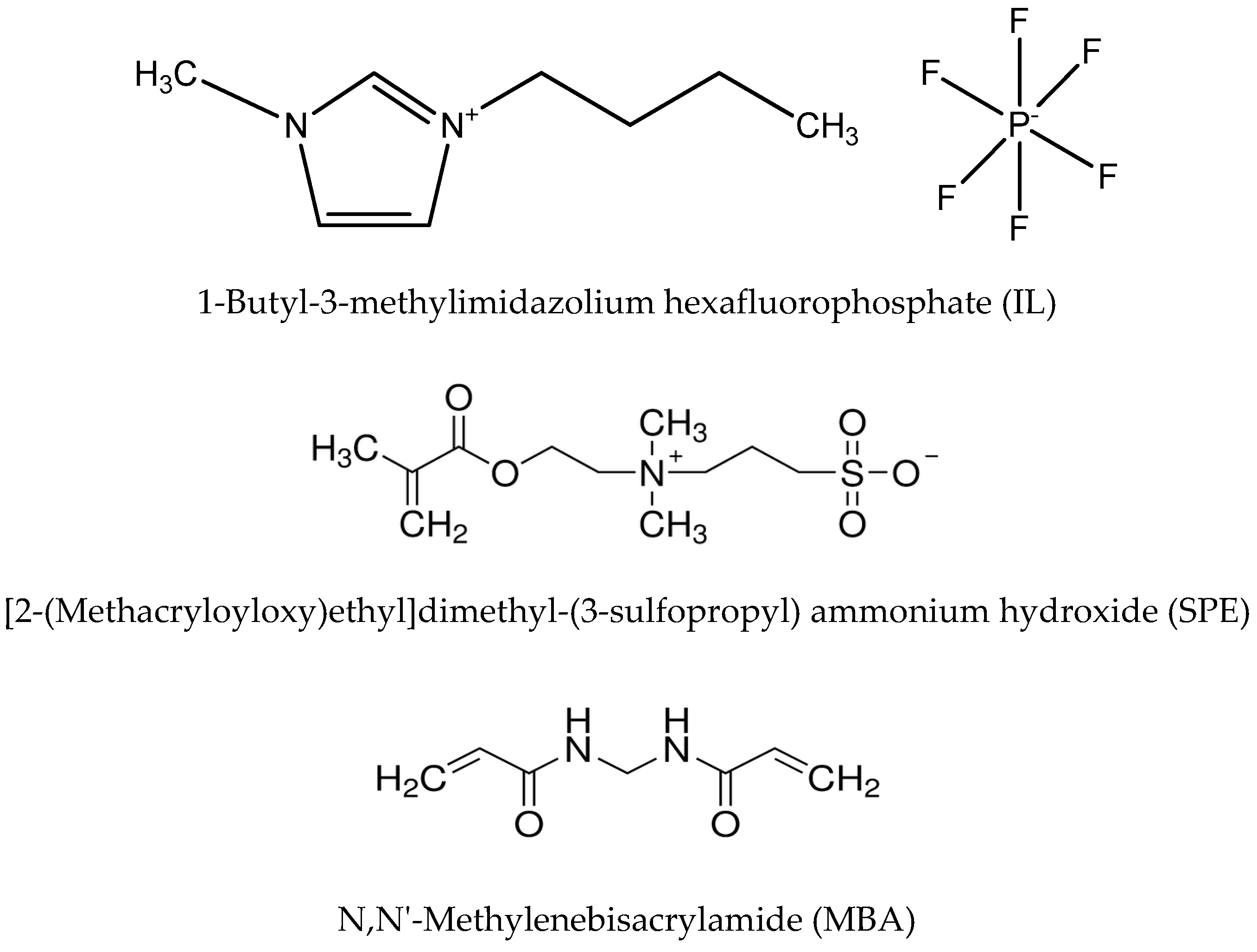
2.1. Materials
2.2. Preparation of Hydrogel Composite Membranes
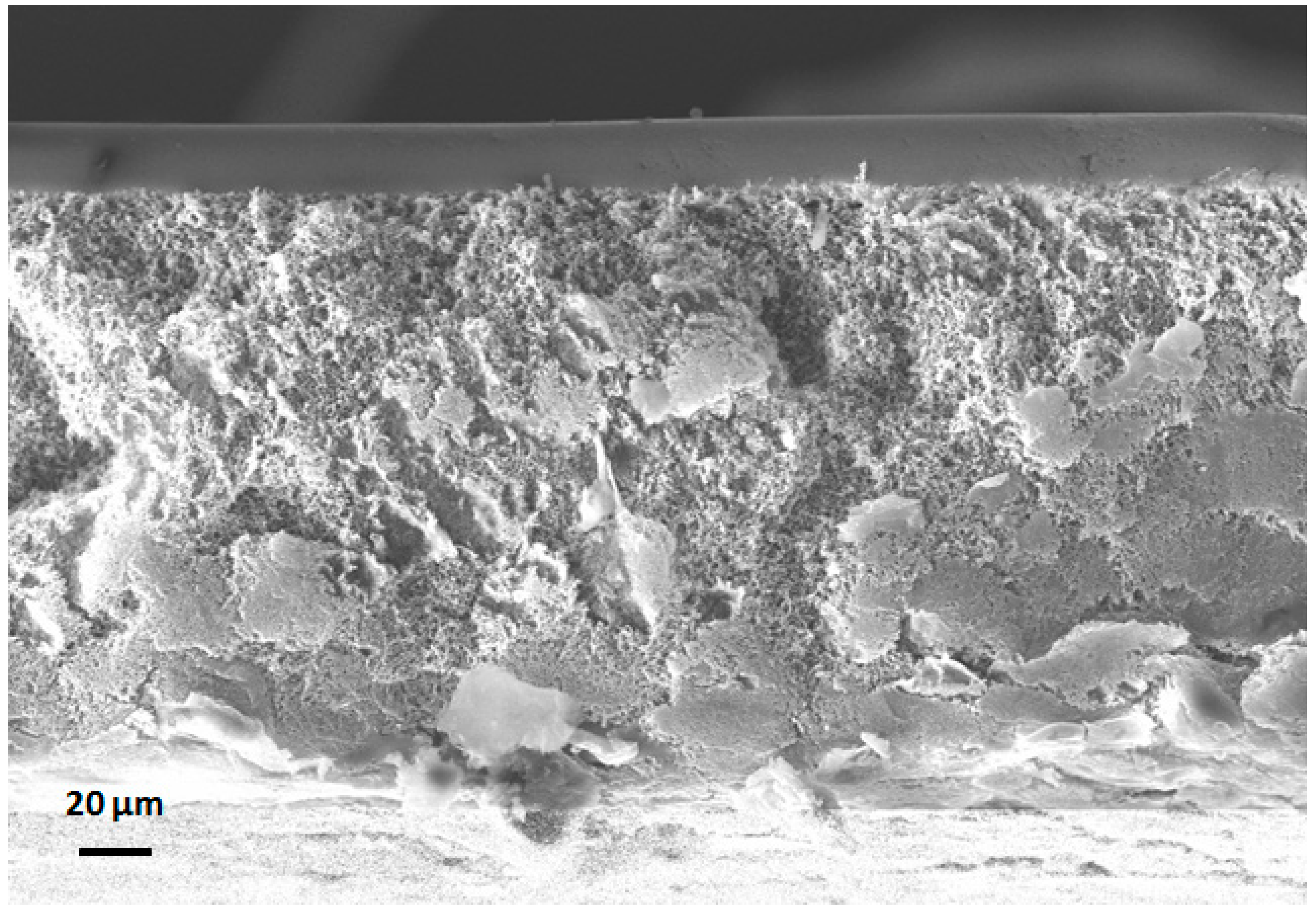
2.3. Membrane Morphology Examination
2.4. Water Contact Angle
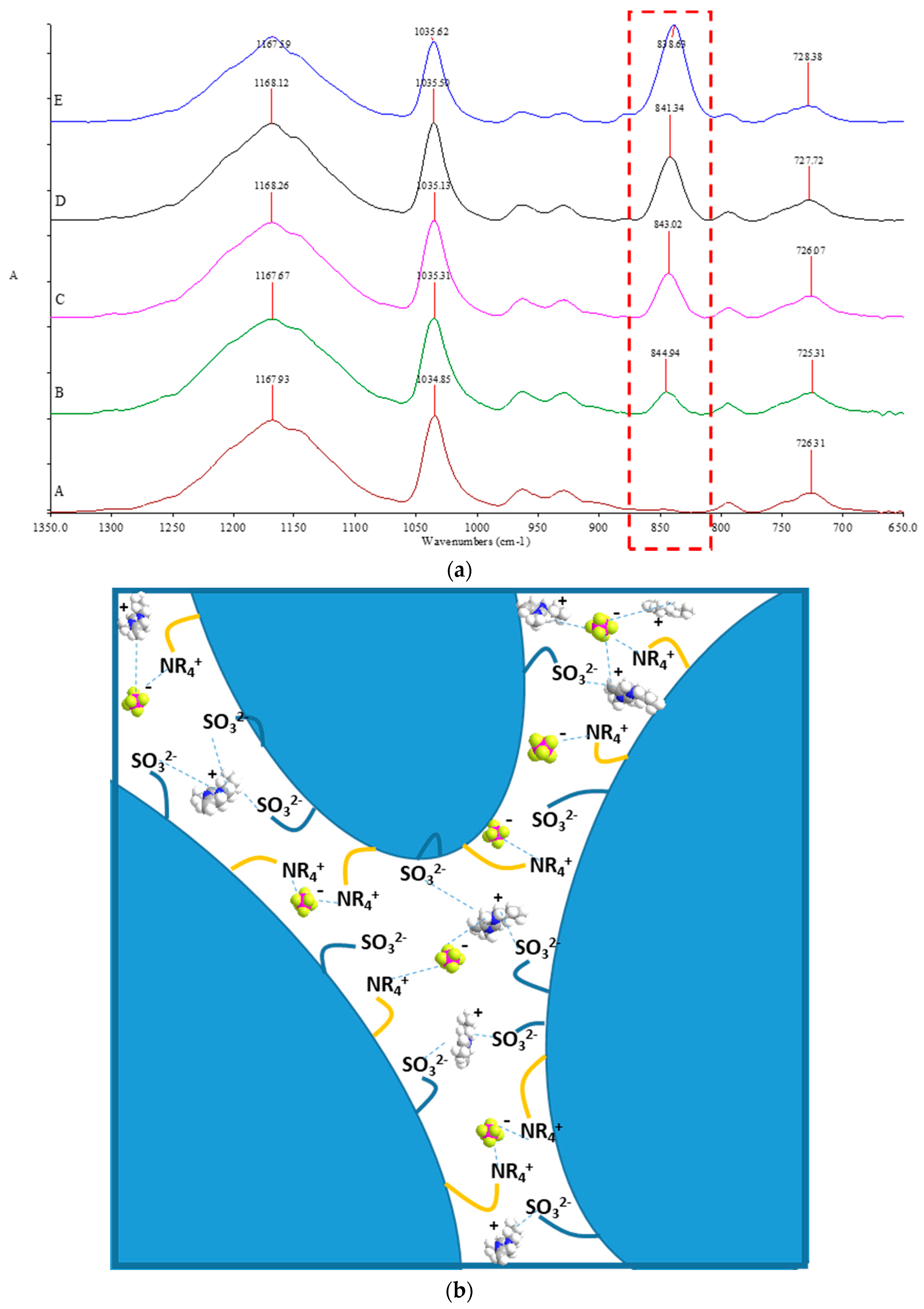
2.5. Chemical Surface Analysis
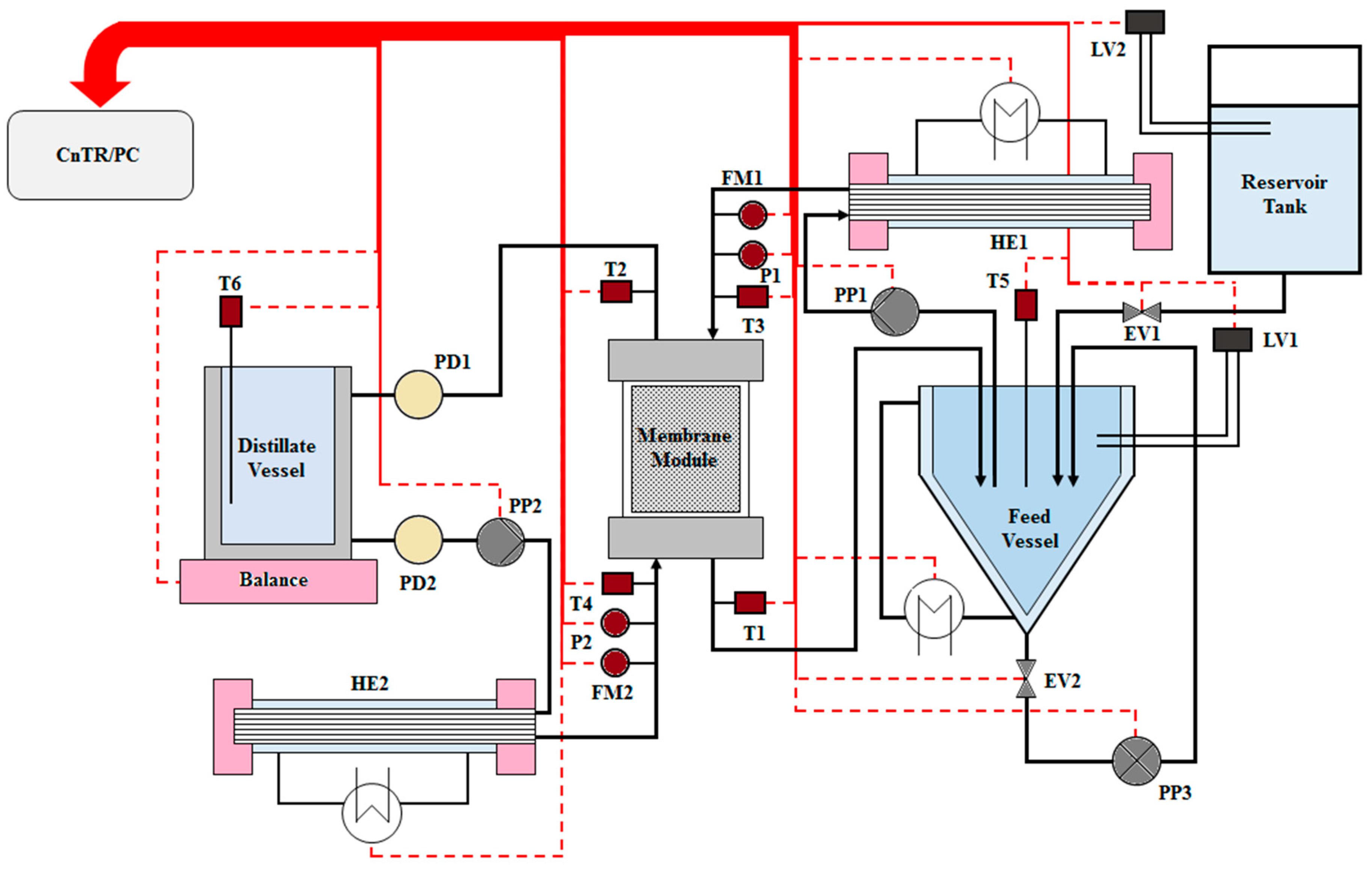
2.6. Direct Contact Membrane Distillation Experiments
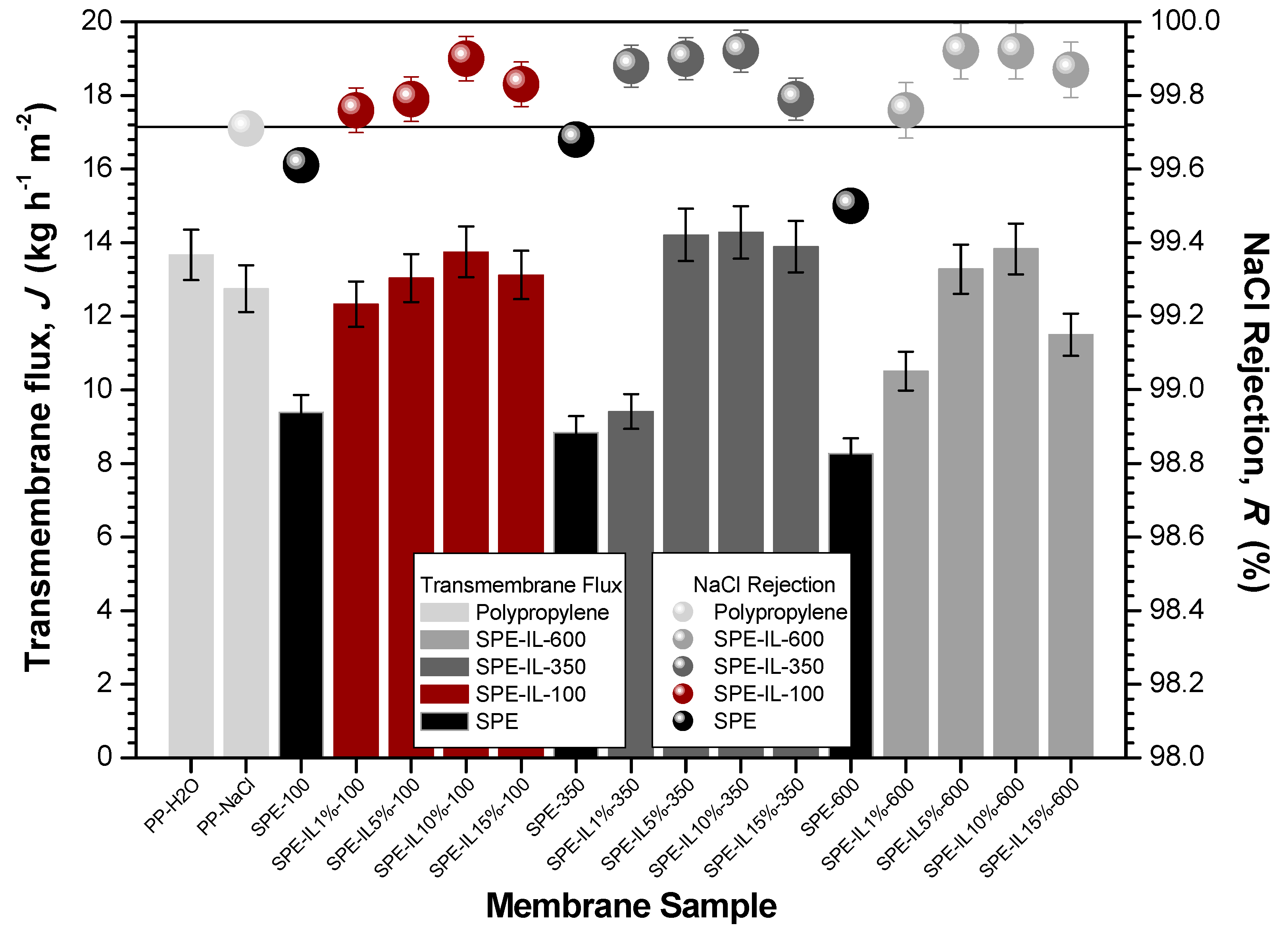
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osada, Y.; Gong, J.P. Soft and Wet Materials: Polymer Gels. Adv. Mater. 1998, 10, 827. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Tong, J.; Anderson, J.L. Partitioning and diffusion of proteins and linear polymers in polyacrylamide gels. Biophys. J. 1996, 70, 1505–1513. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Surface-Tethered pH-Responsive Hydrogel Thin Films as Size-Selective Layers on Nanoporous Asymmetric Membranes. Chem. Mater. 2009, 21, 4323–4331. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Polotsky, A.A.; Plamper, F.A.; Borisov, O.V. Collapse-to-Swelling Transitions in pH- and Thermoresponsive Microgels in Aqueous Dispersions: The Thermodynamic Theory. Macromolecules 2013, 46, 8702–8709. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling Behaviors of pH- and Salt-Responsive Cellulose-Based Hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.T.; Uenonishio, S. Collapse of Gels in an Electric Field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Mosiewicz, K.A.; Kolb, L.; van der Vlies, A.J.; Martino, M.M.; Lienemann, P.S.; Hubbell, J.A.; Ehrbar, M.; Lutolf, M.P. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013, 12, 1072–1078. [Google Scholar] [CrossRef]
- Frey, W.; Meyer, D.E.; Chilkoti, A. Dynamic addressing of a surface pattern by a stimuli-responsive fusion protein. Adv. Mater. 2003, 15, 248–251. [Google Scholar] [CrossRef]
- Mart, R.J.; Osborne, R.D.; Stevens, M.M.; Ulijn, R.V. Peptide-based stimuli-responsive biomaterials. Soft Matter 2006, 2, 822–835. [Google Scholar] [CrossRef]
- Wilson, A.N.; Guiseppi-Elie, A. Bioresponsive Hydrogels. Adv. Healthcare Mater. 2013, 2, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar] [CrossRef]
- Salehi, S.M.; Di Profio, G.; Fontananova, E.; Nicoletta, F.P.; Curcio, E.; De Filpo, G. Membrane Distillation by Novel Hydrogel Composite Membranes. J. Membr. Sci. 2016, 504, 220–229. [Google Scholar] [CrossRef]
- Di Profio, G.; Polino, M.; Nicoletta, F.P.; Belviso, B.D.; Caliandro, R.; Fontananova, E.; De Filpo, G.; Curcio, E.; Drioli, E. Tailored hydrogel membranes for efficient protein crystallization. Adv. Funct. Mater. 2014, 24, 1582–1590. [Google Scholar] [CrossRef]
- Salehi, S.M.; Manjua, A.C.; Belviso, B.D.; Portugal, C.A.M.; Coelhoso, I.M.; Mirabelli, V.; Fontananova, E.; Caliandro, R.; Crespo, J.G.; Curcio, E.; et al. Hydrogel Composite Membranes Incorporating Iron Oxide Nanoparticles as Topographical Designers for Controlled Heteronucleation of Proteins. Cryst. Growth Des. 2018, 18, 3317–3327. [Google Scholar] [CrossRef]
- Di Profio, G.; Salehi, S.M.; Caliandro, R.; Guccione, P.; Nico, G.; Curcio, E.; Fontananova, E. Bioinspired synthesis of CaCO3 superstructures through a novel hydrogel composite membranes mineralization platform: A comprehensive view. Adv. Mater. 2016, 28, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, V.; Salehi, S.M.; Angiolillo, L.; Belviso, B.D.; Conte, A.; del Nobile, M.A.; Di Profio, G.; Caliandro, R. Enzyme Crystals and Hydrogel Composite Membranes as New Active Food Packaging Material. Glob. Chall. 2018, 2, 1700089. [Google Scholar] [CrossRef]
- Israelachvili, J.; Pashley, R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature 1982, 300, 341–342. [Google Scholar] [CrossRef]
- Tsao, Y.; Evans, D.; Wennerstrom, H. Long-range attractive force between hydrophobic surfaces observed by atomic force microscopy. Science 1993, 262, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Ma, Z.; Hong, Y.; Ma, L.; Su, M. Superhydrophobic membranes with ordered arrays of nanospiked microchannels for water desalination. Langmuir 2009, 25, 5446–5450. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Wang, Z.; Elimelech, M.; Lin, S. Environmental applications of interfacial materials with special wettability. Environ. Sci. Technol. 2016, 50, 2132–3150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, J.; Hou, D.; Lin, S. Tailoring surface charge and wetting property for robust oil-fouling mitigation in membrane distillation. J. Membr. Sci. 2016, 516, 113–122. [Google Scholar] [CrossRef]
- Pashley, R.M. Hydration forces between mica surfaces in aqueous electrolyte solutions. J. Colloid Interface Sci. 1981, 80, 153–162. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Fe, H.; Lu, D.; Cheng, W.; Zhang, T.; Lu, X.; Liu, Q.; Jiang, J.; Technology, P.; Cheng, W.; Zhang, T.; et al. Hydrophilic Fe2O3 dynamic membrane mitigating fouling of support ceramic membrane in ultrafiltration of oil/water emulsion. Sep. Purif. Technol. 2016, 165, 1–9. [Google Scholar]
- Lin, S.; Nejati, S.; Boo, C.; Hu, Y.; Osuji, C.O.; Elimelech, M. Omniphobic membrane for robust membrane distillation. Environ. Sci. Technol. Lett. 2014, 1, 443–447. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Engineering surface energy and nanostructure of microporous films for expanded membrane distillation applications. Environ. Sci. Technol. 2016, 50, 8112–8119. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, D.; Lin, S. Composite membrane with underwater-oleophobic surface for anti-oil-fouling membrane distillation. Environ. Sci. Technol. 2016, 50, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.J.; Yang, M.C.; Li, Y.L. Prevention of surfactant wetting with agarose hydrogel layer for direct contact membrane distillation used in dyeing wastewater treatment. J. Membr. Sci. 2015, 475, 511–520. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Akbari, A.; Peyravi, M.; Jahanshahi, M. PDADMAC/PAA semi-IPN hydrogel-coated PVDF membrane for robust anti-wetting in membrane distillation. J. Ind. Eng. Chem. 2019, 74, 14–25. [Google Scholar] [CrossRef]
- Talaty, E.R.; Raja, S.; Storhaug, V.J.; Do1lle, A.; Carper, W.R. Raman and Infrared Spectra and ab Initio Calculations of C2–4MIM Imidazolium Hexafluorophosphate Ionic Liquids. J. Phys. Chem. B 2004, 108, 13177–13184. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Vydrov, O.A.; Magee, J.W.; Frenkel, M. Thermodynamic Properties of 1-Butyl-3-methylimidazolium Hexafluorophosphate in the Ideal Gas State. J. Chem. Eng. Data 2003, 48, 457–462. [Google Scholar] [CrossRef]
- Peng, P.; Fane, A.G.; Li, X. Desalination by membrane distillation adopting a hydrophilic membrane. Desalination 2005, 173, 45–54. [Google Scholar] [CrossRef]
- Hagmeyer, G.; Gimbel, R. Modelling the salt rejection of nanofiltration membranes for ternary ion mixtures and for single salts at different pH values. Desalination 1998, 117, 247–256. [Google Scholar] [CrossRef]
- Garcia-Aleman, J.; Dickson, J.M. Permeation of mixed-salt solutions with commercial and pore-filled nanofiltration membranes: Membrane charge inversion phenomena. J. Membr. Sci. 2004, 239, 163–172. [Google Scholar] [CrossRef]
- Schaep, J.; van der Bruggen, B.; Vandecasteele, C.; Wilms, D. Influence of ion size and charge in nanofiltration. Sep. Purif. Technol. 1998, 14, 155–162. [Google Scholar] [CrossRef]
- Gan, Q.; Rooney, D.; Xue, M.; Thompson, G.; Zou, Y. An experimental study of gas transport and separation properties of ionic liquids supported on nanofiltration membranes. J. Membr. Sci. 2006, 280, 948–956. [Google Scholar] [CrossRef]
| Membrane Sample Code | Monomer (wt.%) | Crosslinker (wt.%) | Photoinitiator (wt.%) | H2O (wt.%) | IL (wt.%) | Casting Thickness (µm) |
|---|---|---|---|---|---|---|
| SPE-100 | 10.0 | 1.0 | 0.3 | 88.7 | 0 | 100 |
| SPE-IL1%-100 | 10.0 | 1.0 | 0.3 | 88.6 | 0.1 | 100 |
| SPE-IL5%-100 | 10.0 | 1.0 | 0.3 | 88.2 | 0.5 | 100 |
| SPE-IL10%-100 | 10.0 | 1.0 | 0.3 | 87.7 | 1.0 | 100 |
| SPE-IL15%-100 | 10.0 | 1.0 | 0.3 | 87.2 | 1.5 | 100 |
| SPE-350 | 10.0 | 1.0 | 0.3 | 88.7 | 0 | 350 |
| SPE-IL1%-350 | 10.0 | 1.0 | 0.3 | 88.7 | 0.1 | 350 |
| SPE-IL5%-350 | 10.0 | 1.0 | 0.3 | 88.6 | 0.5 | 350 |
| SPE-IL10%-350 | 10.0 | 1.0 | 0.3 | 88.2 | 1.0 | 350 |
| SPE-IL15%-350 | 10.0 | 1.0 | 0.3 | 87.7 | 1.5 | 350 |
| SPE-600 | 10.0 | 1.0 | 0.3 | 88.7 | 0 | 600 |
| SPE-IL1%-600 | 10.0 | 1.0 | 0.3 | 88.7 | 0.1 | 600 |
| SPE-IL5%-600 | 10.0 | 1.0 | 0.3 | 88.6 | 0.5 | 600 |
| SPE-IL10%-600 | 10.0 | 1.0 | 0.3 | 88.2 | 1.0 | 600 |
| SPE-IL15%-600 | 10.0 | 1.0 | 0.3 | 87.7 | 1.5 | 600 |
| Membrane Sample Code | Water Contact Angle (°) |
|---|---|
| PP support | 138.0 ± 1.0 |
| SPE-350 | 67.9 ± 3.4 |
| SPE-IL1%-350 | 61.1 ± 0.9 |
| SPE-IL5%-350 | 45.6 ± 1.9 |
| SPE-IL10%-350 | 44.5 ± 2.0 |
| SPE-IL15%-350 | 40.8 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majidi Salehi, S.; Santagada, R.; Depietra, S.; Fontananova, E.; Curcio, E.; Di Profio, G. Ionic Liquid Hydrogel Composite Membranes (IL-HCMs). ChemEngineering 2019, 3, 47. https://doi.org/10.3390/chemengineering3020047
Majidi Salehi S, Santagada R, Depietra S, Fontananova E, Curcio E, Di Profio G. Ionic Liquid Hydrogel Composite Membranes (IL-HCMs). ChemEngineering. 2019; 3(2):47. https://doi.org/10.3390/chemengineering3020047
Chicago/Turabian StyleMajidi Salehi, Shabnam, Rosangela Santagada, Stefania Depietra, Enrica Fontananova, Efrem Curcio, and Gianluca Di Profio. 2019. "Ionic Liquid Hydrogel Composite Membranes (IL-HCMs)" ChemEngineering 3, no. 2: 47. https://doi.org/10.3390/chemengineering3020047
APA StyleMajidi Salehi, S., Santagada, R., Depietra, S., Fontananova, E., Curcio, E., & Di Profio, G. (2019). Ionic Liquid Hydrogel Composite Membranes (IL-HCMs). ChemEngineering, 3(2), 47. https://doi.org/10.3390/chemengineering3020047








