Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD)
Abstract
1. Introduction
2. Membrane Distillation
2.1. History of MD Process
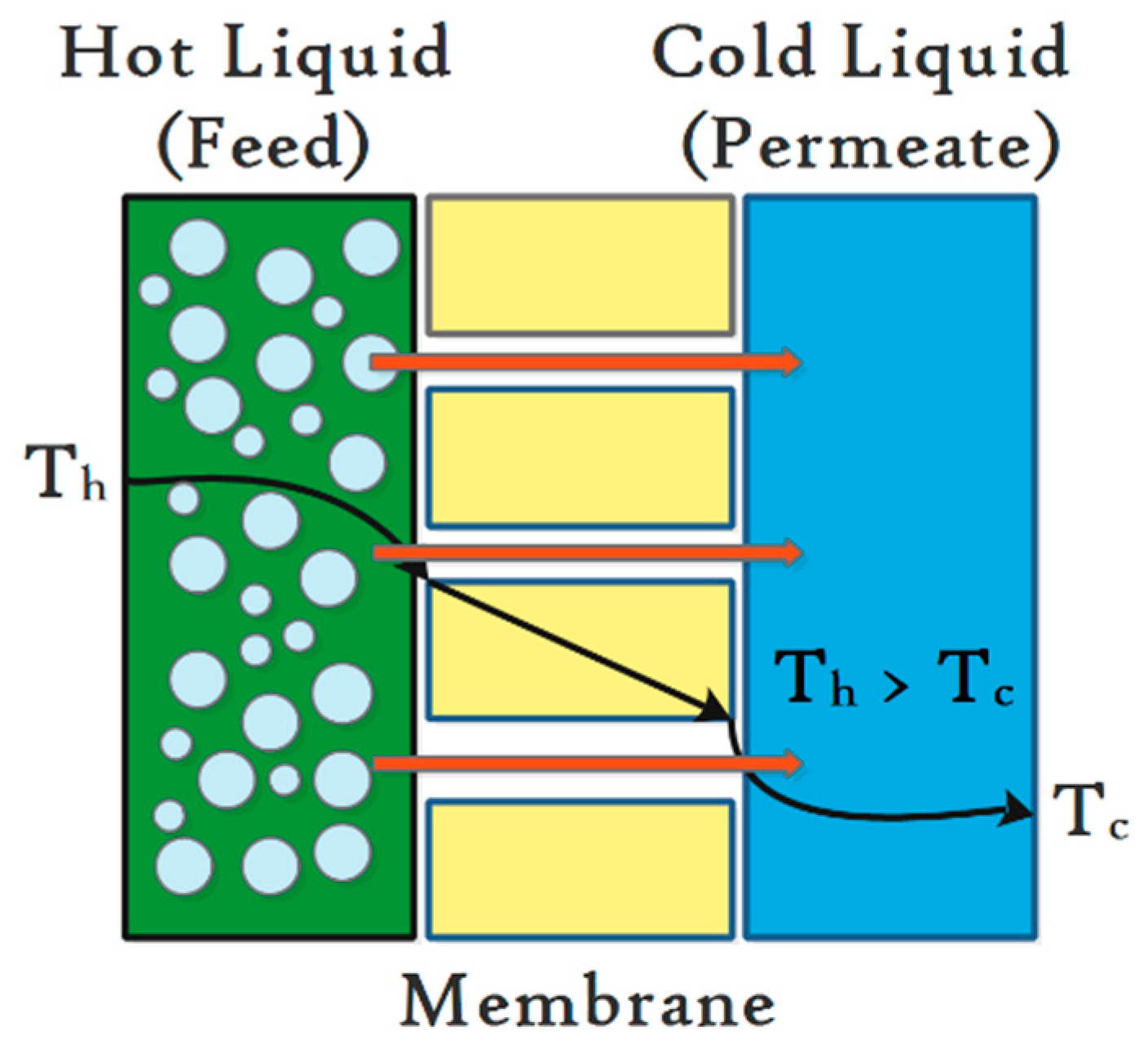
2.2. Definition of MD
2.3. Limitation and Benefits of MD Process
2.4. Membrane Characteristics
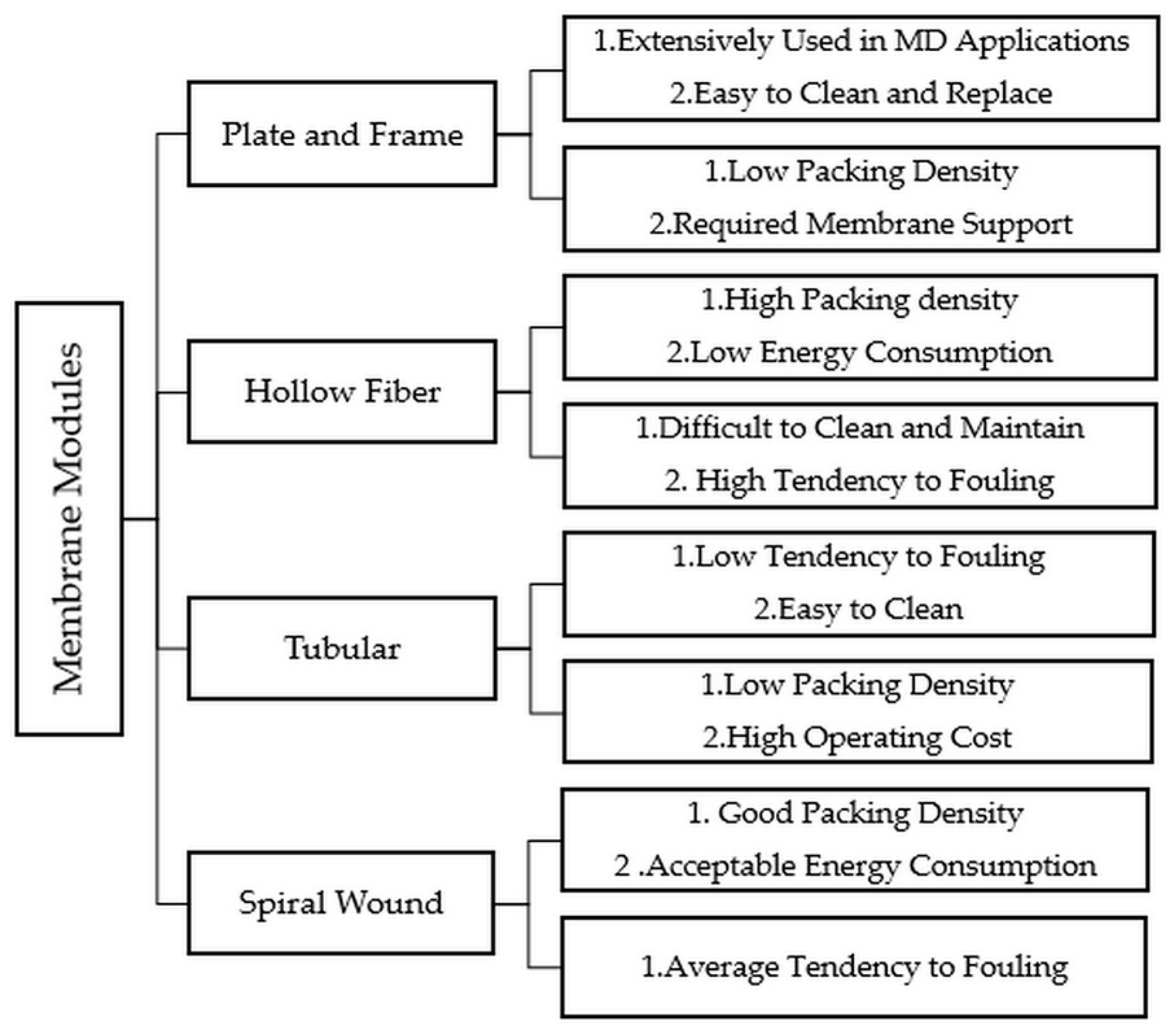
2.5. Membrane Materials and Modules
3. Conventional MD Configurations
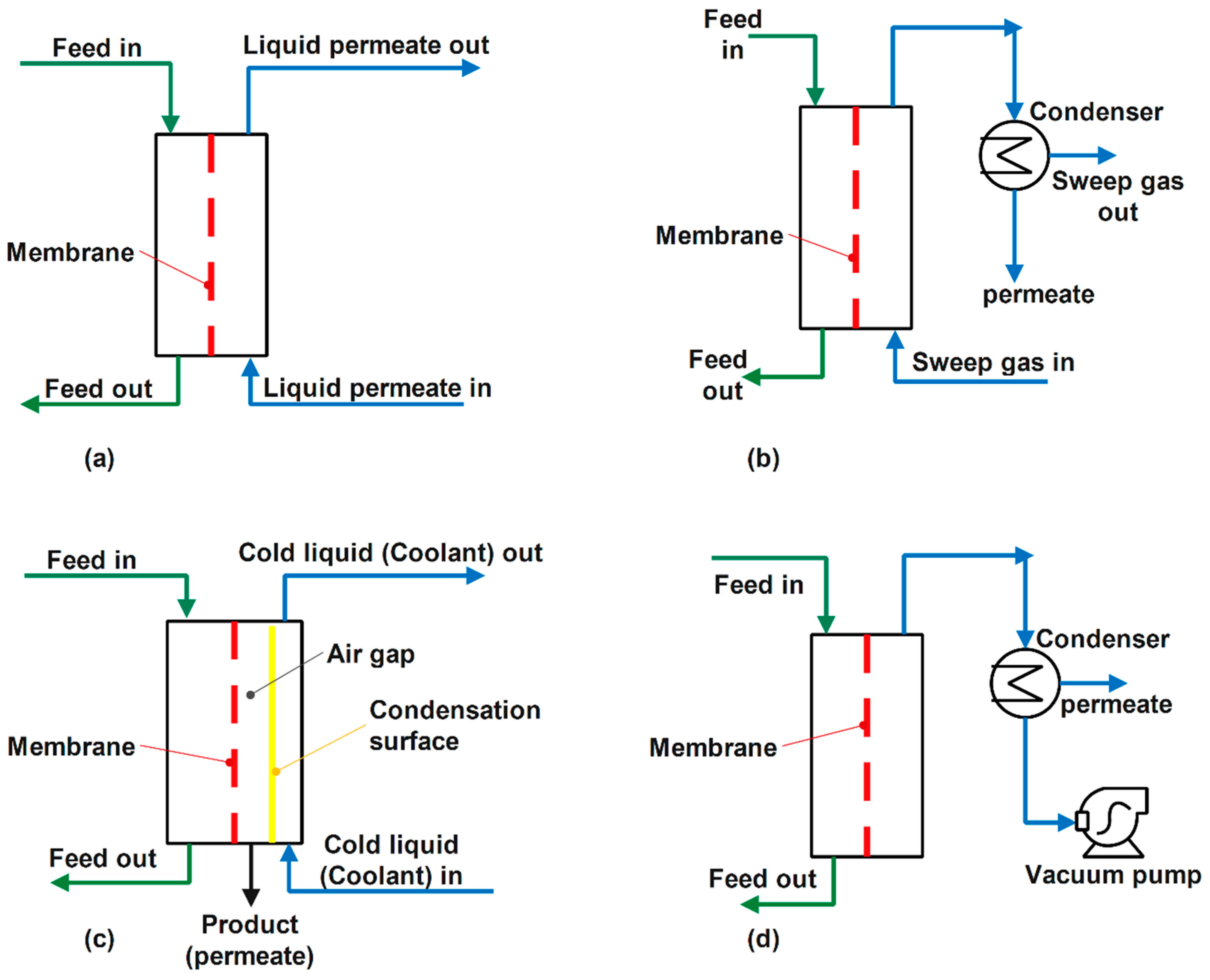
3.1. Direct Contact Membrane Distillation (DCMD)
3.2. Air Gap Membrane Distillation (AGMD)
3.3. Sweeping Gas Membrane Distillation (SGMD)
3.4. Vacuum Membrane Distillation (VMD)
4. New MD Configurations
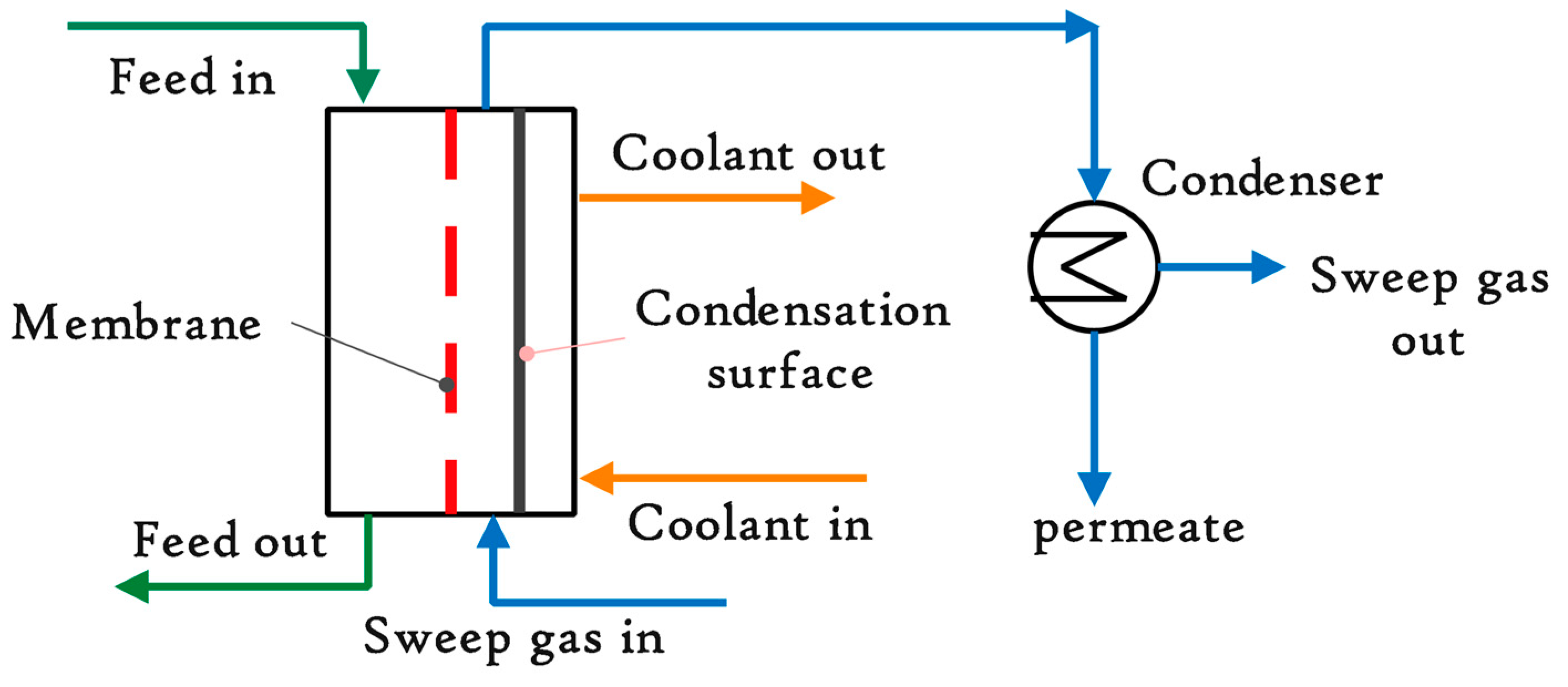
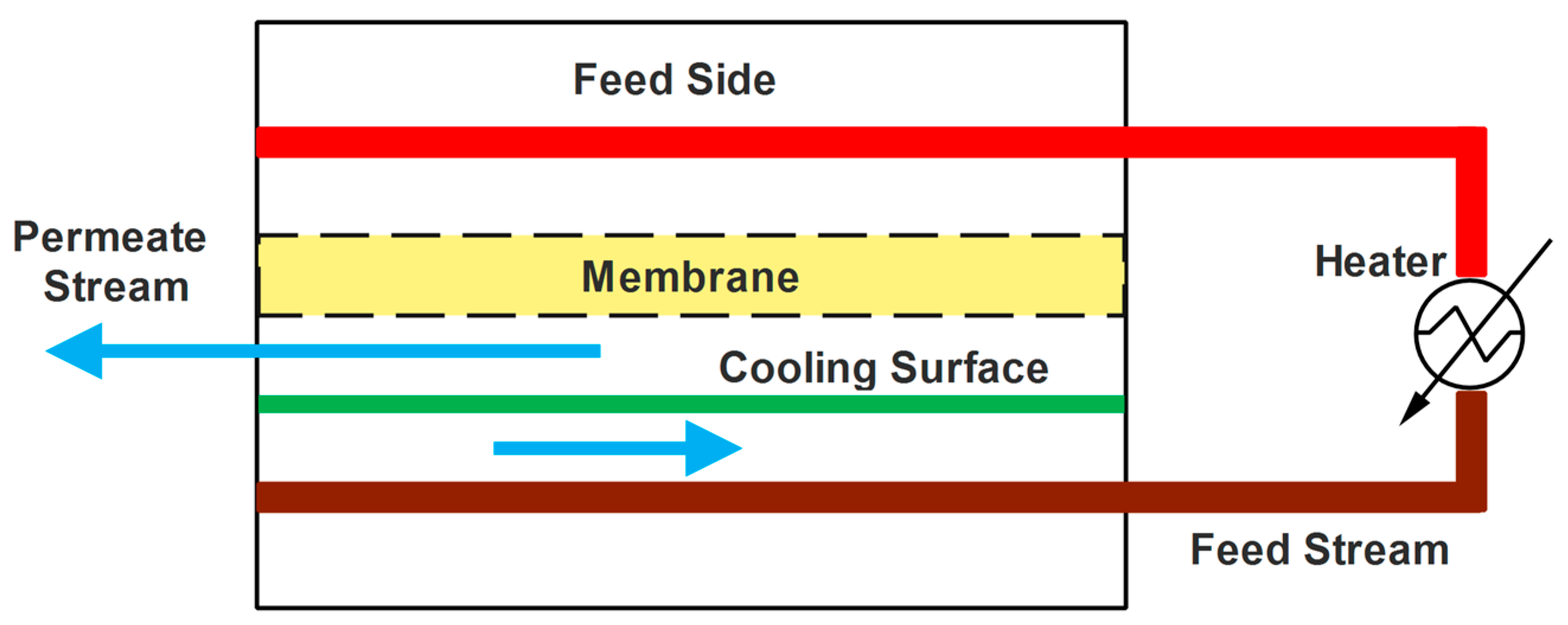
4.1. Thermostatic Sweeping Gas Membrane Distillation (TSGMD)
4.2. Multi-Effect Membrane Distillation (MEMD)
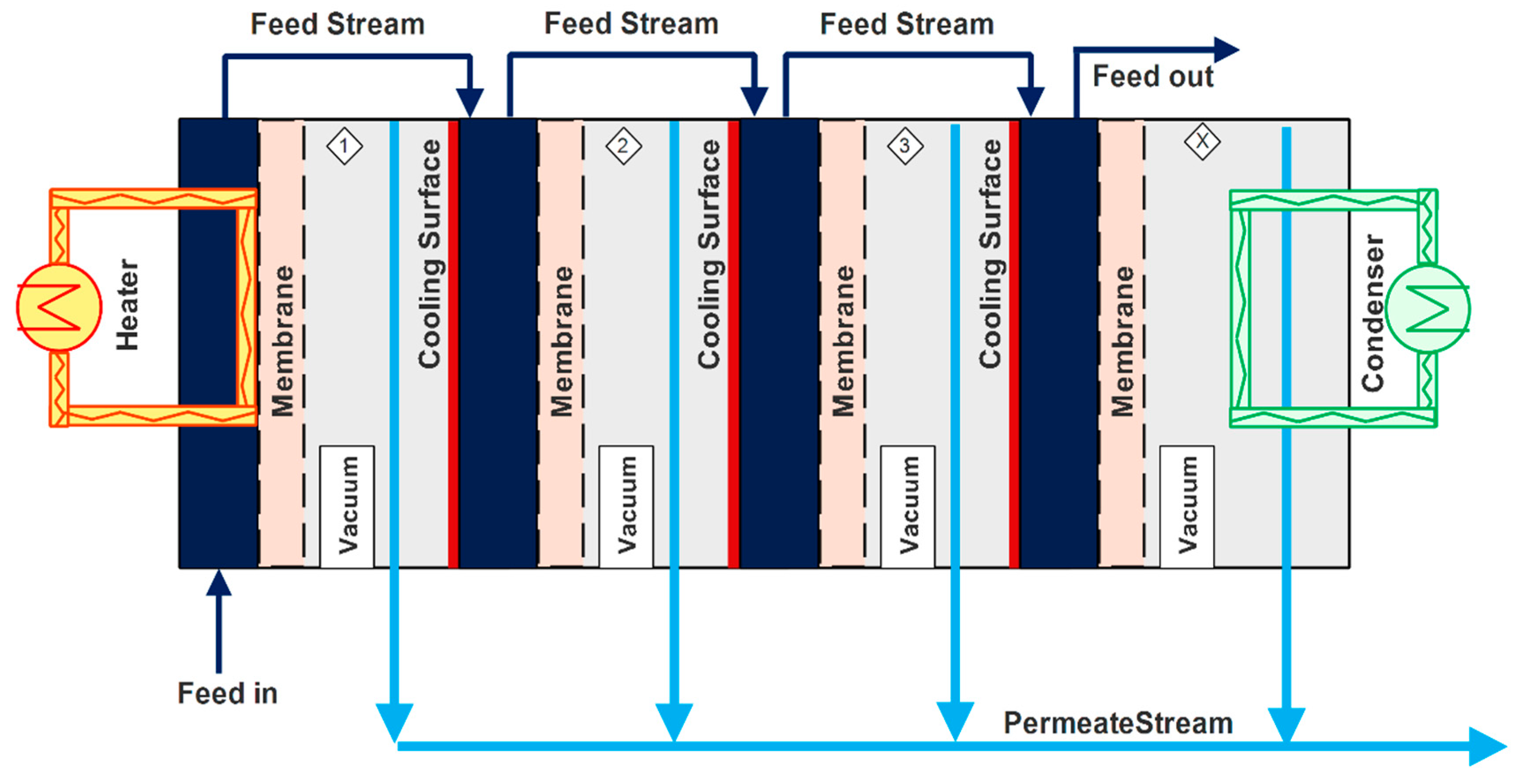
4.3. Vacuum Multi-Effect Membrane Distillation (V-MEMD)
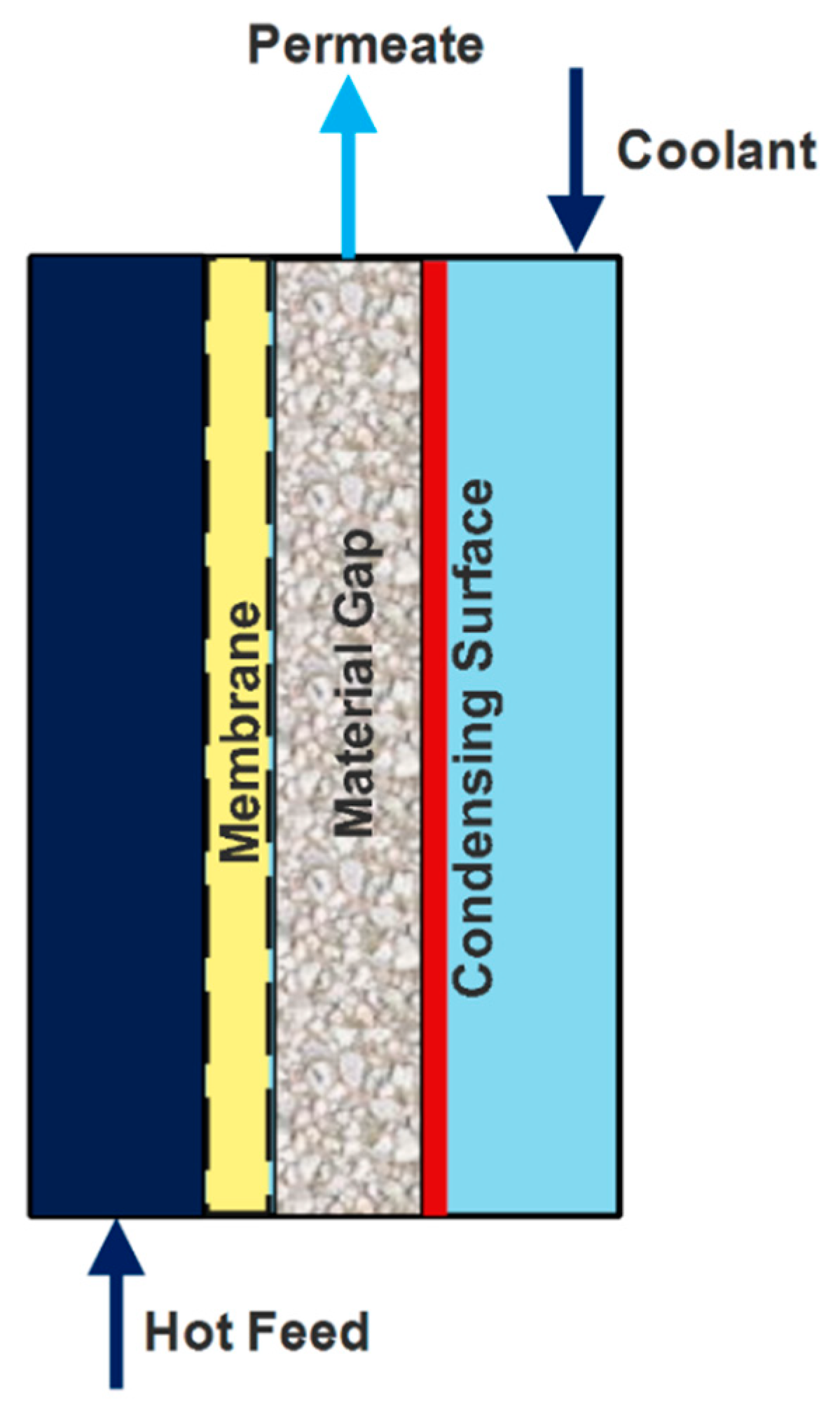
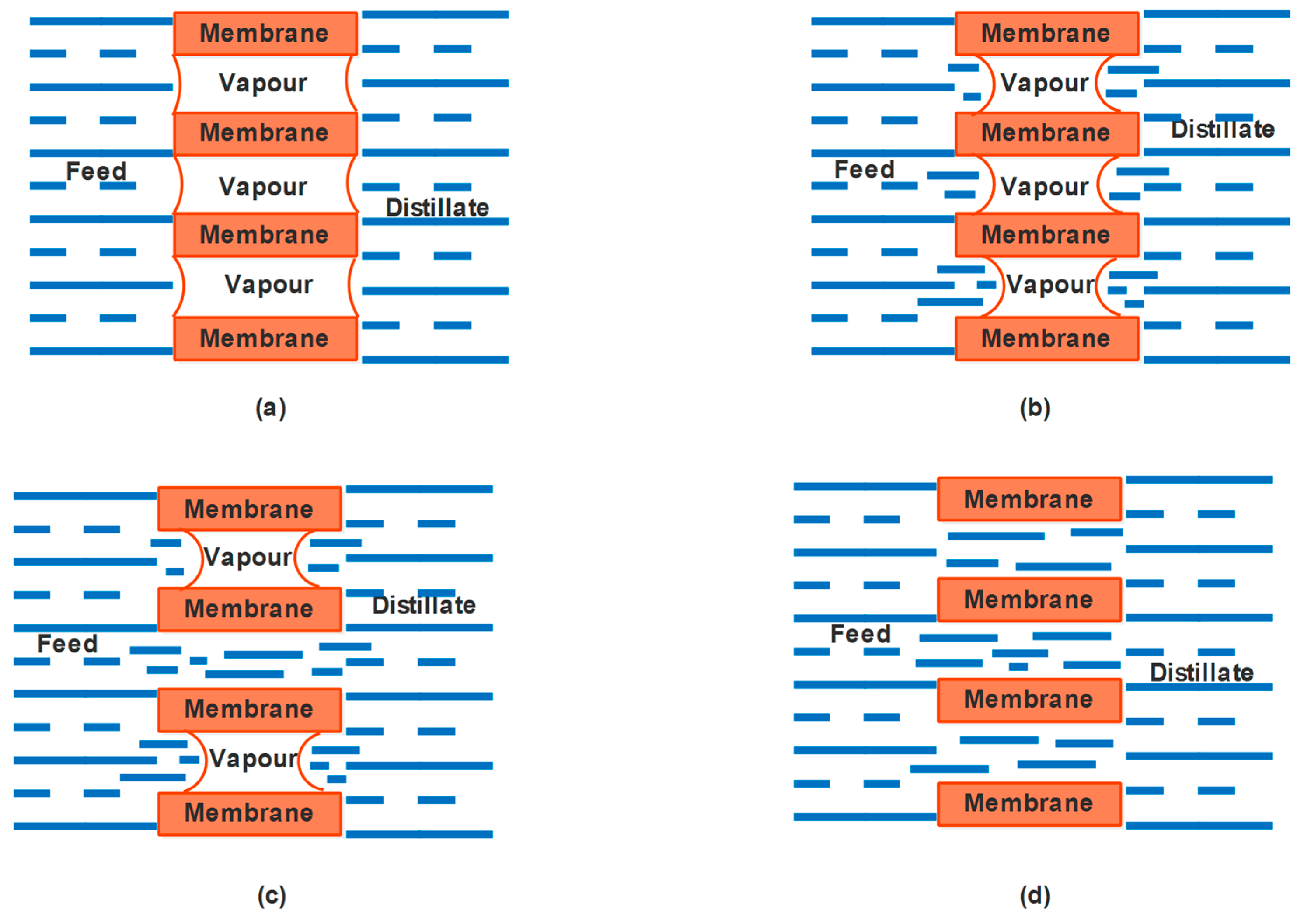
4.4. Material-Gap Membrane Distillation (MGMD)
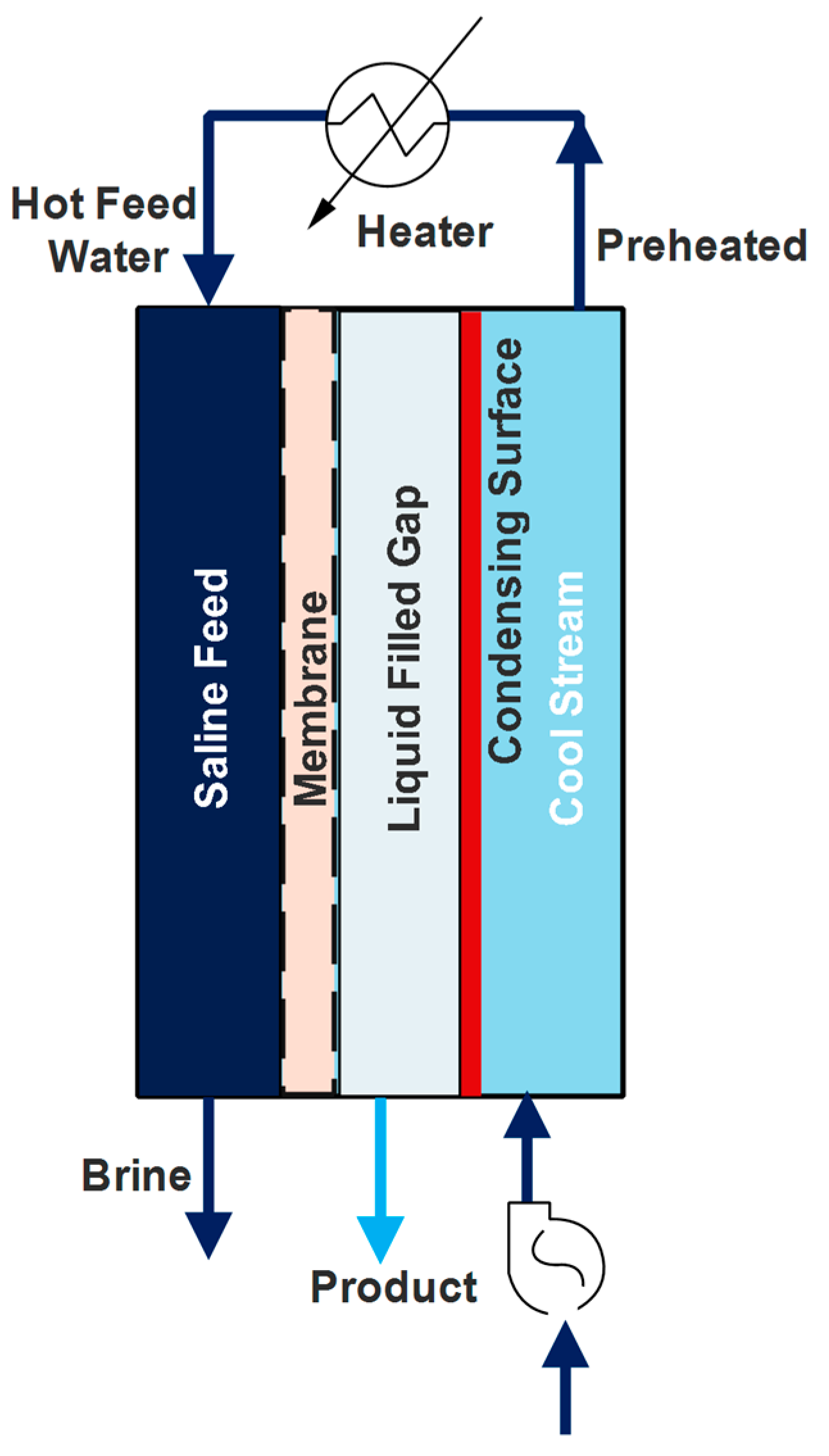
4.5. Permeate-Gap Membrane Distillation (PGMD)
5. Application of MD
The Application of MD in Water and Wastewater Treatment
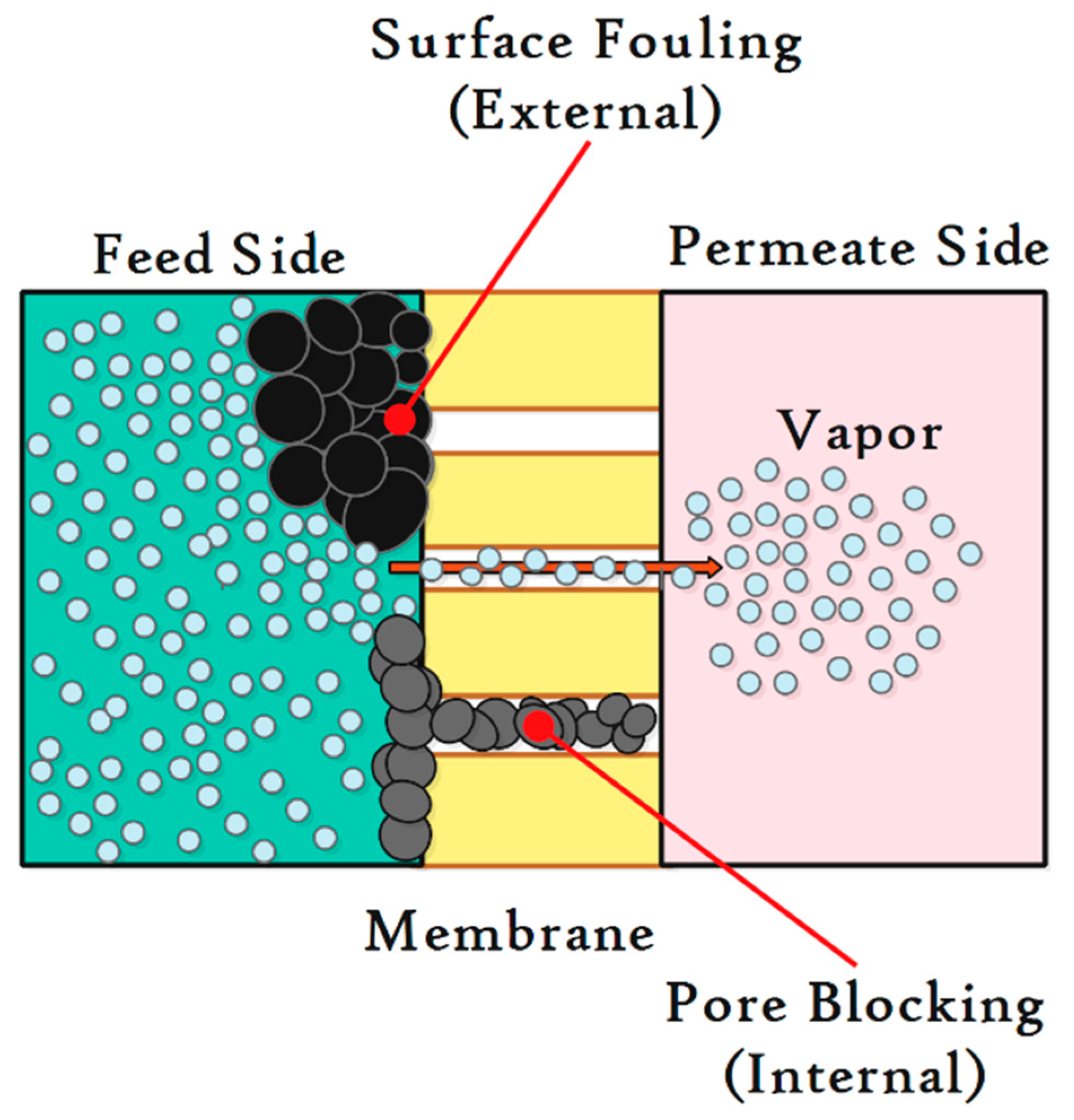
6. Fouling Phenomenon in MD Process
7. Wetting Phenomenon in MD Process
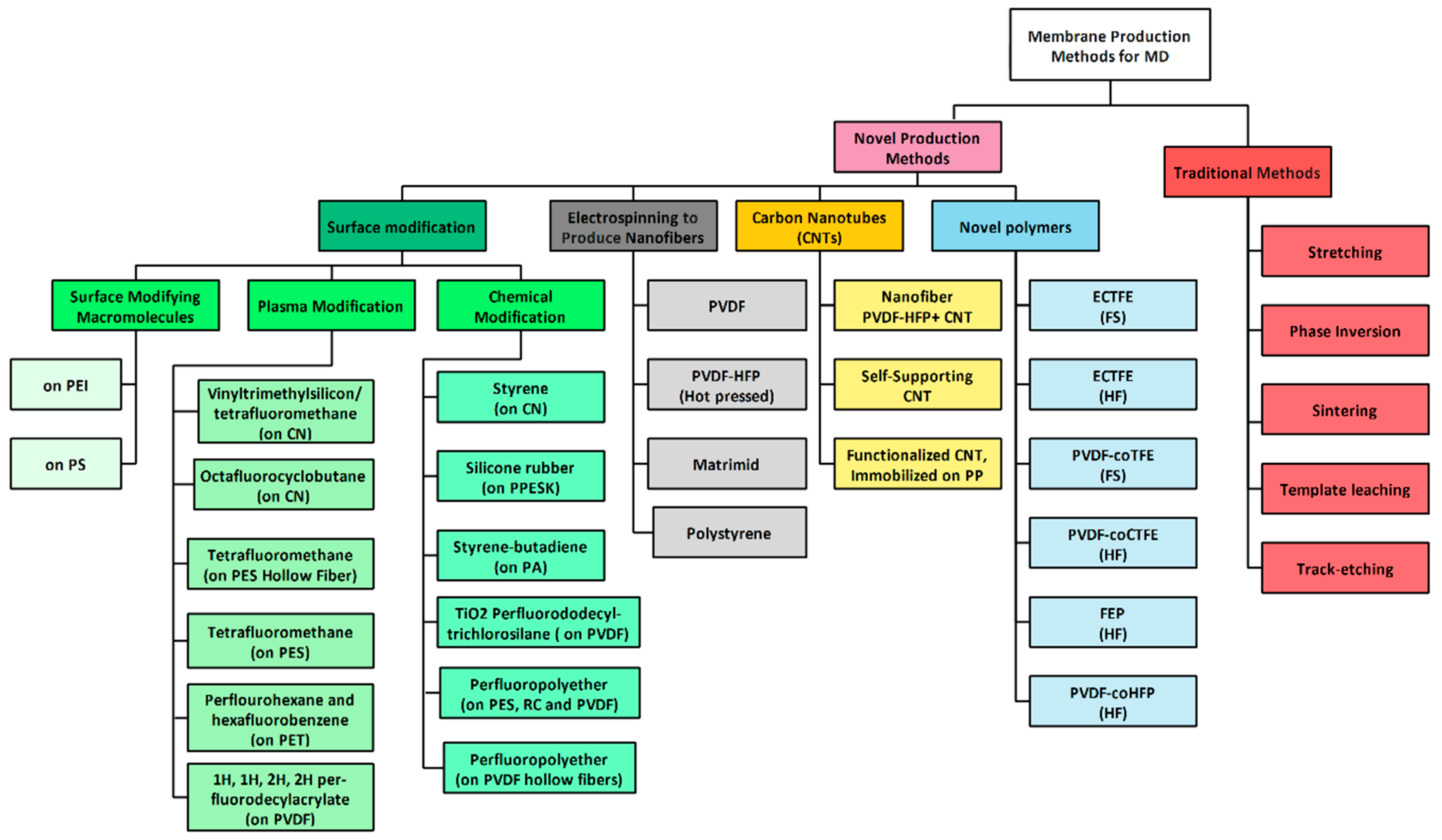
8. Novel Approaches to MD Technology
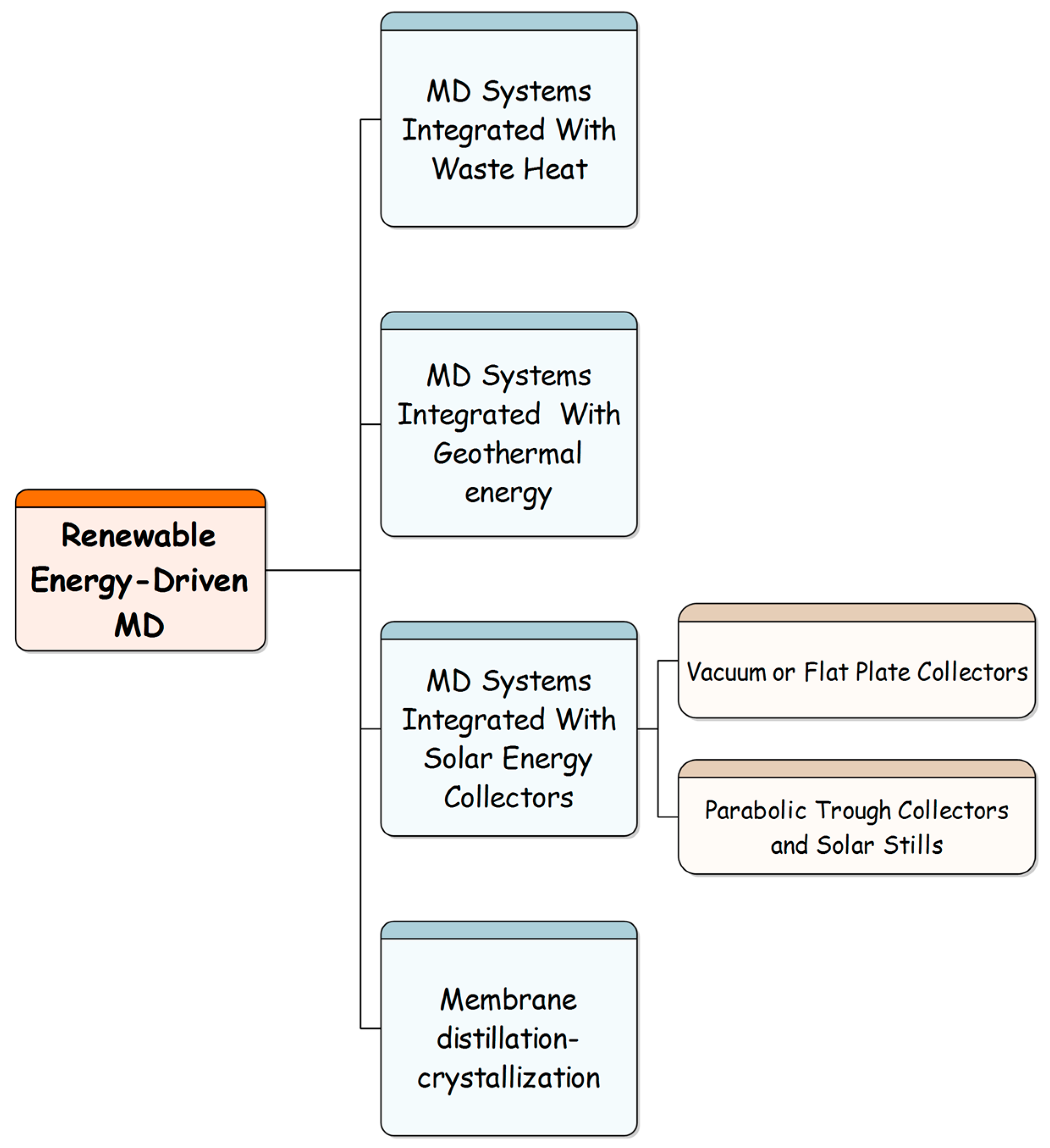
9. Economic Analysis of MD Process
10. Future Trends and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Symbols
| AGMD | Air Gap Membrane Distillation |
| COD | Chemical Oxygen Demand |
| CN | Cellulose Nitrate |
| CNT | Carbon Nanotube |
| DCMD | Direct Contact Membrane Distillation |
| ECTFE | Poly (ethene-co-chlorotrifluoroethene) |
| ED | Electrodialysis |
| FEP | poly (vinylidene fluoride-co-chlorotrifluoroethylene) |
| FO | Forward Osmosis |
| FS | Flat sheet |
| HF | Hollow fiber |
| LEPW | Liquid Entry Pressure of Water |
| LGMD | Liquid-Gap membrane distillation |
| MD | Membrane Distillation |
| MEE | Multiple-Effect Evaporation |
| MED | Multiple-Effect Distillation |
| MGMD | Material-Gap Membrane Distillation |
| MSF | Multi-Stage Flash |
| NF | Nanofiltration |
| NOM | Natural Organic Matter |
| PES | Polyethersulfone |
| PET | Poly(ethylene terephthalate) |
| PGMD | Permeate-Gap Membrane Distillation |
| PP | Polypropylene |
| PS | Polysulfone |
| PPESK | Poly(phthalazinone ether sulfone ketone) |
| PTFE | Polytetrafluoroethylene |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene fluoride |
| PVDF-co-CTFE | Pol (vinylidene fluoride-co-chlorotrifluoroethylene( |
| PVDF-co-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| PVDF-co-TFE | Poly(vinylidene fluoride-cotetrafluoroethylene) |
| RC | Regenerated cellulose |
| RO | Reverse Osmosis |
| SGMD | Sweeping Gas Membrane Distillation |
| SEE | Single-Effect Evaporation |
| TDS | Total Dissolved Solids |
| TOC | Total Organic Carbon |
| TSGMD | Thermostatic Sweeping Gas Membrane Distillation |
| TSS | Total suspended solids |
| VMD | Vacuum Membrane Distillation |
| V-MEMD | Vacuum Multi-Effect Membrane Distillation |
| ZTIMD | Zero Thermal Input Membrane Distillation |
| δ | Thickness |
| ε | Porosity |
References
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The future of science: Food and water for life. Am. Soc. Plant Biol. 2009. [Google Scholar] [CrossRef]
- Bejaoui, I.; Mnif, A.; Hamrouni, B. Performance of reverse osmosis and nanofiltration in the removal of fluoride from model water and metal packaging industrial effluent. Sep. Sci. Technol. 2014, 49, 1135–1145. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rahimpour, M.R. 10–Membrane reactors for biodiesel production and processing. In Membrane Reactors for Energy Applications and Basic Chemical Production; Basile, A., Di Paola, L., Hai, F.l., Piemonte, V., Eds.; Woodhead Publishing: London, UK, 2015; pp. 289–312. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Introduction. In The MBR Book, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2011; Chapter 1; pp. 1–54. [CrossRef]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Ismail, A.F. Membranes and Membrane Processes: Fundamentals. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Mozia, S., Molinari, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 2; pp. 45–70. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Shirazi, A.; Mahdi, M.; Kargari, A. A review on applications of membrane distillation (MD) process for wastewater treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar]
- Khayet, M.; Wang, R. Mixed Matrix Polytetrafluoroethylene/Polysulfone Electrospun Nanofibrous Membranes for Water Desalination by Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 24275–24287. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Air gap membrane distillation: A detailed study of high saline solution. Desalination 2017, 403, 179–186. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Netke, T.; Fane, A.G.; Chew, J.W. Understanding oily wastewater treatment via membrane distillation. J. Membr. Sci. 2017, 539, 284–294. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane operations for process intensification in desalination. Appl. Sci. 2017, 7, 100. [Google Scholar] [CrossRef]
- Bodell, B.R. Distillation of Saline Water Using Silicone Rubber Membrane. U.S. Patents US3361645A, 9 August 1968. [Google Scholar]
- Findley, M. Vaporization through porous membranes. Ind. Eng. Chem. Process Des. Dev. 1967, 6, 226–230. [Google Scholar] [CrossRef]
- Gore, D.W. Gore-Tex membrane distillation. In Proceedings of the 10th Annual Conference Water, Honolulu, HI, USA, 25–29 July 1982; pp. 25–29. [Google Scholar]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Ma, Q.; Ahmadi, A.; Cabassud, C. Direct integration of a vacuum membrane distillation module within a solar collector for small-scale units adapted to seawater desalination in remote places: Design, modeling & evaluation of a flat-plate equipment. J. Membr. Sci. 2018, 564, 617–633. [Google Scholar] [CrossRef]
- Ozbey-Unal, B.; Imer, D.Y.; Keskinler, B.; Koyuncu, I. Boron removal from geothermal water by air gap membrane distillation. Desalination 2018, 433, 141–150. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-D.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Shi, L.; Fane, A.G.; Debowski, M. Performance improvement of PVDF hollow fiber-based membrane distillation process. J. Membr. Sci. 2011, 369, 437–447. [Google Scholar] [CrossRef]
- Wang, P.; Teoh, M.M.; Chung, T.-S. Morphological architecture of dual-layer hollow fiber for membrane distillation with higher desalination performance. Water Res. 2011, 45, 5489–5500. [Google Scholar] [CrossRef]
- Jacob, P.; Laborie, S.; Cabassud, C. Visualizing and evaluating wetting in membrane distillation: New methodology and indicators based on Detection of Dissolved Tracer Intrusion (DDTI). Desalination 2018, 443, 307–322. [Google Scholar] [CrossRef]
- Ali, A.; Tsai, J.-H.; Tung, K.-L.; Drioli, E.; Macedonio, F. Designing and optimization of continuous direct contact membrane distillation process. Desalination 2018, 426, 97–107. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C. Desalination by Membrane Distillation. In Emerging Membrane Technology for Sustainable Water Treatment; Hankins, N.P., Singh, R., Eds.; Elsevier: Boston, MA, USA, 2016; Chapter 4; pp. 77–109. [Google Scholar] [CrossRef]
- Terki, L.; Kujawski, W.; Kujawa, J.; Kurzawa, M.; Filipiak-Szok, A.; Chrzanowska, E.; Khaled, S.; Madani, K. Implementation of osmotic membrane distillation with various hydrophobic porous membranes for concentration of sugars solutions and preservation of the quality of cactus pear juice. J. Food Eng. 2018, 230, 28–38. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Yu, J.; Ma, R.; Yang, Z. Concentrating the extract of traditional Chinese medicine by direct contact membrane distillation. J. Membr. Sci. 2008, 310, 539–549. [Google Scholar] [CrossRef]
- Khayet, M. Treatment of radioactive wastewater solutions by direct contact membrane distillation using surface modified membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Mejia Mendez, D.L.; Castel, C.; Lemaitre, C.; Favre, E. Membrane distillation (MD) processes for water desalination applications. Can dense selfstanding membranes compete with microporous hydrophobic materials? Chem. Eng. Sci. 2018, 188, 84–96. [Google Scholar] [CrossRef]
- Kiss, A.A.; Kattan Readi, O.M. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Banat, F. Desalination by solar powered membrane distillation systems. Desalination 2013, 308, 186–197. [Google Scholar] [CrossRef]
- Luo, W.; Phan, H.V.; Li, G.; Hai, F.I.; Price, W.E.; Elimelech, M.; Nghiem, L.D. An osmotic membrane bioreactor–membrane distillation system for simultaneous wastewater reuse and seawater desalination: Performance and implications. Environ. Sci. Technol. 2017, 51, 14311–14320. [Google Scholar] [CrossRef]
- Susanto, H. Towards practical implementations of membrane distillation. Chem. Eng. Process. Process Intensif. 2011, 50, 139–150. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Khayet, M. Membrane distillation. In Advanced Membrane Technology and Applications; Elsevier: Amsterdam, The Netherlands, 2008; pp. 297–369. [Google Scholar]
- Zhang, J.; Gray, S. Effect of applied pressure on performance of PTFE membrane in DCMD. J. Membr. Sci. 2011, 369, 514–525. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Martı́nez, L.; Florido-Dı́az, F.; Hernandez, A.; Prádanos, P. Characterisation of three hydrophobic porous membranes used in membrane distillation: Modelling and evaluation of their water vapour permeabilities. J. Membr. Sci. 2002, 203, 15–27. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.; Garcıa-Payo, M.; Fernández-Pineda, C. Air gap membrane distillation of sucrose aqueous solutions. J. Membr. Sci. 1999, 155, 291–307. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. 3-Membrane distillation—Principles, applications, configurations, design, and implementation. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 55–106. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. 4–Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 107–456. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Membrane Distillation (MD). In Progress in Filtration and Separation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 61–99. [Google Scholar]
- Hassan, A.S.; Fath, H.E. Review and assessment of the newly developed MD for desalination processes. Desalin. Water Treat. 2013, 51, 574–585. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Martinez, L.; Florido-Diaz, F. Theoretical and experimental studies on desalination using membrane distillation. Desalination 2001, 139, 373–379. [Google Scholar] [CrossRef]
- Wang, K.Y.; Foo, S.W.; Chung, T.-S. Mixed matrix PVDF hollow fiber membranes with nanoscale pores for desalination through direct contact membrane distillation. Ind. Eng. Chem. Res. 2009, 48, 4474–4483. [Google Scholar] [CrossRef]
- Su, M.; Teoh, M.M.; Wang, K.Y.; Su, J.; Chung, T.-S. Effect of inner-layer thermal conductivity on flux enhancement of dual-layer hollow fiber membranes in direct contact membrane distillation. J. Membr. Sci. 2010, 364, 278–289. [Google Scholar] [CrossRef]
- Asadi, R.Z.; Suja, F.; Tarkian, F.; Mashhoon, F.; Rahimi, S.; Jameh, A.A. Solar desalination of Gas Refinery wastewater using membrane distillation process. Desalination 2012, 291, 56–64. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced water treatment: Application of air gap membrane distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Summers, E.K.; Arafat, H.A. Energy efficiency comparison of single-stage membrane distillation (MD) desalination cycles in different configurations. Desalination 2012, 290, 54–66. [Google Scholar] [CrossRef]
- Duong, H.C.; Chivas, A.R.; Nelemans, B.; Duke, M.; Gray, S.; Cath, T.Y.; Nghiem, L.D. Treatment of RO brine from CSG produced water by spiral-wound air gap membrane distillation—A pilot study. Desalination 2015, 366, 121–129. [Google Scholar] [CrossRef]
- Khalifa, A.; Lawal, D.; Antar, M.; Khayet, M. Experimental and theoretical investigation on water desalination using air gap membrane distillation. Desalination 2015, 376, 94–108. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating energy consumption of air gap membrane distillation for seawater desalination at pilot scale level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Li, P.; Li, W.; Wang, F. Comparative study of air gap and permeate gap membrane distillation using internal heat recovery hollow fiber membrane module. Desalination 2018, 426, 42–49. [Google Scholar] [CrossRef]
- Kalla, S.; Upadhyaya, S.; Singh, K. Principles and advancements of air gap membrane distillation. Rev. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; Sanmartino, J.A.; Khayet, M.; García-Payo, C.; Chaouch, M. Fouling in membrane distillation, osmotic distillation and osmotic membrane distillation. Appl. Sci. 2017, 7, 334. [Google Scholar] [CrossRef]
- Rivier, C.; Garcı́a-Payo, M.; Marison, I.; Von Stockar, U. Separation of binary mixtures by thermostatic sweeping gas membrane distillation: I. Theory and simulations. J. Membr. Sci. 2002, 201, 1–16. [Google Scholar] [CrossRef]
- Perfilov, V.; Fila, V.; Sanchez Marcano, J. A general predictive model for sweeping gas membrane distillation. Desalination 2018, 443, 285–306. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Kazerooni, N.M.; Parhoudeh, M. Water Treatment by Renewable Energy-Driven Membrane Distillation. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–211. [Google Scholar]
- Shukla, S.; Méricq, J.; Belleville, M.; Hengl, N.; Benes, N.; Vankelecom, I.; Marcano, J.S. Process intensification by coupling the Joule effect with pervaporation and sweeping gas membrane distillation. J. Membr. Sci. 2018, 545, 150157. [Google Scholar] [CrossRef]
- Moore, S.E.; Mirchandani, S.D.; Karanikola, V.; Nenoff, T.M.; Arnold, R.G.; Sáez, A.E. Process modeling for economic optimization of a solar driven sweeping gas membrane distillation desalination system. Desalination 2018, 437, 108–120. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Blanco, J.; Zaragoza, G.; Alarcón, D.-C.; Palenzuela, P.; Ibarra, M.; Gernjak, W. Experimental analysis of an air gap membrane distillation solar desalination pilot system. J. Membr. Sci. 2011, 379, 386–396. [Google Scholar] [CrossRef]
- Li, L.; Sirkar, K.K. Studies in vacuum membrane distillation with flat membranes. J. Membr. Sci. 2017, 523, 225–234. [Google Scholar] [CrossRef]
- Chen, Q.; Ja, M.K.; Li, Y.; Chua, K. Thermodynamic optimization of a vacuum multi-effect membrane distillation system for liquid desiccant regeneration. Appl. Energy 2018, 230, 960–973. [Google Scholar] [CrossRef]
- Walton, J.; Lu, H.; Turner, C.; Solis, S.; Hein, H. Solar and waste heat desalination by membrane distillation. In Desalination and Water Purification Research and Development Program Report; Research and Development Office: El Paso, TX, USA, 2004; p. 20. [Google Scholar]
- Xie, Z.; Duong, T.; Hoang, M.; Nguyen, C.; Bolto, B. Ammonia removal by sweep gas membrane distillation. Water Res. 2009, 43, 1693–1699. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Guijt, C.M.; de Haan, A.B. Desalination and water recycling by air gap membrane distillation. Desalination 2006, 187, 291–301. [Google Scholar] [CrossRef]
- Souhaimi, M.K.; Matsuura, T. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Tan, Y.Z.; Han, L.; Chew, N.G.P.; Chow, W.H.; Wang, R.; Chew, J.W. Membrane distillation hybridized with a thermoelectric heat pump for energy-efficient water treatment and space cooling. Appl. Energy 2018, 231, 1079–1088. [Google Scholar] [CrossRef]
- Garcı́a-Payo, M.; Rivier, C.; Marison, I.; Von Stockar, U. Separation of binary mixtures by thermostatic sweeping gas membrane distillation: II. Experimental results with aqueous formic acid solutions. J. Membr. Sci. 2002, 198, 197–210. [Google Scholar] [CrossRef]
- Bamufleh, H.; Abdelhady, F.; Baaqeel, H.M.; El-Halwagi, M.M. Optimization of multi-effect distillation with brine treatment via membrane distillation and process heat integration. Desalination 2017, 408, 110–118. [Google Scholar] [CrossRef]
- Jansen, A.; Assink, J.; Hanemaaijer, J.; Van Medevoort, J.; Van Sonsbeek, E. Development and pilot testing of full-scale membrane distillation modules for deployment of waste heat. Desalination 2013, 323, 55–65. [Google Scholar] [CrossRef]
- Boutikos, P.; Mohamed, E.S.; Mathioulakis, E.; Belessiotis, V. A theoretical approach of a vacuum multi-effect membrane distillation system. Desalination 2017, 422, 25–41. [Google Scholar] [CrossRef]
- Andrés-Mañas, J.A.; Ruiz-Aguirre, A.; Acién, F.G.; Zaragoza, G. Assessment of a pilot system for seawater desalination based on vacuum multi-effect membrane distillation with enhanced heat recovery. Desalination 2018, 443, 110–121. [Google Scholar] [CrossRef]
- Zhao, K.; Heinzl, W.; Wenzel, M.; Büttner, S.; Bollen, F.; Lange, G.; Heinzl, S.; Sarda, N. Experimental study of the memsys vacuum-multi-effect-membrane-distillation (V-MEMD) module. Desalination 2013, 323, 150–160. [Google Scholar] [CrossRef]
- Heinzl, W.; Büttner, S.; Lange, G. Industrialized modules for MED Desalination with polymer surfaces. Desalin. Water Treat. 2012, 42, 177–180. [Google Scholar] [CrossRef]
- Mohamed, E.S.; Boutikos, P.; Mathioulakis, E.; Belessiotis, V. Experimental evaluation of the performance and energy efficiency of a vacuum multi-effect membrane distillation system. Desalination 2017, 408, 70–80. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Alsaadi, A.A.; Amy, G.L. Material gap membrane distillation: A new design for water vapor flux enhancement. J. Membr. Sci. 2013, 448, 240–247. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; AlMarzooqi, F.A.; Arafat, H.A.; Lienhard V, J.H. Energy efficiency of permeate gap and novel conductive gap membrane distillation. J. Membr. Sci. 2016, 502, 171–178. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination using membrane distillation: Experimental studies on full scale spiral wound modules. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; Lienhard V, J.H. Membrane distillation model based on heat exchanger theory and configuration comparison. Appl. Energy 2016, 184, 491–505. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.K.; Bryjak, M.; Cichosz, M.; Kujawski, W. Removal of volatile organic compounds from aqueous solutions applying thermally driven membrane processes. 2. Air gap membrane distillation. J. Membr. Sci. 2016, 499, 245–256. [Google Scholar] [CrossRef]
- Kalla, S.; Upadhyaya, S.; Singh, K.; Baghel, R. Experimental and mathematical study of air gap membrane distillation for aqueous HCl azeotropic separation. J. Chem. Technol. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Mondal, S.; Macedonio, F.; Drioli, E. A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 2016, 505, 167–173. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: thermal efficiency, sensitivity study and cost estimation. J. Membr. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Khalifa, A.; Ahmad, H.; Antar, M.; Laoui, T.; Khayet, M. Experimental and theoretical investigations on water desalination using direct contact membrane distillation. Desalination 2017, 404, 22–34. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Lazarides, H.N. Osmotic concentration of liquid foods. J. Food Eng. 2001, 49, 201–206. [Google Scholar] [CrossRef]
- Alves, V.; Coelhoso, I. Orange juice concentration by osmotic evaporation and membrane distillation: A comparative study. J. Food Eng. 2006, 74, 125–133. [Google Scholar] [CrossRef]
- Kimura, S.; Nakao, S.-I.; Shimatani, S.-I. Transport phenomena in membrane distillation. J. Membr. Sci. 1987, 33, 285–298. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Kulozik, U.; Duke, M. Performance assessment of membrane distillation for skim milk and whey processing. J. Dairy Sci. 2014, 97, 56–71. [Google Scholar] [CrossRef]
- Criscuoli, A.; Zhong, J.; Figoli, A.; Carnevale, M.; Huang, R.; Drioli, E. Treatment of dye solutions by vacuum membrane distillation. Water Res. 2008, 42, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Banat, F.; Al-Asheh, S.; Qtaishat, M. Treatment of waters colored with methylene blue dye by vacuum membrane distillation. Desalination 2005, 174, 87–96. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F. Dye wastewater treatment by direct contact membrane distillation using polyvinylidene fluoride hollow fiber membranes. J. Polym. Eng. 2015, 35, 471–479. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, S.; Hung, Y.-T. Treatment of pulp and paper mill wastes. In Waste Treatment in the Process Industries; Springer: Berlin, Gremnay, 2006; pp. 453–497. [Google Scholar]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.-S. Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Carbon nanotube enhanced membrane distillation for simultaneous generation of pure water and concentrating pharmaceutical waste. Sep. Purif. Technol. 2012, 90, 239–245. [Google Scholar] [CrossRef]
- Jia, F.; Li, J.; Wang, J.; Sun, Y. Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann. Nucl. Energy 2017, 103, 363–368. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J. Treatment of radioactive wastewater using direct contact membrane distillation. J. Hazard. Mater. 2013, 261, 307–315. [Google Scholar] [CrossRef]
- Reis, B.G.; Araújo, A.L.B.; Amaral, M.C.S.; Ferraz, H.C. Comparison of Nanofiltration and Direct Contact Membrane Distillation as an alternative for gold mining effluent reclamation. Chem. Eng. Process. Process Intensif. 2018, 133, 24–33. [Google Scholar] [CrossRef]
- Woldemariam, D.; Kullab, A.; Khan, E.U.; Martin, A. Recovery of ethanol from scrubber-water by district heat-driven membrane distillation: Industrial-scale technoeconomic study. Renew. Energy 2018, 128, 484–494. [Google Scholar] [CrossRef]
- Onishi, V.C.; Fraga, E.S.; Reyes-Labarta, J.A.; Caballero, J.A. Desalination of shale gas wastewater: Thermal and membrane applications for zero-liquid discharge. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 399–431. [Google Scholar]
- Aminfard, S.; Davidson, F.T.; Webber, M.E. Multi-layered spatial methodology for assessing the technical and economic viability of using renewable energy to power brackish groundwater desalination. Desalination 2019, 450, 12–20. [Google Scholar] [CrossRef]
- Borsani, R.; Rebagliati, S. Fundamentals and costing of MSF desalination plants and comparison with other technologies. Desalination 2005, 182, 29–37. [Google Scholar] [CrossRef]
- Turek, M.; Dydo, P. Hybrid membrane-thermal versus simple membrane systems. Desalination 2003, 157, 51–56. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Integrated membrane desalination systems with membrane crystallization units for resource recovery: A new approach for mining from the sea. Crystals 2016, 6, 36. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K.; Strmecky, T. Hybrid processes for treatment of landfill leachate: Coagulation/UF/NF-RO and adsorption/UF/NF-RO. Sep. Purif. Technol. 2016, 168, 39–46. [Google Scholar] [CrossRef]
- Lokare, O.R.; Tavakkoli, S.; Rodriguez, G.; Khanna, V.; Vidic, R.D. Integrating membrane distillation with waste heat from natural gas compressor stations for produced water treatment in Pennsylvania. Desalination 2017, 413, 144–153. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168. [Google Scholar] [CrossRef]
- Boukhriss, M.; Khemili, S.; Hamida, M.B.B.; Bacha, H.B. Simulation and experimental study of an AGMD membrane distillation pilot for the desalination of seawater or brackish water with zero liquid discharged. Heat Mass Transf. 2018, 1–11. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Lan, C.Q.; Matsuura, T. Zero thermal input membrane distillation, a zero-waste and sustainable solution for freshwater shortage. Appl. Energy 2017, 187, 910–928. [Google Scholar] [CrossRef]
- Huang, X.; Luo, X.; Chen, J.; Yang, Z.; Chen, Y.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Synthesis and dual-objective optimization of industrial combined heat and power plants compromising the water–energy nexus. Appl. Energy 2018, 224, 448–468. [Google Scholar] [CrossRef]
- Moradi, R.; Monfared, S.M.; Amini, Y.; Dastbaz, A. Vacuum enhanced membrane distillation for trace contaminant removal of heavy metals from water by electrospun PVDF/TiO2 hybrid membranes. Korean J. Chem. Eng. 2016, 33, 2160–2168. [Google Scholar] [CrossRef]
- Rácz, G.; Kerker, S.; Schmitz, O.; Schnabel, B.; Kovacs, Z.; Vatai, G.; Ebrahimi, M.; Czermak, P. Experimental determination of liquid entry pressure (LEP) in vacuum membrane distillation for oily wastewaters. Membr. Water Treat. 2015, 6, 237–249. [Google Scholar] [CrossRef]
- Carnevale, M.; Gnisci, E.; Hilal, J.; Criscuoli, A. Direct contact and vacuum membrane distillation application for the olive mill wastewater treatment. Sep. Purif. Technol. 2016, 169, 121–127. [Google Scholar] [CrossRef]
- Mokhtar, N.; Lau, W.; Ismail, A.; Kartohardjono, S.; Lai, S.; Teoh, H. The potential of direct contact membrane distillation for industrial textile wastewater treatment using PVDF-Cloisite 15A nanocomposite membrane. Chem. Eng. Res. Des. 2016, 111, 284–293. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.; Zhao, X. Removal of nuclides and boron from highly saline radioactive wastewater by direct contact membrane distillation. Desalination 2016, 394, 101–107. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Lincicome, D.; Wickramasinghe, S.R. Combined electrocoagulation and membrane distillation for treating high salinity produced waters. J. Membr. Sci. 2018. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Wang, J.; Song, J.; Li, X.-M.; He, T. Preparation of omniphobic PVDF membrane with hierarchical structure for treating saline oily wastewater using direct contact membrane distillation. J. Membr. Sci 2018, 555, 197–205. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Li, X.; Wang, X.; Drioli, E.; Wang, Z.; Zhao, S. Optimization of novel composite membranes for water and mineral recovery by vacuum membrane distillation. Desalination 2018, 440, 39–47. [Google Scholar] [CrossRef]
- Peydayesh, M.; Kazemi, P.; Bandegi, A.; Mohammadi, T.; Bakhtiari, O. Treatment of bentazon herbicide solutions by vacuum membrane distillation. J. Water Process Eng. 2015, 8, e17–e22. [Google Scholar] [CrossRef]
- Wu, Y.; Kang, Y.; Zhang, L.; Qu, D.; Cheng, X.; Feng, L. Performance and fouling mechanism of direct contact membrane distillation (DCMD) treating fermentation wastewater with high organic concentrations. J. Environ. Sci. 2018, 65, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Naidu, G.; Jeong, S.; Vigneswaran, S.; Hwang, T.-M.; Choi, Y.-J.; Kim, S.-H. A review on fouling of membrane distillation. Desalin. Water Treat. 2016, 57, 10052–10076. [Google Scholar] [CrossRef]
- Bush, J.A.; Vanneste, J.; Cath, T.Y. Comparison of membrane distillation and high-temperature nanofiltration processes for treatment of silica-saturated water. J. Membr. Sci 2019, 570, 258–269. [Google Scholar] [CrossRef]
- Tow, E.W.; Warsinger, D.M.; Trueworthy, A.M.; Swaminathan, J.; Thiel, G.P.; Zubair, S.M.; Myerson, A.S. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 2018, 556, 352–364. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Shan, H.; Liu, J.; Li, X.; Li, Y.; Tezel, F.H.; Li, B.; Wang, S. Nanocoated amphiphobic membrane for flux enhancement and comprehensive anti-fouling performance in direct contact membrane distillation. J. Membr. Sci. 2018, 567, 166–180. [Google Scholar] [CrossRef]
- Gryta, M. Direct contact membrane distillation with crystallization applied to NaCl solutions. Chem. Pap. Slovak Acad. Sci. 2002, 56, 14–19. [Google Scholar]
- Guillen-Burrieza, E.; Thomas, R.; Mansoor, B.; Johnson, D.; Hilal, N.; Arafat, H. Effect of dry-out on the fouling of PVDF and PTFE membranes under conditions simulating intermittent seawater membrane distillation (SWMD). J. Membr. Sci. 2013, 438, 126–139. [Google Scholar] [CrossRef]
- Gryta, M. Polyphosphates used for membrane scaling inhibition during water desalination by membrane distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Cath, T. A scaling mitigation approach during direct contact membrane distillation. Sep. Purif. Technol. 2011, 80, 315–322. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Di Profio, G.; Sulaiman, A.O.; Fontananova, E.; Drioli, E. Membrane distillation operated at high seawater concentration factors: Role of the membrane on CaCO3 scaling in presence of humic acid. J. Membr. Sci. 2010, 346, 263–269. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S. Interaction of humic substances on fouling in membrane distillation for seawater desalination. Chem. Eng. J. 2015, 262, 946–957. [Google Scholar] [CrossRef]
- Sutzkover-Gutman, I.; Hasson, D. Feed water pretreatment for desalination plants. Desalination 2010, 264, 289–296. [Google Scholar] [CrossRef]
- Vedavyasan, C.V. Pretreatment trends—An overview. Desalination 2007, 203, 296–299. [Google Scholar] [CrossRef]
- Prihasto, N.; Liu, Q.-F.; Kim, S.-H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Gryta, M. Alkaline scaling in the membrane distillation process. Desalination 2008, 228, 128–134. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Wang, R.; Fane, A.G. Performance enhancement and scaling control with gas bubbling in direct contact membrane distillation. Desalination 2013, 308, 47–55. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Cath, T.Y. Sustainable operation of membrane distillation for enhancement of mineral recovery from hypersaline solutions. J. Membr. Sci. 2014, 454, 426–435. [Google Scholar] [CrossRef]
- Xu, J.; Lange, S.; Bartley, J.; Johnson, R. Alginate-coated microporous PTFE membranes for use in the osmotic distillation of oily feeds. J. Membr. Sci. 2004, 240, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, B.; Wang, Q.; Wang, S. Fabrication and characterization of superhydrophobic poly (vinylidene fluoride) membrane for direct contact membrane distillation. Desalination 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Lyster, E.; Kim, M.-m.; Au, J.; Cohen, Y. A method for evaluating antiscalant retardation of crystal nucleation and growth on RO membranes. J. Membr. Sci. 2010, 364, 122–131. [Google Scholar] [CrossRef]
- Burgoyne, A.; Vahdati, M.M. Direct Contact Membrane Distillation. Sep. Sci. Technol. 2000, 35, 1257–1284. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Samhaber, W.M. Wetting prevention in membrane distillation through superhydrophobicity and recharging an air layer on the membrane surface. J. Membr. Sci. 2017, 530, 42–52. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Matsuura, T. 13-Modelling of pore wetting in membrane distillation compared with pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 385–413. [Google Scholar] [CrossRef]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane fouling and wetting in a DCMD process for RO brine concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Mavukkandy, M.; Bilad, M.; Arafat, H. Understanding wetting phenomena in membrane distillation and how operational parameters can affect it. J. Membr. Sci. 2016, 515, 163–174. [Google Scholar] [CrossRef]
- Damtie, M.M.; Kim, B.; Woo, Y.C.; Choi, J.-S. Membrane distillation for industrial wastewater treatment: Studying the effects of membrane parameters on the wetting performance. Chemosphere 2018, 206, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Z.; Carlson, K.H.; Lee, J.; Tong, T. Membrane fouling and reusability in membrane distillation of shale oil and gas produced water: Effects of membrane surface wettability. J. Membr. Sci. 2018, 567, 199–208. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Coating techniques for membrane distillation: An experimental assessment. Sep. Purif. Technol. 2018, 193, 38–48. [Google Scholar] [CrossRef]
- Chen, L.-H.; Chen, Y.-R.; Huang, A.; Chen, C.-H.; Su, D.-Y.; Hsu, C.-C.; Tsai, F.-Y.; Tung, K.-L. Nanostructure depositions on alumina hollow fiber membranes for enhanced wetting resistance during membrane distillation. J. Membr. Sci. 2018, 564, 227–236. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Boo, C.; Elimelech, M. Thermal desalination membranes: Carbon nanotubes keep up the heat. Nat. Nanotechnol. 2017, 12, 501. [Google Scholar] [CrossRef]
- Ashoor, B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Lin, S.; Yip, N.Y.; Elimelech, M. Direct contact membrane distillation with heat recovery: Thermodynamic insights from module scale modeling. J. Membr. Sci. 2014, 453, 498–515. [Google Scholar] [CrossRef]
- Long, R.; Lai, X.; Liu, Z.; Liu, W. Direct contact membrane distillation system for waste heat recovery: Modelling and multi-objective optimization. Energy 2018, 148, 1060–1068. [Google Scholar] [CrossRef]
- Lee, J.-G.; Bak, C.-u.; Thu, K.; Ghaffour, N.; Kim, Y.-D. Effect of seawater-coolant feed arrangement in a waste heat driven multi-stage vacuum membrane distillation system. Sep. Purif. Technol. 2018, 9, 18–20. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M. Energy efficiency of membrane distillation up to high salinity: Evaluating critical system size and optimal membrane thickness. Appl. Energy 2018, 211, 715–734. [Google Scholar] [CrossRef]
- Yang, X.; Fane, A.G.; Wang, R. Membrane distillation: Now and future. In Desalination; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 373–424. [Google Scholar]
- Kesieme, U.K.; Milne, N.; Aral, H.; Cheng, C.Y.; Duke, M. Economic analysis of desalination technologies in the context of carbon pricing, and opportunities for membrane distillation. Desalination 2013, 323, 66–74. [Google Scholar] [CrossRef]
- Soomro, M.I.; Kim, W.-S. Performance and economic investigations of solar power tower plant integrated with direct contact membrane distillation system. Energy Convers. Manag. 2018, 174, 626–638. [Google Scholar] [CrossRef]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. A techno-economic assessment of membrane distillation for treatment of Marcellus shale produced water. Desalination 2017, 416, 24–34. [Google Scholar] [CrossRef]
- Hitsov, I.; Sitter, K.D.; Dotremont, C.; Nopens, I. Economic modelling and model-based process optimization of membrane distillation. Desalination 2018, 436, 125–143. [Google Scholar] [CrossRef]
- Soomro, M.I.; Kim, W.-S. Parabolic-trough plant integrated with direct-contact membrane distillation system: Concept, simulation, performance, and economic evaluation. Sol. Energy 2018, 173, 348–361. [Google Scholar] [CrossRef]
| Patent | Highlights |
|---|---|
| Desulfurization waste water zero-discharging treatment technology for coal-fired power plants Publication number: CN105712557A Publication date: 2016-06-29 | The present invention relates to a zero-discharging treatment device for desulfurization wastewater that consists of a nanofiltration system, calcium removal sedimentation pool set, a heavy metal and magnesium removal pool set, an evaporating crystallizer, and a membrane distillation system. Crystal salt gained from evaporating crystallization can be entirely recycled, and treatment and operating cost are extremely reduced. |
| A membrane distillation system that is used for concentration of desulfurization waste water Publication number: CN204981458U Publication date: 2016-01-20 | The utility model reveals the MD system that is applied for the handling of the concentration of desulfurized wastewater, with this considered to have some benefits such as a simple structure, safe operation, and a smaller area. |
| Salt-containing wastewater treatment system Publication number: CN205133326U Publication date: 2016-04-06 | The present utility model includes a salt-containing wastewater treatment system that contains an electrodialysis device for receiving and handling salted wastewater, which is connected to the MD system. |
| Process and system for produced water treatment Publication number: US20170096356A1 Publication date: 2017-04-06 | This invention relates to a system and process for produced water treatment. A heat exchanger, a vacuum tank, an adsorption-desorption, and MD crystallization process are in this process combination. The efficiency related to costs of maintenance and energy consumption is considered. |
| Modularly installed energy-saving membrane distillation wastewater treatment device and method Publication number: CN106865663A Publication date: 2017-06-20 | The present invention represents a modularly installed energy-saving MD wastewater treatment device which consists of a post-treatment and primary treatment module. The proposed device assembles easily and has suitable energy-saving effects for the treatment of different types of sewage. |
| Energy -saving membrane distillation effluent treatment plant of modularization installation Publication number: CN206635064U Publication date: 2017-11-14 | The utility model relates to an energy-saving MD sewage treatment plant of modularization installation and consists of post-processing and primary treatment modules. The applied used equipment in the device is energy conserving, convenient, and effective for all types of effluent treatment. |
| High -concentration organic wastewater treatment system Publication number: CN206318843U Publication date: 2017-07-11 | The present utility model reveals a highly concentrated organic wastewater treatment system that consists of a liquid bath of raw material, anaerobic biological treatment device, and a positive osmotic membrane. Highly concentrated organic wastewater from a bioreactor is handled in the combined MD system. This system proposes greater recovery of the pure water rate and reduces the concentration of organic matters. |
| Multi-stage submerged membrane distillation water treatment apparatus and a resource recovery method Publication number: US20170313610A1 Publication date: 2017-11-02 | This investigation related to a submerging multi-stage membrane distillation water treatment device. |
| Wastewater treatment system Publication number: CN207243660U Publication date: 2018-04-17 | This utility model shows a wastewater treatment system that consists of an MD unit connected to magnetism loaded flocculation unit. This proposed wastewater treatment system has some benefits like simple equipment, efficient sewage treatment, and a lower energy consumption. |
| Concentrated decrement device of vacuum membrane distillation wastewater that frequently flows backwards Publication number: CN207734625U Publication date: 2018-08-17 | This invention concerns a zero-discharging wastewater treatment device, which is particularly related to a vacuum membrane distillation (VDM). MD has some effective benefits, for instance, it enhances the service life, and shows great abilities to treat wastewater with high salinity. |
| Slot-type solar sea water desalination device based on membrane distillation Publication number: CN107720863A Publication date: 2018-02-23 | The invention reveals a slot-type solar seawater desalination device integrated with membrane distillation. The required heat energy of the process is supplied from solar energy emitted by a slot-type condenser mirror, in which solar energy is reflected and condensed onto an arc heat collection tube. |
| Porous membrane for membrane distillation, and method for operating membrane distillation module Publication number: WO2018174279A1 Publication date: 2018-09-27 | A membrane distillation device, with a hydrophobic porous hollow fiber membrane, and a condenser for condensing water vapor is invented for water treatment. The membrane has an average pore diameter of 0.01–1 μm. |
| Hollow fiber membrane module for direct contact membrane distillation-based desalinization Publication number: WO2018195534A1 Publication date: 2018-10-25 | The invention is a desalination system by direct contact membrane distillation integrated with a cylindrical cross-flow module comprising high-flux composite hydrophobic hollow fiber membranes. A model is developed and directed to the system and shows the observed water vapor production rates for various feed brine temperatures at different feed brine flow rates. |
| A membrane distillation technique and method for treating radioactive waste water systems Publication number: CN108597636A Publication date: 2018-09-28 | The invention shows a seed film distillation procedure and technology for processing radioactive waste including (pretreatment, preheating, membrane separation, condensation process) by accumulating the wastewater |
| Multistage immersion type membrane distillation water treatment apparatus and a resource recovery method using the same number of oil resources Publication number: KR101870350B1 Publication date: 2018-06-22 | This invention provides a multistage immersion-type membrane distillation water treatment system and a viable resource recovery technique applying the same number of oil resources which can substantially reduce the heat energy. |
| Advantages | Disadvantages | Reference |
|---|---|---|
| Low operating temperature (the process liquid is not essentially heated up to the boiling temperatures) | Lower permeate flux compared to other commercialized separation processes, such as RO. | [9,19,33,34,35] |
| Lower hydrostatic pressure required compare to pressure-driven membrane separation processes such as reverse osmosis (RO). | [9,19,33] | |
| High rejection (99–100%) for macromolecules, non-volatile compounds (colloids, salts), and inorganic ions. In fact, 100% separation happens, theoretically. | High susceptibility of permeate flux to temperature and concentration polarization effects, partial or total pore wetting, and membrane scaling and fouling. | [19,36,37] |
| Lower requirements on the mechanical properties of the membrane. | [18,33] | |
| Larger pore size and less chemical interaction between process solution and membrane lead to less fouling. | High heat loss (by conduction) and energy consumption | [33,36] |
| Alternative low-grade energy sources like waste heat, solar energy, and geometrical heat can be utilized. | Pore wetting risk | [8,38] |
| The possibility to combine with some other separation processes in order to build an integrated separating system, like an RO unit or ultrafiltration | Unclear economic and energy costs for different MD applications and configurations, just when waste heat is available MD becomes cost competitive. | [8,33] |
| An efficient method to eliminate heavy metals and organic from wastewater. | [33] | |
| It is an effective and safe process to remove radioactive waste. | The lack of commercially available MD modulus manufactured for large-scale applications and high-performance membrane. | [33,39] |
| MD is able to work with a saturated solution or high solute concentration in a liquid stream | [33] | |
| Fewer vapor spaces needed in comparison with common distillation process so MD can be used at a smaller scale. | Having less producers of MD technology | [8,33] |
| Reduced sensibility to concentration polarization. | [8,33,38] | |
| High concentration polarization or osmotic pressure does not limit performance. | ||
| Having low cost and less sophisticated installation and construction (because of lower operating temperature and pressure), leads to a full level of automation. | Limitations of MD permeate flux, due to a further mass transfer resistance caused by trapped air through the membrane | [18,33] |
| Being less sensitive to membrane pollution or concentration polarization and without a pretreatment stage. | [8,33,36] |
| Membrane Trade Name | Material | Manufacturer | δ (μm) | ε (%) | LEPW (kPa) | Reference |
|---|---|---|---|---|---|---|
| TF200 | PTFE */PP ** | Gelman | 178 | 80 | 282 | |
| TF450 | PTFE/PP | Gelman | 178 | 80 | 138 | [8,18,43] |
| TF1000 | PTFE/PP | Gelman | 178 | 80 | 48 | |
| PT20 | PTFE/PP | Gore | 64 ± 5 | 90 ± 1 | 3.68 ± 0.01 | [8] |
| PT45 | PTFE/PP | Gore | 77 ± 8 | 89 ± 4 | 2.88 ± 0.01 | |
| TS1.0 | PTFE/PP | Osmonics Corp. | 175 | 70 | - | |
| TS22 | PTFE/PP | Osmonics Corp. | 175 | 70 | - | [18] |
| TS45 | PTFE/PP | Osmonics Corp. | 175 | 70 | - | |
| Taflen | PTFE/PP | Gelman | 60 | 50 | - | |
| FGLP | PTFE/PE | Millipore | 130 | 70 | 280 | |
| FHLP | PTFE/PE *** | Millipore | 175 | 85 | 124 | |
| GVHP | PVDF **** | Millipore | 110 | 75 | 204 | |
| PV22 | PVDF | Millipore | 126 ± 7 | 62 ± 2 | 2.29 ± 0.03 | [8,44] |
| PV45 | PVDF | Millipore | 116 ± 9 | 66 ± 2 | 1.10 ± 0.04 | |
| HVHP (Durapore) | PVDF | Millipore | 140 | 75 | 105 | |
| GVSP | PVDF | Millipore | 108 | 80 | - | [18] |
| Gore | PTFE | Gore | 64 | 90 | 368 | |
| Gore | PTFE | Gore | 77 | 89 | 288 | |
| Teknokrama | PTFE | Teknokrama | - | 80 | - | |
| Teknokrama | PTFE | Teknokrama | - | 80 | - | |
| Teknokrama | PTFE | Teknokrama | - | 80 | - | |
| G-4.0-6-7 | PTFE | GoreTex Sep GmbH | 100 | 80 | 463 | |
| Sartorious | PTFE | Sartorious | 70 | 70 | - | |
| MD080CO2N | PP | Enka Microdyn | 650 | 70 | - | |
| MD020TP2N | PP | Enka Microdyn | 1550 | 70 | - | [8,18] |
| Accurel® | PP | Enka A.G. | 400 | 74 | - | |
| Celgard X-20 | PP | Hoechst Celanese Co | 25 | 35 | - | |
| Accurel® S6/2 | PP | AkzoNobel | 450 | 70 | 1.4 | |
| Enka | PP | Sartorious | 100 | 75 | - | |
| Enka | PP | Sartorious | 140 | 75 | - | [18] |
| 3MA | PP | 3M Corporation | 91 | 66 | - | |
| 3MB | PP | 3M Corporation | 81 | 76 | - | |
| 3MC | PP | 3M Corporation | 76 | 79 | - | |
| 3MD | PP | 3M Corporation | 86 | 80 | - | |
| 3ME | PP | 3M Corporation | 79 | 85 | - | |
| Membrana | PP | Membrana, Germany | 91 | - | - | |
| PP22 | PP | Osmonics Corp. | 150 | 70 | - | |
| Metricel | PP | Gelman | 90 | 55 | - | |
| Celgard 2400 | PP | Hoechst Celanese Co. | 25 | 38 | - | |
| Celgard 2500 | PP | Hoechst Celanese Co. | 28 | 45 | - | |
| EHF270FA-16 | PE | Mitsubishi | 55 | 70 | - |
| Method of Treatment | Advantages | Disadvantages | Reference |
|---|---|---|---|
| DCMD | Simplest operation Least required equipment Simplest MD configuration | Not suitable for removing non-volatile organics and dissolved gasses (water must be permeating flux) Highest heat loss by conduction among other configurations | [33,38,48] |
| AGMD | High flexibility in MD configuration Less conductive heat loss Less tendency to fouling High flux Without wetting on the permeate side | Creation of additional resistance to mass transfer Hard module designing Minimum obtained output ratio | [8,9,55] |
| SGMD | A suitable configuration for removing contaminant (volatile component and dissolved gasses) Without wetting from the permeate side Lower thermal polarization | Large condenser needed due to the small volume of permeate diffuses in a large sweep gas volume Low flux | [8,9,33,70,71] |
| VMD | Negligible conductive heat loss High flux Suitable for aroma compounds recovery | Pore wetting risk Higher fouling Vacuum pump and external condenser | [8,9,33] |
| Area | Application | MD Configuration | Reference |
|---|---|---|---|
| Chemical industry | Removing volatile organic compounds from water Acid concentrating Crystallization Azeotropic mixtures separation | VMD DCMD SGMD AGMD | [33,87,88,89] |
| Desalination | Producing pure water from brackish water | VMD DCMD SGMD AGMD | [33,34,90,91] |
| Food industry (Juice and Dairy) | Juice concentrating Processing of milk Temperature sensitive materials | VMD DCMD AGMD | [33,92,93,94,95] |
| Textile industry | Dye removal Wastewater treatment | VMD DCMD | [33,96,97,98] |
| Pulp and paper industry | Removing sodium sulfate, organic and inorganic compounds, adsorbable organic halogens (AOX), color, phenolic compounds, and chemical oxygen demand (COD) from wastewater | DCMD | [99,100,101] |
| Pharmaceutical and biomedical industries | Wastewater treatment Water removing from protein and blood solutions | DCMD | [30,33,102,103] |
| Nuclear industry | Producing pure water Wastewater treatment Radioactive solutions concentrating | DCMD VMD | [31,33,104,105] |
| Gold mining | Reusing mining effluents Removing hazardous metals and ions such as sulfate from mining effluents | DCMD | [106] |
| Bioethanol production plants | Recovery of ethanol from scrubber-water | AGMD | [107] |
| Feed | Membrane Configuration | Membrane Material | Contaminant | Removal Efficiency (%) | Scale | Reference |
|---|---|---|---|---|---|---|
| Radioactive wastewater (SrCl2) | VMD | PP | Sr2+ | Over 99.60 | laboratory-scale | [104] |
| Metal solution (salts of Co (II), Zn (II), Cu (II), Ni (II), Cd (II) and Pb (II)) | VMD | Poly(vinylidenefluoride)-titanium tetraisopropoxide PVDF-TTIP | Heavy metals | Total removal | laboratory-scale | [119] |
| Distilled water and crude oil | VMD | PVDF | Total Organic Carbon (TOC) | 93.4–97 | laboratory-scale | [120] |
| Olive Mill WasteWater (OMWW) | DCMD & VMD | PP | Polyphenols TOC | 99.6 89 and 99.6 | laboratory-scale | [121] |
| Industrial textile wastewater | DCMD | PVDF-Cloisite 15A nanocomposite | Colour Total Dissolved Solids(TDS) Chemical Oxygen Demand(COD) | 95.3 93.7 90.8 | laboratory-scale | [122] |
| Synthetic dye solution | DCMD | PVDF modified by ethylene glycol (EG) | RB5 | 99.86 | laboratory-scale | [98] |
| Highly saline radioactive wastewater | DCMD | PP | Nuclides (Co(II), Sr(II), Cs(I)) and boron (B) | >99.97% | laboratory-scale | [123] |
| Synthetic wastewater and Seawater | Osmotic membrane bioreactor (OMBR)—(DCMD) hybrid system | PTFE active layer and a PP supporting layer | 30 trace organic contaminants | >90% | laboratory-scale | [37] |
| Geothermal water | AGMD | PP, PTFE and PVDF | Boron | 99.5% | laboratory-scale | [21] |
| High salinity hydraulic fracturing produced water (HFPW) | Combined Electrocoagulation (EC) and DCMD | Ethylene chlorotrifluoroethylene (ECTFE) | Turbidity, Total suspended solids (TSS) and TOC | 96%, 91% and 61%, respectively | laboratory-scale | [124] |
| Industrial dyeing wastewater | DCMD combined with physicochemical and biological treatment | PTFE and PVDF | COD and color removal | 96% and 100% respectively | laboratory-scale | [49] |
| Saline oily wastewater | DCMD | PVDF modified with silica nanoparticles and Polystyrene (PS) microspheres | Oil and gas emulsified wastewater | Highly desirable | laboratory-scale | [125] |
| Mining wastewater | VMD | PVDF membrane was coated by Hyflon AD materials (Hyflon AD40L, Hyflon AD40H) | Mining waste | Highly efficient | laboratory-scale | [126] |
| Bentazon herbicide solutions | VMD | PTFE | Bentazon | Very effective | laboratory-scale | [127] |
| Fermentation wastewater | DCMD | PP | COD, TOC | 95% | laboratory-scale | [128] |
| Detection Method(s) | Prevention Process(es) | Reference |
|---|---|---|
|
| [35,61,128,129,132,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biniaz, P.; Torabi Ardekani, N.; Makarem, M.A.; Rahimpour, M.R. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering 2019, 3, 8. https://doi.org/10.3390/chemengineering3010008
Biniaz P, Torabi Ardekani N, Makarem MA, Rahimpour MR. Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering. 2019; 3(1):8. https://doi.org/10.3390/chemengineering3010008
Chicago/Turabian StyleBiniaz, Parisa, Niloofar Torabi Ardekani, Mohammad Amin Makarem, and Mohammad Reza Rahimpour. 2019. "Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD)" ChemEngineering 3, no. 1: 8. https://doi.org/10.3390/chemengineering3010008
APA StyleBiniaz, P., Torabi Ardekani, N., Makarem, M. A., & Rahimpour, M. R. (2019). Water and Wastewater Treatment Systems by Novel Integrated Membrane Distillation (MD). ChemEngineering, 3(1), 8. https://doi.org/10.3390/chemengineering3010008




