Improved Kinetics and Water Recovery with Propane as Co-Guest Gas on the Hydrate-Based Desalination (HyDesal) Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
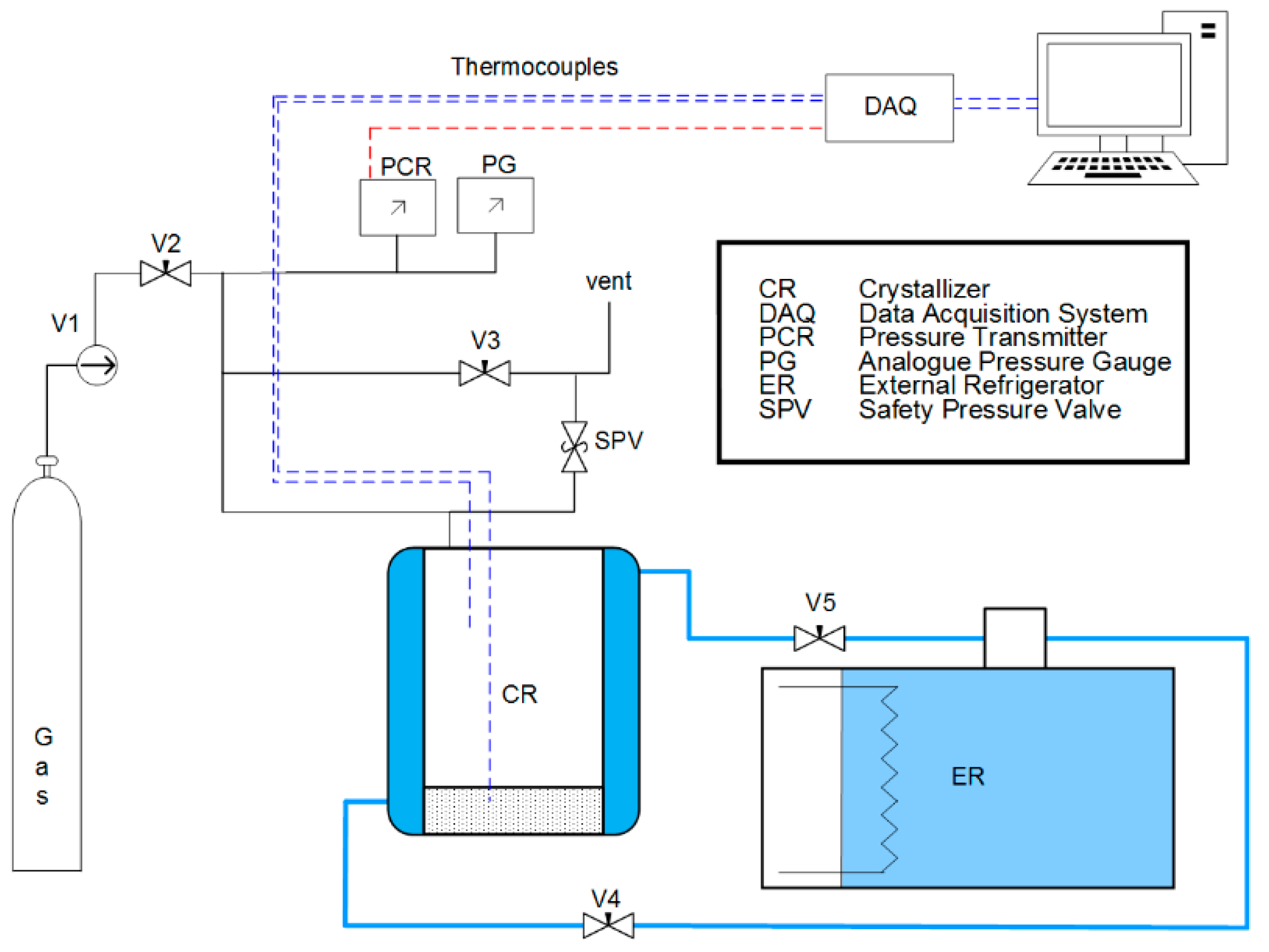
2.2. Apparatus
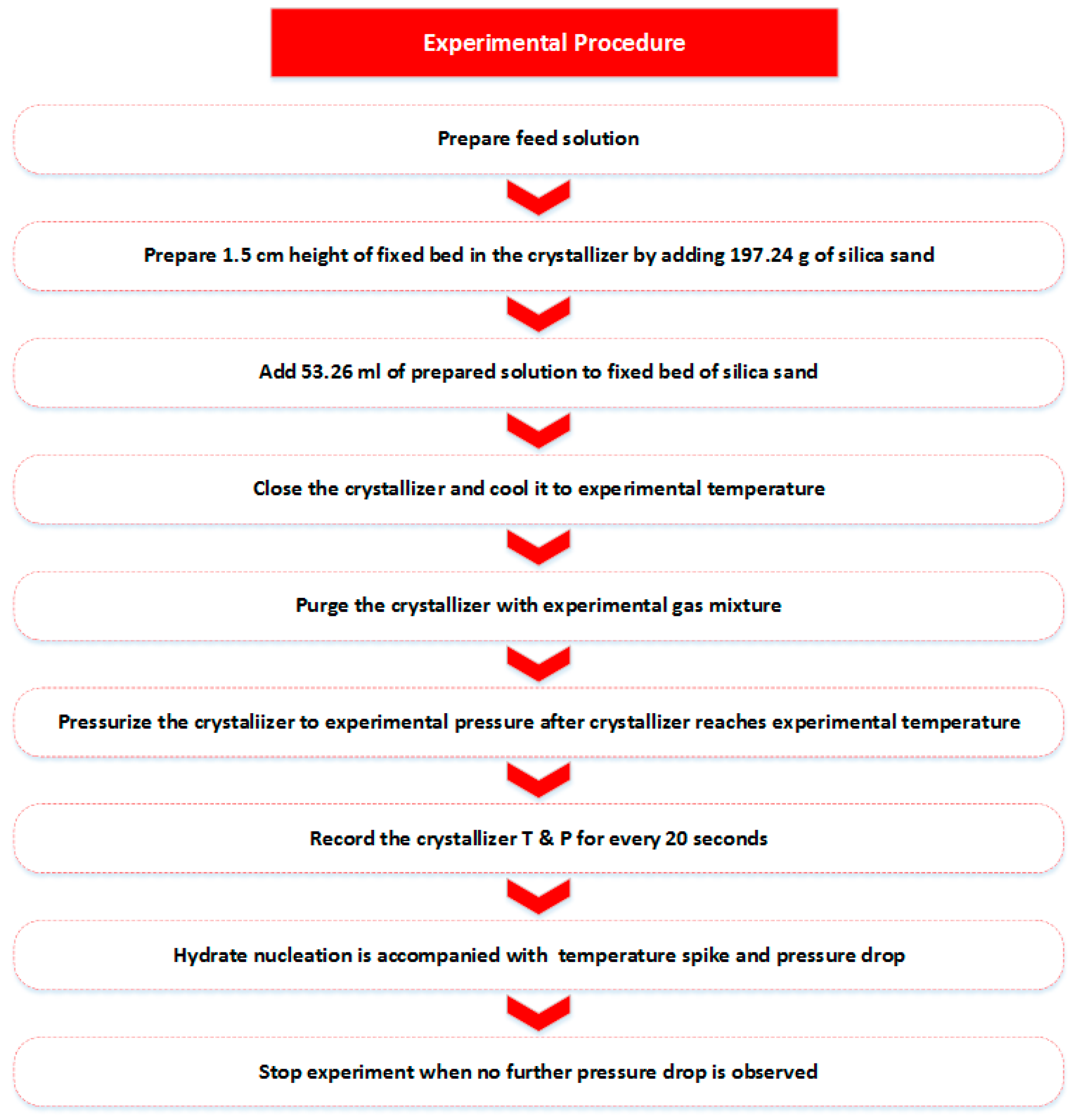
2.3. Procedure
2.3.1. Preparation of Solution
2.3.2. Preparation of Silica and Bed
2.3.3. Hydrate Formation Procedure
2.4. Calculation of Water Recovery
3. Results and Discussion
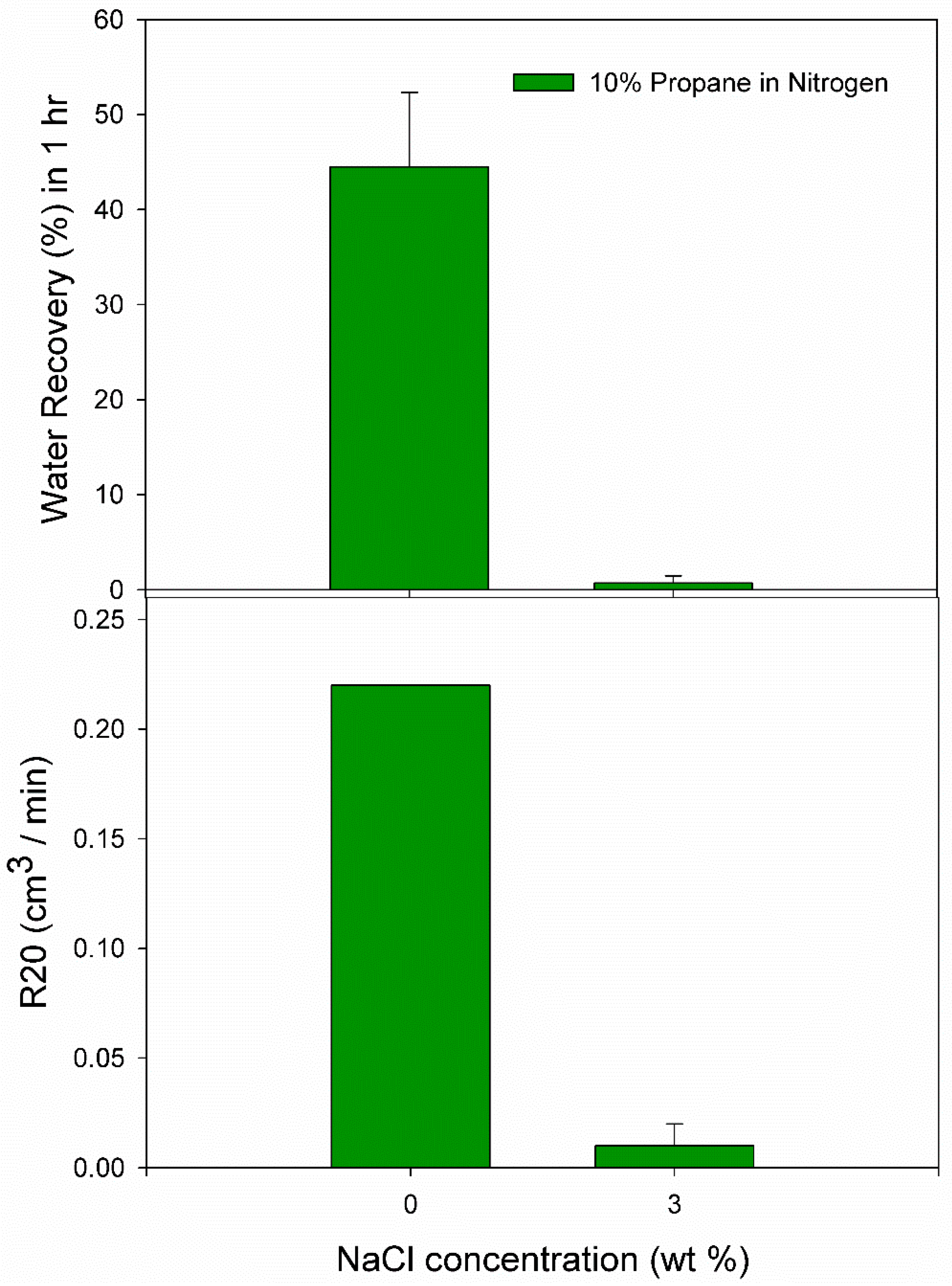
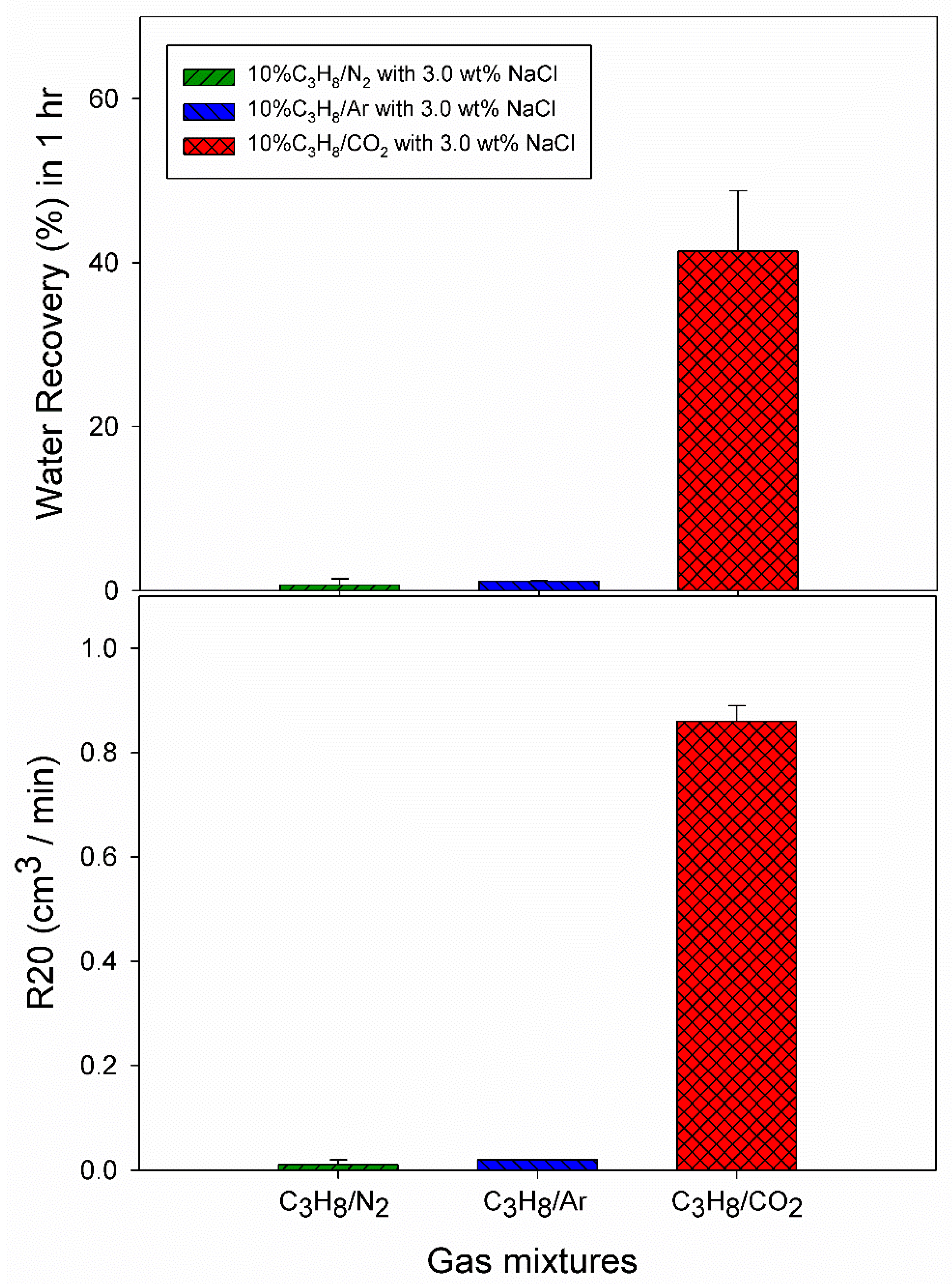
3.1. Effect of 10% Propane in Nitrogen Gas Mixture (G1)
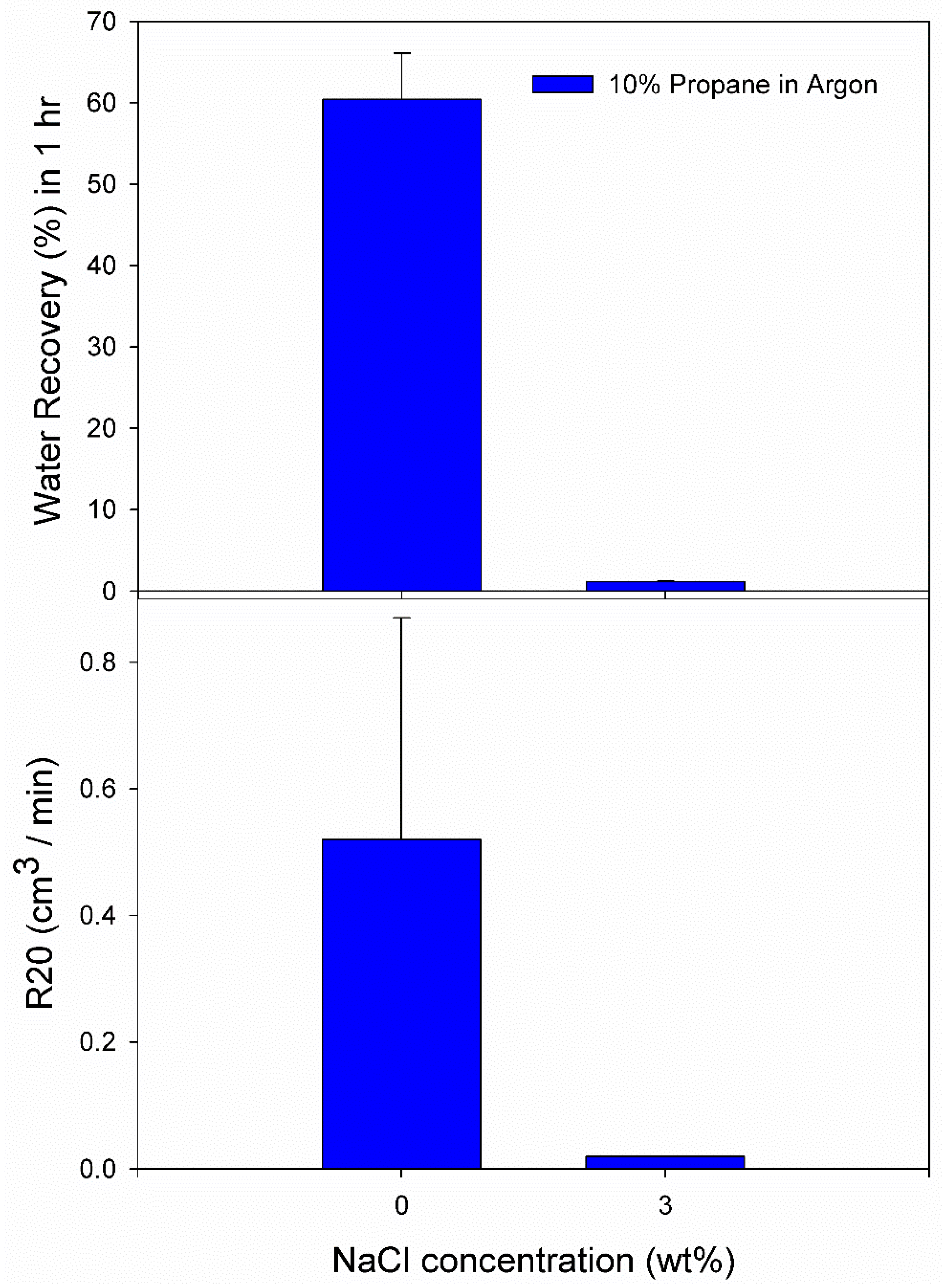
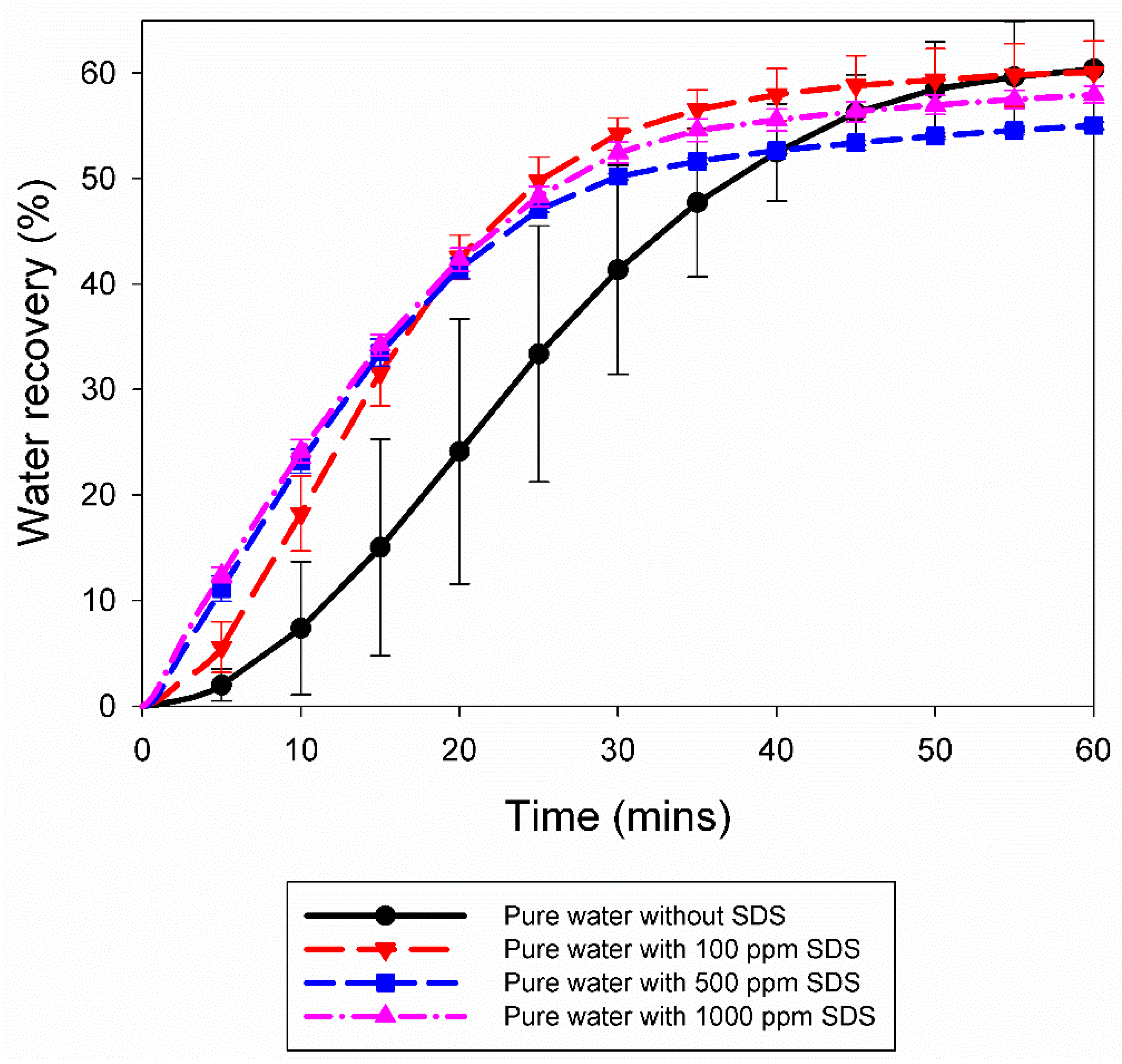
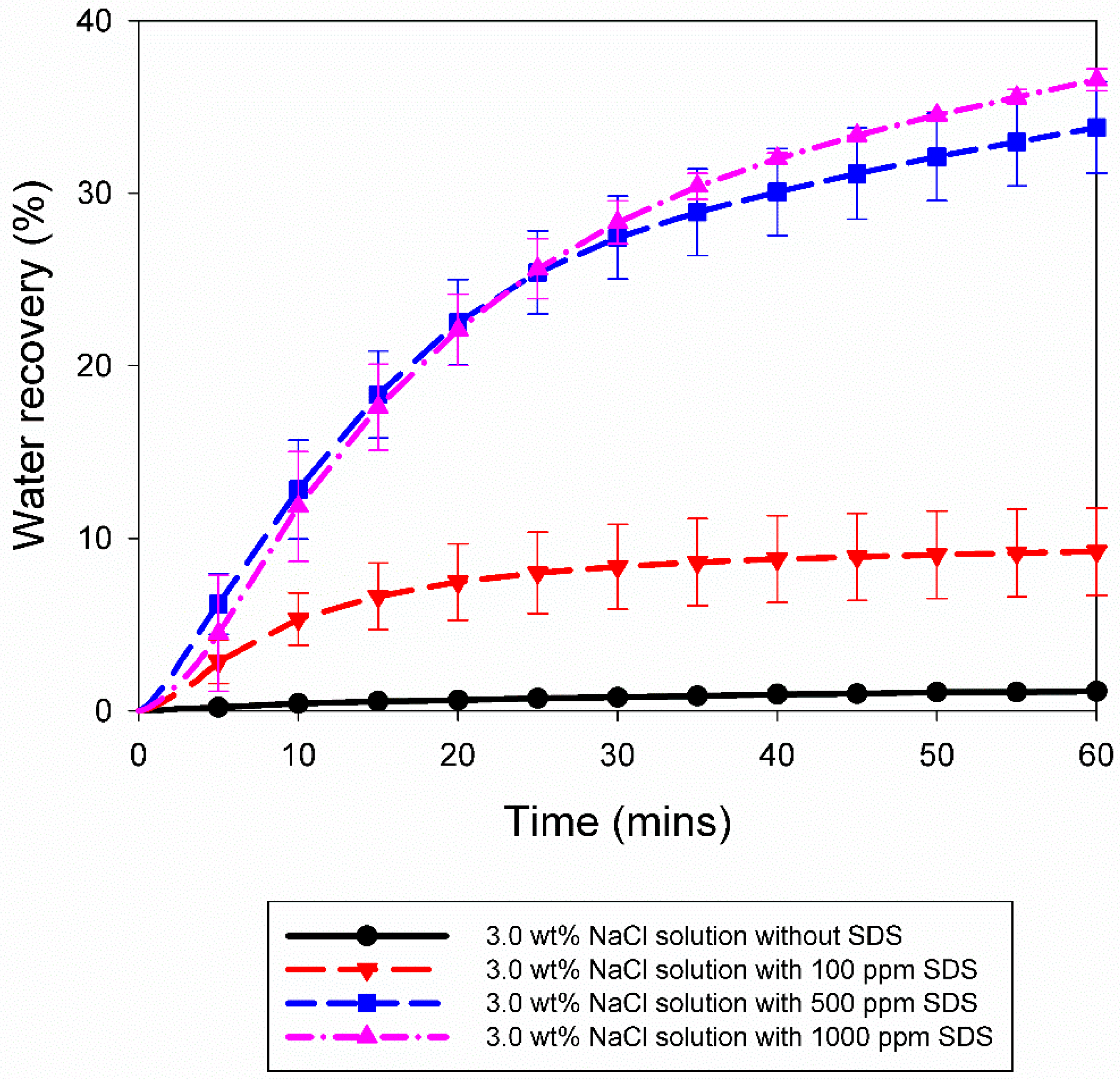
3.2. Effect of 10% Propane in Argon Gas Mixture (G2)
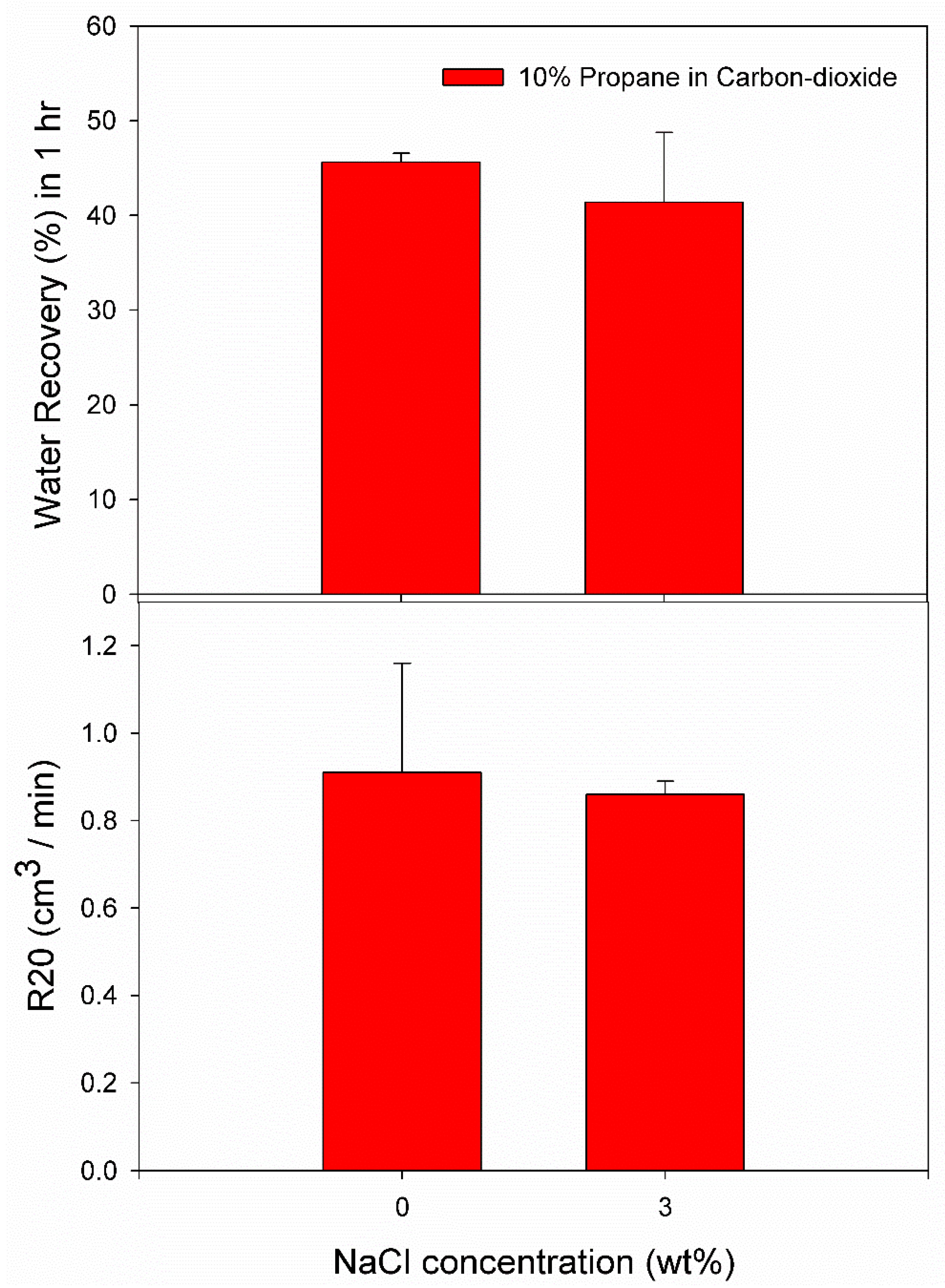
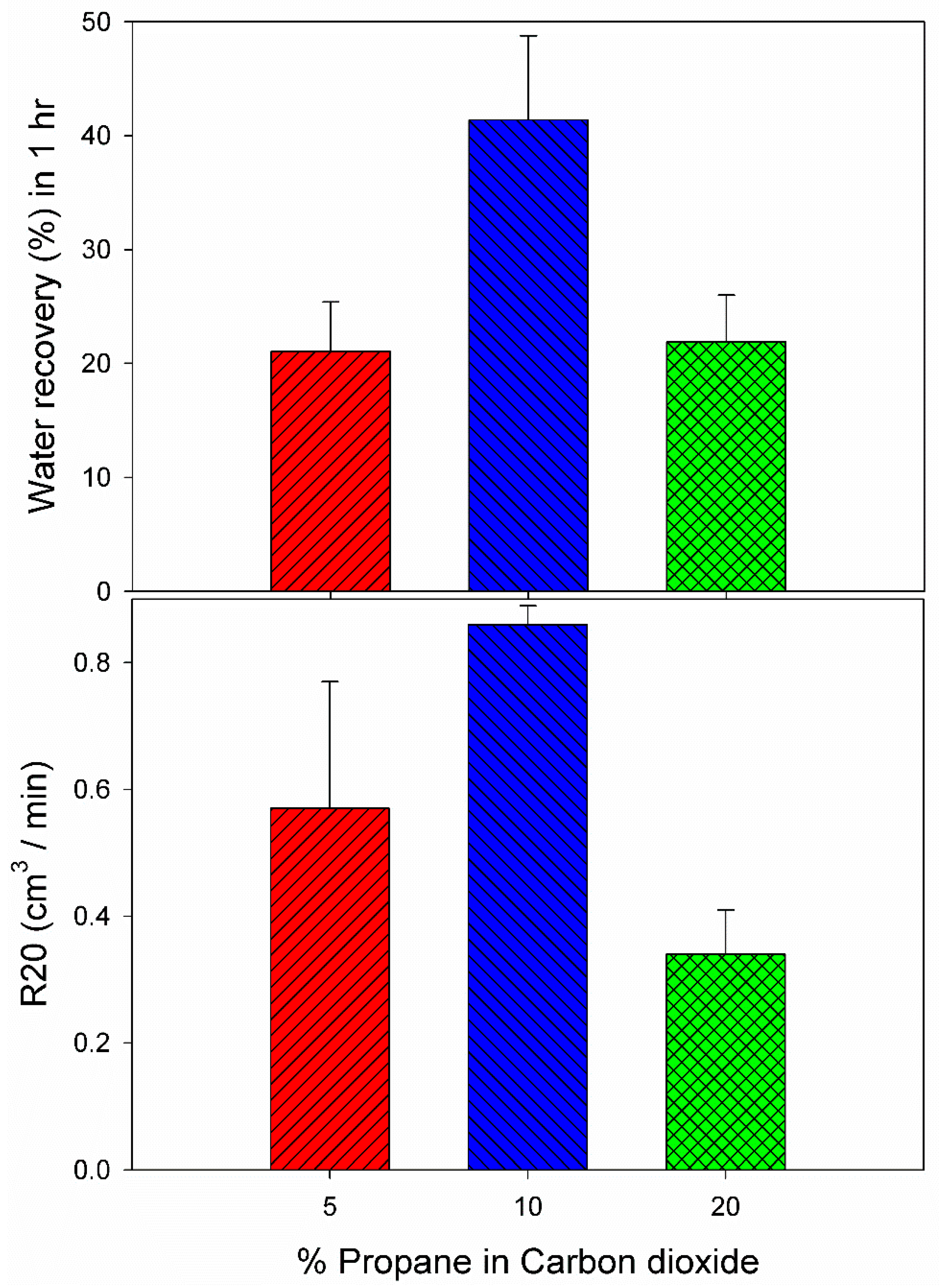
3.3. Effect of 10% Propane in Carbon Dioxide Gas Mixture (G3)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Water, U.N. Coping with Water Scarcity: Challenge of the Twenty-First Century; Prepared for World Water Day; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Montgomery, M.A.; Elimelech, M. Water And Sanitation in Developing Countries: Including Health in the Equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Feeley, T.J., III; Skone, T.J.; Stiegel, G.J., Jr.; McNemar, A.; Nemeth, M.; Schimmoller, B.; Murphy, J.T.; Manfredo, L. Water: A critical resource in the thermoelectric power industry. Energy 2008, 33, 1–11. [Google Scholar] [CrossRef]
- Postel, S.; Bawa, K.; Kaufman, L.; Peterson, C.H.; Carpenter, S.; Tillman, D.; Dayton, P.; Alexander, S.; Lagerquist, K.; Goulder, L. Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Marchal, V.; Dellink, R.; van Vuuren, D.; Clapp, C.; Château, J.; Lanzi, E.; Magné, B.; van Vliet, J. OECD Environmental Outlook to 2050: The Consequences of Inaction; OECD: Paris, France, 2012. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Al-Sahali, M.; Ettouney, H. Developments in thermal desalination processes: Design, energy, and costing aspects. Desalination 2007, 214, 227–240. [Google Scholar] [CrossRef]
- Drewes, J.E.; Cath, T.Y.; Xu, P.; Graydon, J.; Veil, J.; Snyder, S. An Integrated Framework for Treatment and Management of Produced Water; RPSEA Project; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2009; p. 07122.
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012, cts049. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Escobar, I.C. Chapter 14 Conclusion: A Summary of Challenges still Facing Desalination and Water Reuse. In Sustainability Science and Engineering; Isabel, C.E., Andrea, I.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 389–397. [Google Scholar]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Raluy, R.G.; Serra, L.; Uche, J. Life cycle assessment of desalination technologies integrated with renewable energies. Desalination 2005, 183, 81–93. [Google Scholar] [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Eslamimanesh, A.; Mohammadi, A.H.; Richon, D.; Naidoo, P.; Ramjugernath, D. Application of gas hydrate formation in separation processes: A review of experimental studies. J. Chem. Thermodyn. 2012, 46, 62–71. [Google Scholar] [CrossRef]
- Sum, A.K.; Koh, C.A.; Sloan, E.D. Clathrate hydrates: From laboratory science to engineering practice. Ind. Eng. Chem. Res. 2009, 48, 7457–7465. [Google Scholar] [CrossRef]
- Ogawa, T.; Ito, T.; Watanabe, K.; Tahara, K.I.; Hiraoka, R.; Ochiai, J.I.; Ohmura, R.; Mori, Y.H. Development of a novel hydrate-based refrigeration system: A preliminary overview. Appl. Therm. Eng. 2006, 26, 2157–2167. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Pre-combustion capture of carbon dioxide in a fixed bed reactor using the clathrate hydrate process. Energy 2013, 50, 364–373. [Google Scholar] [CrossRef]
- McCormack, R.; Niblock, G. Build and Operate a Clathrate Desalination Pilot Plant; US Bureau of Reclamation Water Treatment Technology Program Report; US Bureau of Reclamation: Denver, CO, USA, 1998.
- McCormack, R.A.; Niblock, G.A. Investigation of High Freezing Temperature, Zero Ozone, and Zero Global Warming Potential, Clathrate Formers for Desalination; US Department of the Interior, Bureau of Reclamation, Technical Service Center, Water Treatment Engineering and Research Group: Burlington, MA, USA, 2000.
- Bradshaw, R.W.; Greathouse, J.A.; Cygan, R.T.; Simmons, B.A.; Dedrick, D.E.; Majzoub, E.H. Desalination Utilizing Clathrate Hydrates (LDRD Final Report); Digital Library: New York, NY, USA, 2008. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Paris, France, 2007. [Google Scholar]
- Mccormack, R.; Ripmeester, J. Clathrate Desalination Process Using an Ultrasonic Actuator. WO Patent 2,013,049,253, 4 April 2013. [Google Scholar]
- Zheng, J.; Zhang, B.-Y.; Wu, Q.; Linga, P. Kinetic Evaluation of Cyclopentane as a Promoter for CO2 Capture via a Clathrate Process Employing Different Contact Modes. ACS Sustain. Chem. Eng. 2018, 6, 11913–11921. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy–Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- He, T.; Nair, S.K.; Babu, P.; Linga, P.; Karimi, I.A. A Novel Conceptual Design of Hydrate Based Desalination (HyDesal) Process by Utilizing LNG Cold Energy. Appl. Energy 2018, 222, 13–24. [Google Scholar] [CrossRef]
- Zheng, J.; Lee, Y.K.; Babu, P.; Zhang, P.; Linga, P. Impact of fixed bed reactor orientation, liquid saturation, bed volume and temperature on the clathrate hydrate process for pre-combustion carbon capture. J. Nat. Gas Sci. Eng. 2016, 35, 1499–1510. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbot, M.M. Introduction to Chemical Engineering Thermodynamics, 6th ed.; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Sloan, E.D., Jr.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mekala, P.; Babu, P.; Sangwai, J.S.; Linga, P. Formation and Dissociation Kinetics of Methane Hydrates in Seawater and Silica Sand. Energy Fuels 2014, 28, 2708–2716. [Google Scholar] [CrossRef]
- Chong, Z.R.; Chan, A.H.M.; Babu, P.; Yang, M.; Linga, P. Effect of NaCl on methane hydrate formation and dissociation in porous media. J. Nat. Gas Sci. Eng. 2015, 27, 178–189. [Google Scholar] [CrossRef]
- Holzammer, C.; Finckenstein, A.; Will, S.; Braeuer, A.S. How Sodium Chloride Salt Inhibits the Formation of CO2 Gas Hydrates. J. Phys. Chem. B 2016, 120, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.H.; Afzal, W.; Richon, D. Gas hydrates of methane, ethane, propane, and carbon dioxide in the presence of single NaCl, KCl, and CaCl2 aqueous solutions: Experimental measurements and predictions of dissociation conditions. J. Chem. Thermodyn. 2008, 40, 1693–1697. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, W.; Liu, Y.; Xie, Y.; Chen, X. The influence of NaCl ions on hydrate structure and thermodynamic equilibrium conditions of gas hydrates. Fluid Phase Equilibria 2012, 325, 6–10. [Google Scholar] [CrossRef]
- Yoslim, J.; Linga, P.; Englezos, P. Enhanced growth of methane-propane clathrate hydrate crystals with sodium dodecyl sulfate, sodium tetradecyl sulfate, and sodium hexadecyl sulfate surfactants. J. Cryst. Growth 2010, 313, 68–80. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Lee, P.Y.; Premasinghe, K.; Linga, P. Effect of Biofriendly Amino Acids on the Kinetics of Methane Hydrate Formation and Dissociation. Ind. Eng. Chem. Res. 2017, 56, 6145–6154. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tan, B.; Wang, W. Methane Storage in a Hydrated Form as Promoted by Leucines for Possible Application to Natural Gas Transportation and Storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Kumar, R.; Linga, P. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application. Appl. Energy 2017, 188, 190–199. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderi Far, A.; Kameli, M. Potato starch as methane hydrate promoter. Fuel 2012, 94, 356–360. [Google Scholar] [CrossRef]
- Maghsoodloo Babakhani, S.; Alamdari, A. Effect of maize starch on methane hydrate formation/dissociation rates and stability. J. Nat. Gas Sci. Eng. 2015, 26, 1–5. [Google Scholar] [CrossRef]
- Rogers, R.E.; Kothapalli, C.; Lee, M.S.; Woolsey, J.R. Catalysis of Gas Hydrates by Biosurfactants in Seawater-Saturated Sand/Clay. Can. J. Chem. Eng. 2003, 81, 973–980. [Google Scholar] [CrossRef]
- Yang, S.H.B.; Babu, P.; Chua, S.F.S.; Linga, P. Carbon dioxide hydrate kinetics in porous media with and without salts. Appl. Energy 2016, 162, 1131–1140. [Google Scholar] [CrossRef]
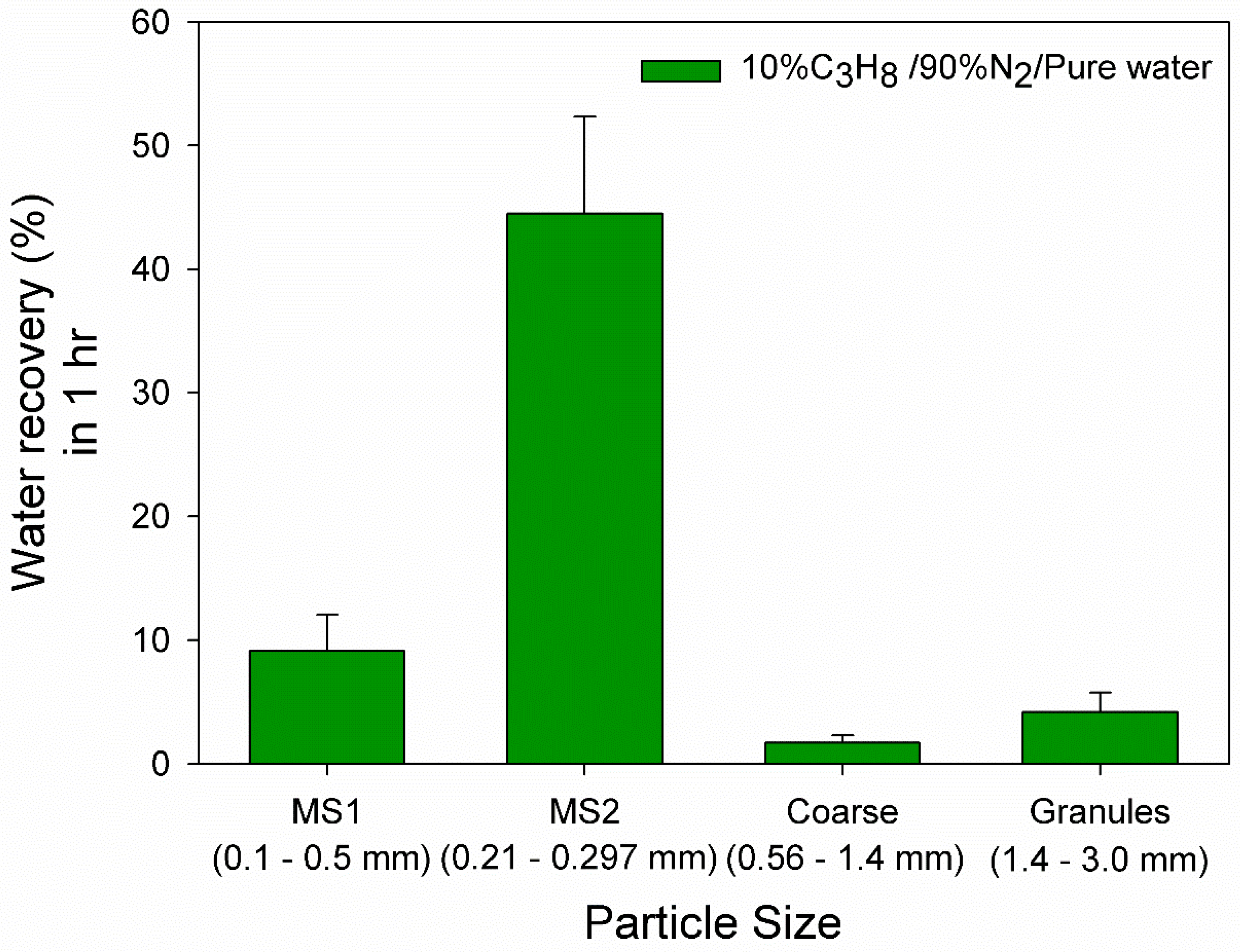
| Medium Sand 1 (MS1) | Medium Sand 2 (MS2) | Coarse Sand | Granular Pebble | |
|---|---|---|---|---|
| Size range (mm) | 0.1–0.5 | 0.21–0.29 | 0.56–1.3 | 1.5–3.0 |
| Bulk density (g/cm3) | 1.56 | 1.61 | 1.53 | 1.49 |
| Pore volume (cm3/g) | 0.27 | 0.27 | 0.238 | 0.258 |
| System with G1 Mixture | Exp. No | Pressure (MPa) | Temperature (K) | Induction Time (IT) (min) | Gas Uptake at IT (mol/mol of Water) | Gas Uptake 60 min from IT (mol/mol of Water) | Water Recovery at 60 min from IT (%) | R20 (cm3/min) |
| Pure Water | N1 | 5.0 | 275.5 | 250.33 | 0.0062 | 0.0438 | 36.34 | 0.21 |
| N2 | 5.0 | 275.5 | 41.67 | 0.0065 | 0.0603 | 51.96 | 0.22 | |
| N3 | 5.0 | 275.5 | 160.33 | 0.0051 | 0.0519 | 45.21 | 0.22 | |
| 3.0 wt% NaCl solution | N4 | 5.0 | 274.2 | 1600 | 0.0035 | 0.0037 | 0.13 | 0.02 |
| N5 | 5.0 | 274.2 | 117.33 | 0.0001 | 0.0014 | 1.24 | 0.00 | |
| Systemwith G2 mixture | Exp. No | Pressure (MPa) | Temperature (K) | Induction Time (IT) (min) | Gas Uptake at IT (mol/mol of Water) | Gas Uptake 60 min from IT (mol/mol of Water) | Water Recovery at 60 min from IT (%) | R20 (cm3/min) |
| Pure Water | A1 | 5.0 | 274.2 | 91.67 | 0.0037 | 0.1017 | 53.41 | 0.29 |
| A2 | 5.0 | 274.2 | 1867.00 | 0.0046 | 0.1222 | 64.73 | 0.35 | |
| A3 | 5.0 | 274.2 | 429.67 | 0.0021 | 0.1060 | 58.90 | 0.92 | |
| Pure water with 100 ppm SDS | A4 | 5.0 | 274.2 | 0.67 | 0.0002 | 0.1025 | 57.99 | 1.00 |
| A5 | 5.0 | 274.2 | 0.33 | 0.0005 | 0.1102 | 62.16 | 1.16 | |
| Pure water with 500 ppm SDS | A6 | 5.0 | 274.2 | 53.33 | 0.0017 | 0.0992 | 55.26 | 1.14 |
| A7 | 5.0 | 274.2 | 24.00 | 0.0005 | 0.0971 | 54.75 | 1.20 | |
| Pure water with 1000 ppm SDS | A8 | 5.0 | 274.2 | 90.67 | 0.0004 | 0.1016 | 57.38 | 1.18 |
| A9 | 5.0 | 274.2 | 1864.33 | 0.0021 | 0.1053 | 58.52 | 1.23 | |
| 3.0 wt% NaCl solution | A10 | 5.5 | 274.2 | 46.00 | 0.0015 | 0.0036 | 1.21 | 0.02 |
| A11 | 5.5 | 274.2 | 3.67 | 0.0012 | 0.0032 | 1.10 | 0.02 | |
| A12 | 5.5 | 274.2 | 4307.00 | 0.0028 | 0.0047 | 1.10 | 0.02 | |
| 3.0 wt% NaCl solution with 100 ppm SDS | A13 | 5.5 | 274.2 | 42.67 | 0.0017 | 0.0132 | 6.50 | 0.17 |
| A14 | 5.5 | 274.2 | 187.67 | 0.0021 | 0.0157 | 7.72 | 0.19 | |
| A15 | 5.5 | 274.2 | 10.00 | 0.0008 | 0.0217 | 11.83 | 0.26 | |
| A16 | 5.5 | 274.2 | 0.33 | 0.0001 | 0.0193 | 10.95 | 0.31 | |
| 3.0 wt% NaCl solution with 500 ppm SDS | A17 | 5.5 | 274.2 | 168.33 | 0.0050 | 0.0679 | 35.67 | 0.69 |
| A18 | 5.5 | 274.2 | 844.67 | 0.0020 | 0.0583 | 31.94 | 0.55 | |
| 3.0 wt% NaCl solution with 1000 ppm SDS | A19 | 5.5 | 274.2 | 1.67 | 0.0001 | 0.0632 | 36.12 | 0.66 |
| A20 | 5.5 | 274.2 | 7.33 | 0.0009 | 0.0662 | 37.05 | 0.52 | |
| Systemwith G3 mixture | Exp. No | Pressure (MPa) | Temperature (K) | Induction Time (IT) (min) | Gas Uptake at IT (mol/mol of Water) | Gas Uptake 60 min from IT (mol/mol of Water) | Water Recovery at 60 min from IT (%) | R20 (cm3/min) |
| Pure Water | C1 | 2.5 | 274.2 | 1004.67 | 0.0164 | 0.0728 | 46.28 | 0.73 |
| C2 | 2.5 | 274.2 | 87.67 | 0.0042 | 0.0590 | 44.99 | 1.09 | |
| 3.0 wt% NaCl solution | C3 | 2.6 | 274.2 | 0.33 | 0.0003 | 0.0444 | 36.18 | 0.78 |
| C4 | 2.6 | 274.2 | 0.00 | 0.0000 | 0.0607 | 49.83 | 0.88 | |
| C5 | 2.6 | 274.2 | 0.00 | 0.0000 | 0.0464 | 38.12 | 0.84 |
| System with (%) Propane in Carbon Dioxide | Exp. No | Pressure (MPa) | Temperature (K) | Induction Time (IT) (min) | Gas Uptake at IT (mol/mol of Water) | Gas Uptake 60 min from IT (mol/mol of Water) | Water Recovery at 60 min from IT (%) | R20 (cm3/min) |
|---|---|---|---|---|---|---|---|---|
| 5 | C6 | 2.85 | 274.2 | 85.00 | 0.0064 | 0.0391 | 24.13 | 0.54 |
| 5 | C7 | 2.85 | 274.2 | 1042.00 | 0.0147 | 0.0391 | 18 | 0.39 |
| 20 | C8 | 2.0 | 274.2 | 2171.00 | 0.0142 | 0.0359 | 18.97 | 0.29 |
| 20 | C9 | 2.0 | 274.2 | 105.67 | 0.0051 | 0.0335 | 24.82 | 0.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nambiar, A.; Babu, P.; Linga, P. Improved Kinetics and Water Recovery with Propane as Co-Guest Gas on the Hydrate-Based Desalination (HyDesal) Process. ChemEngineering 2019, 3, 31. https://doi.org/10.3390/chemengineering3010031
Nambiar A, Babu P, Linga P. Improved Kinetics and Water Recovery with Propane as Co-Guest Gas on the Hydrate-Based Desalination (HyDesal) Process. ChemEngineering. 2019; 3(1):31. https://doi.org/10.3390/chemengineering3010031
Chicago/Turabian StyleNambiar, Abhishek, Ponnivalavan Babu, and Praveen Linga. 2019. "Improved Kinetics and Water Recovery with Propane as Co-Guest Gas on the Hydrate-Based Desalination (HyDesal) Process" ChemEngineering 3, no. 1: 31. https://doi.org/10.3390/chemengineering3010031
APA StyleNambiar, A., Babu, P., & Linga, P. (2019). Improved Kinetics and Water Recovery with Propane as Co-Guest Gas on the Hydrate-Based Desalination (HyDesal) Process. ChemEngineering, 3(1), 31. https://doi.org/10.3390/chemengineering3010031






