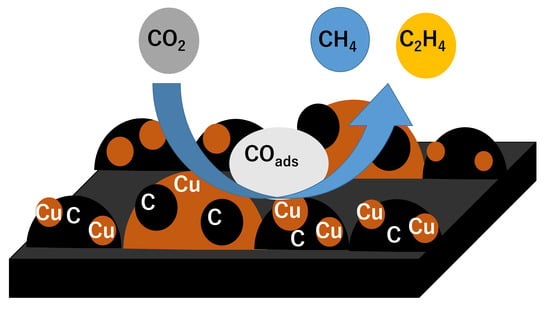
Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Catalyst Preparation
2.3. Electrode Fabrication
2.4. Electrochemical Reduction of CO2
3. Results and Discussion
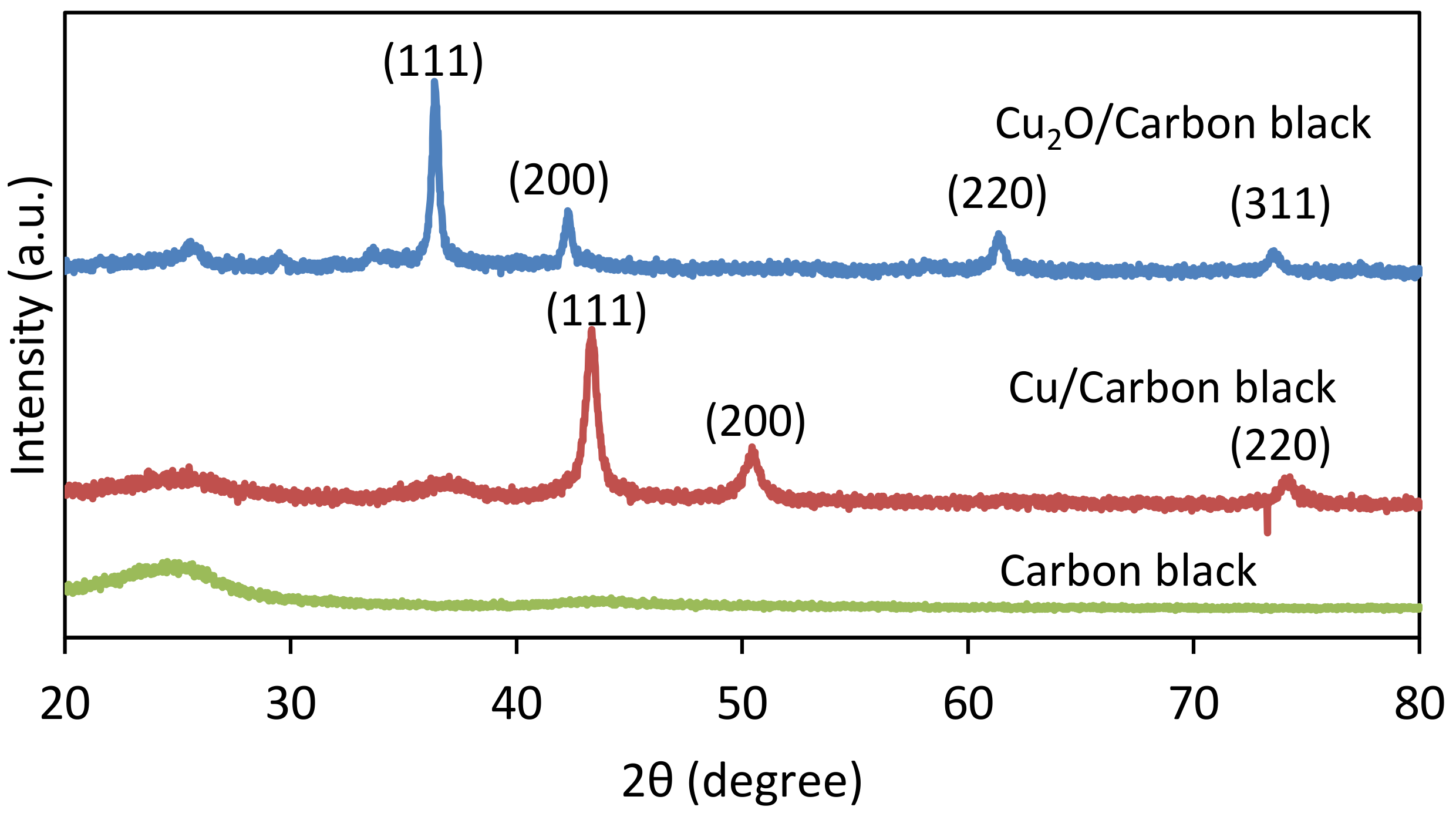
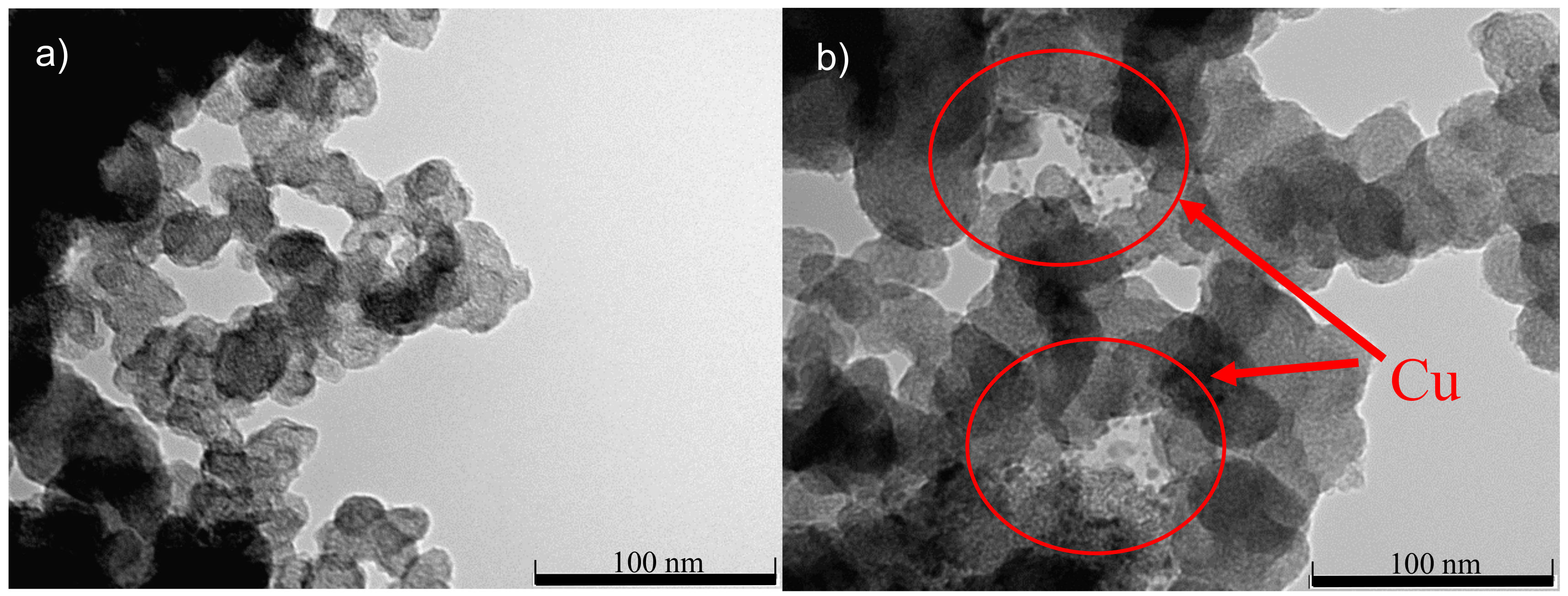
3.1. Physicochemical Characterization
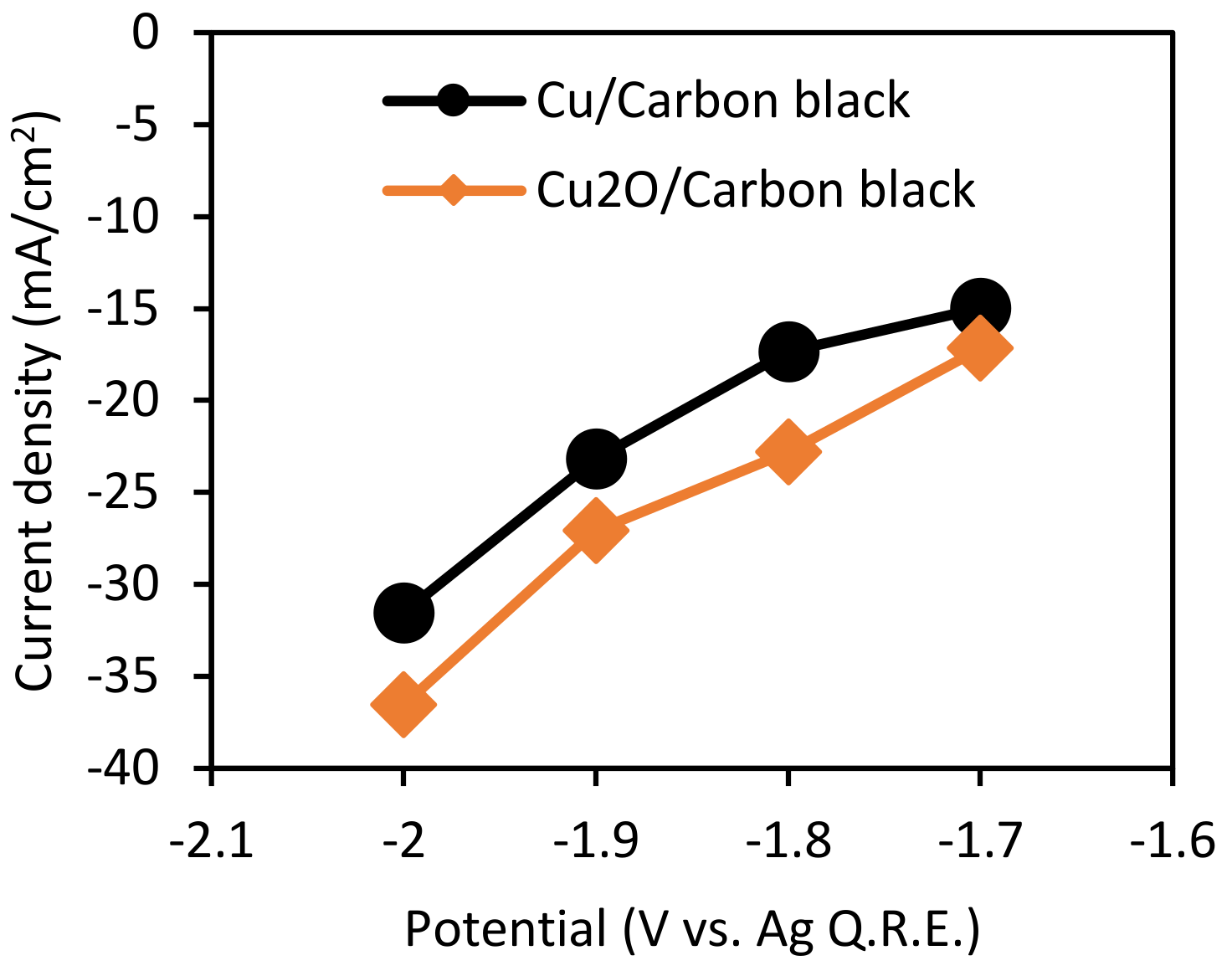
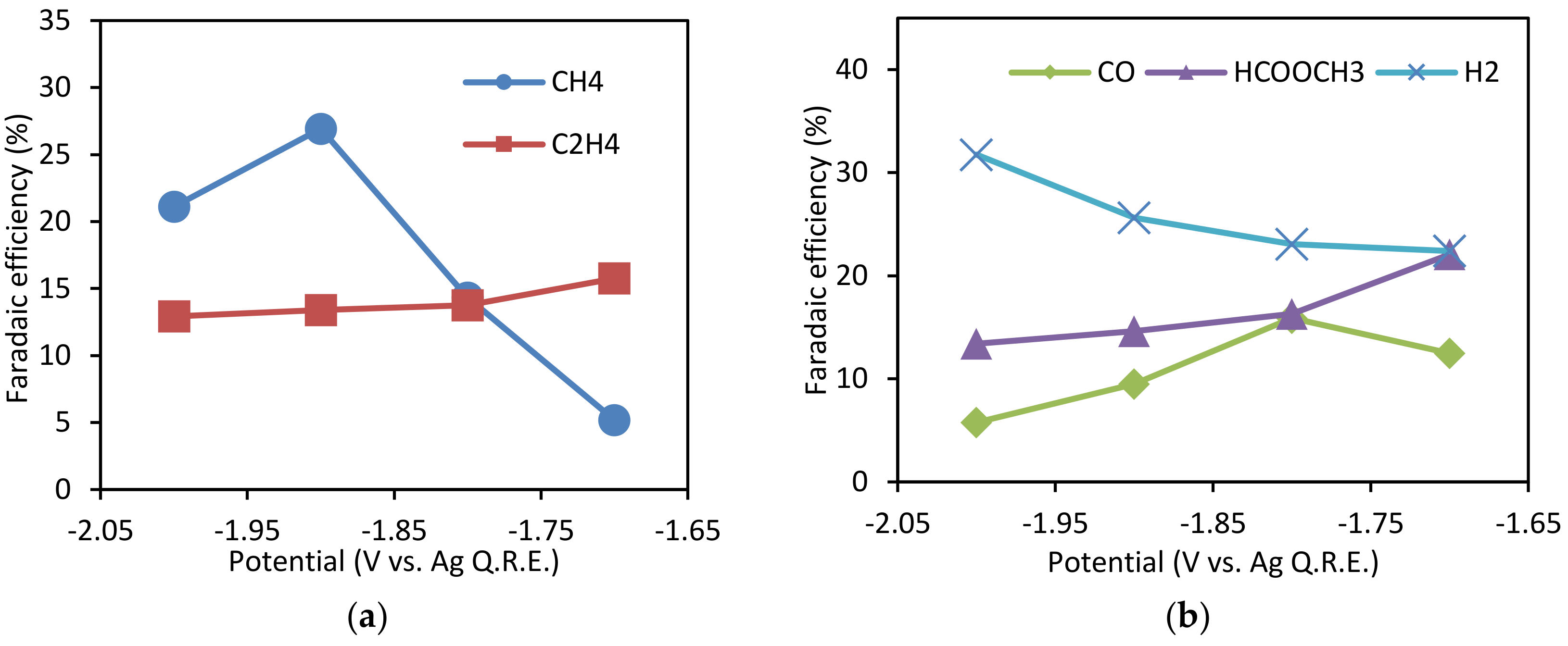
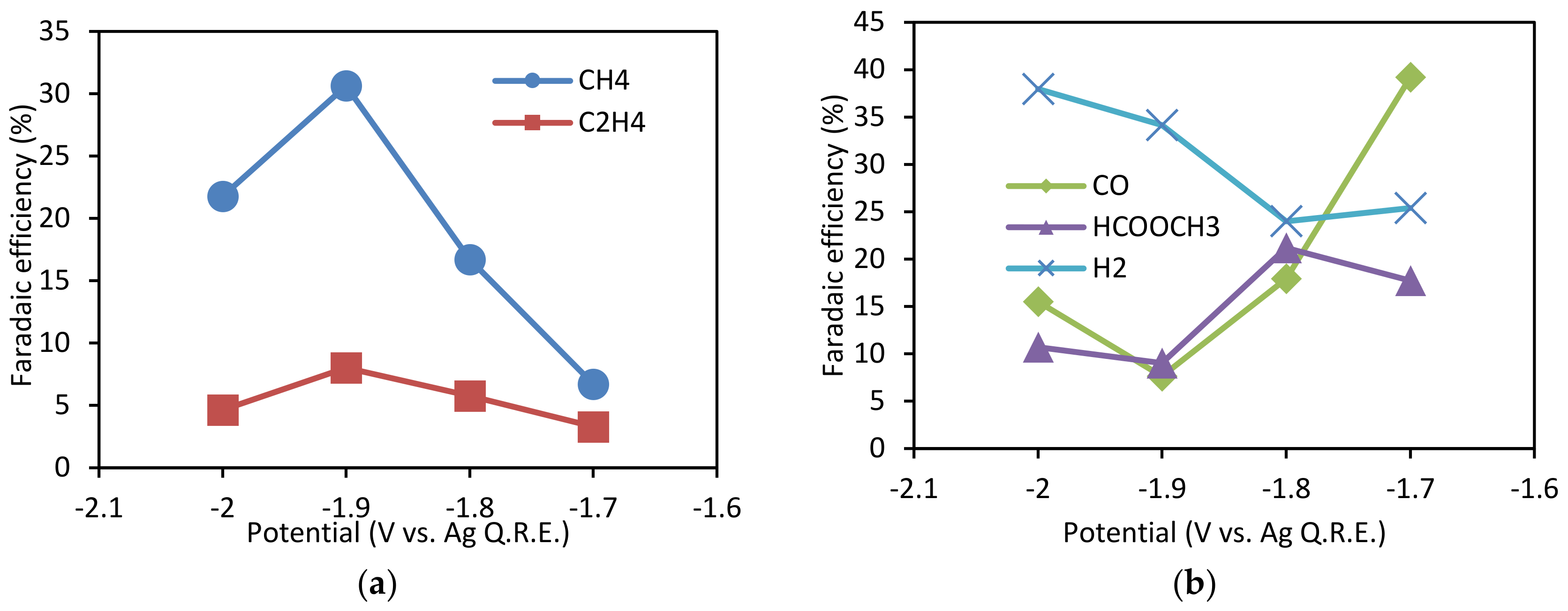
3.2. Effect of Potential
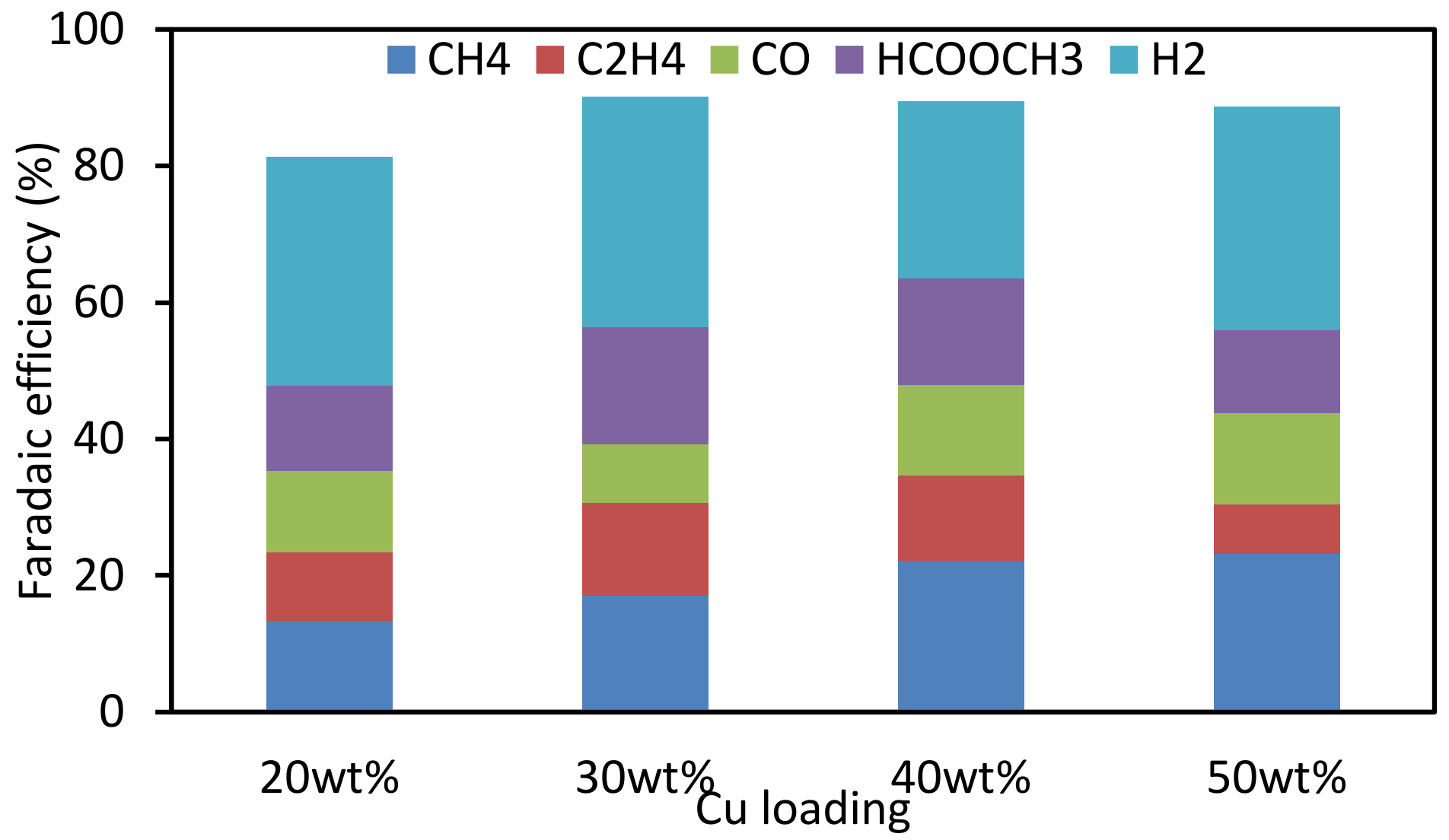
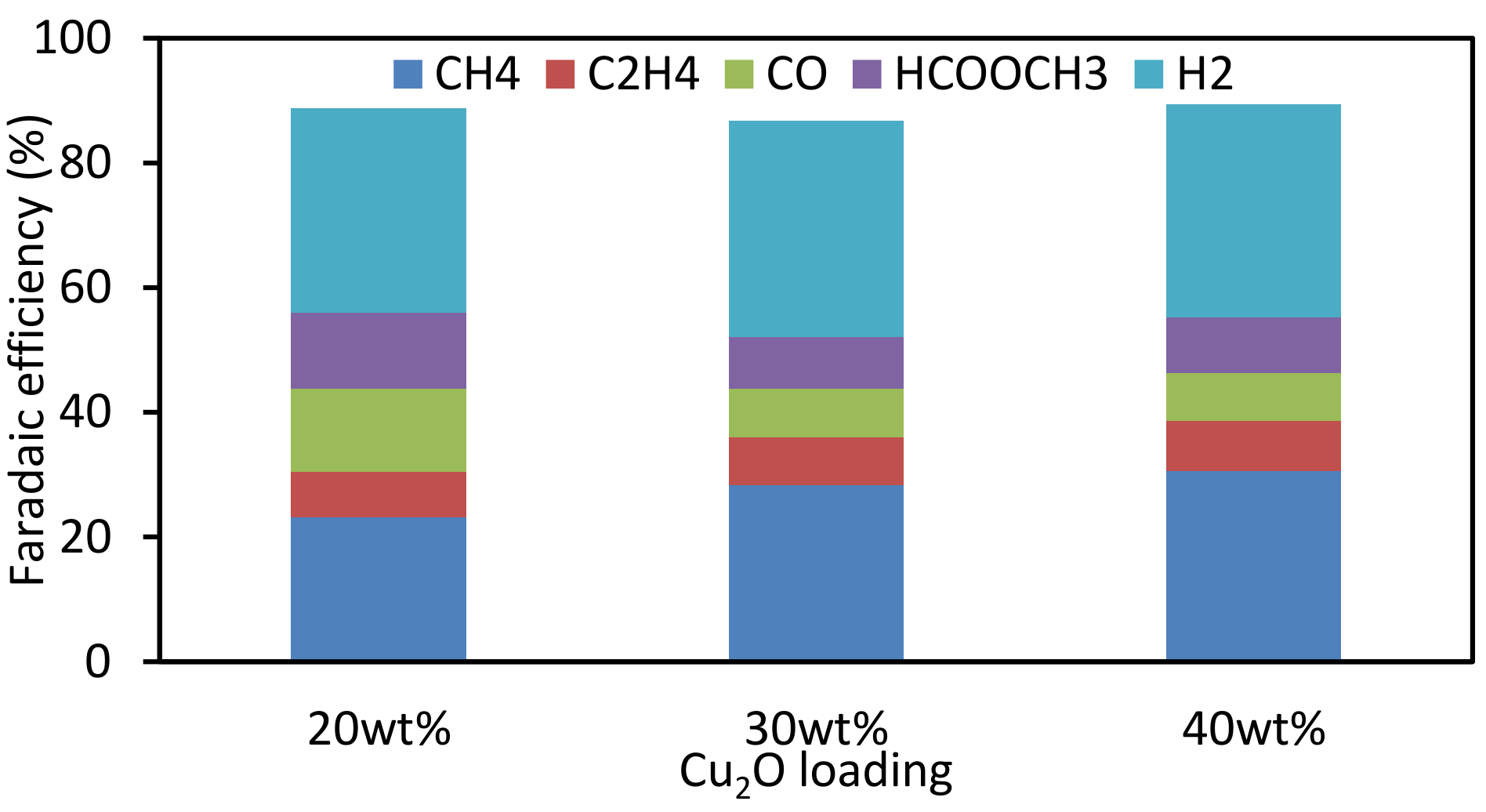
3.3. Effect of Loading Amount
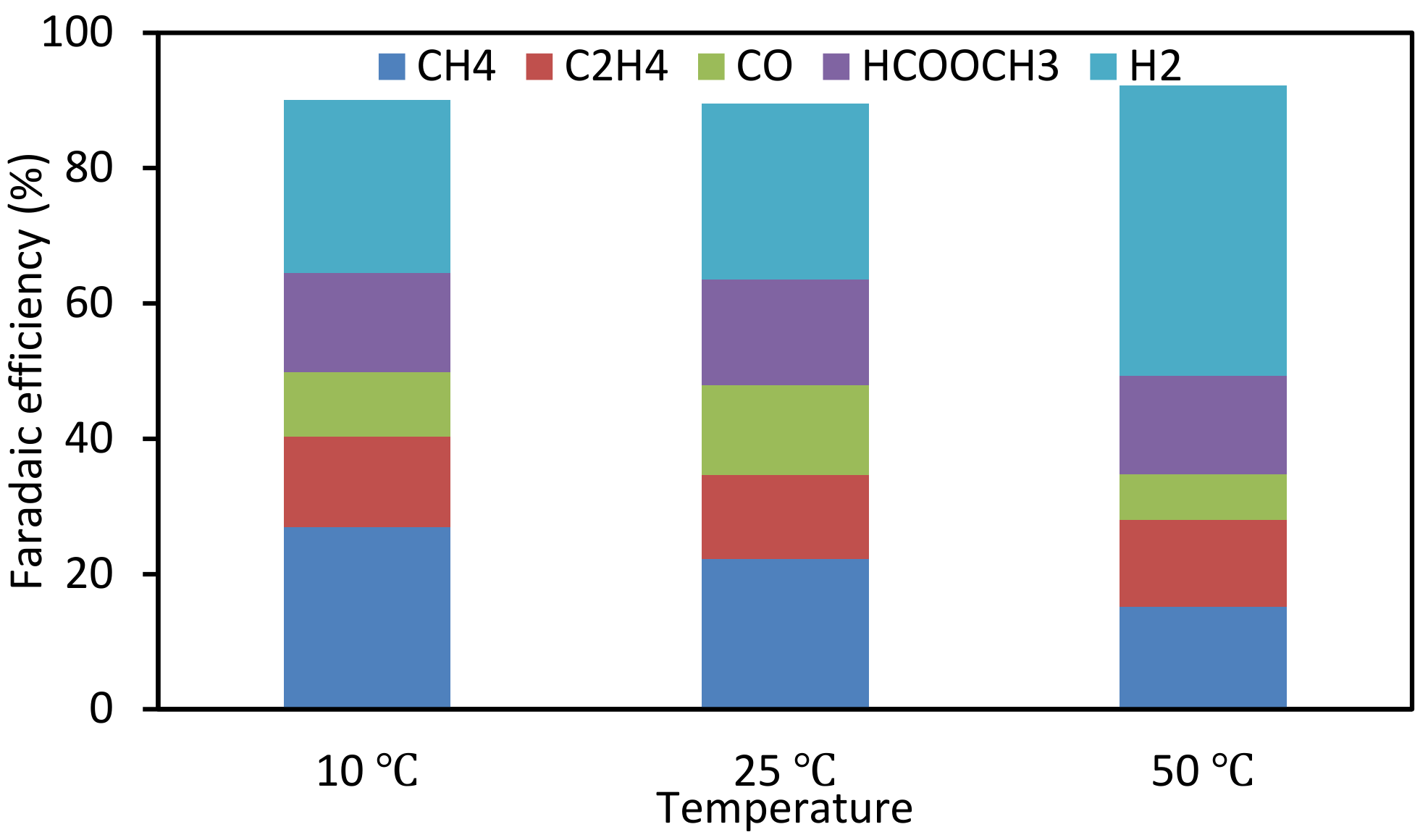
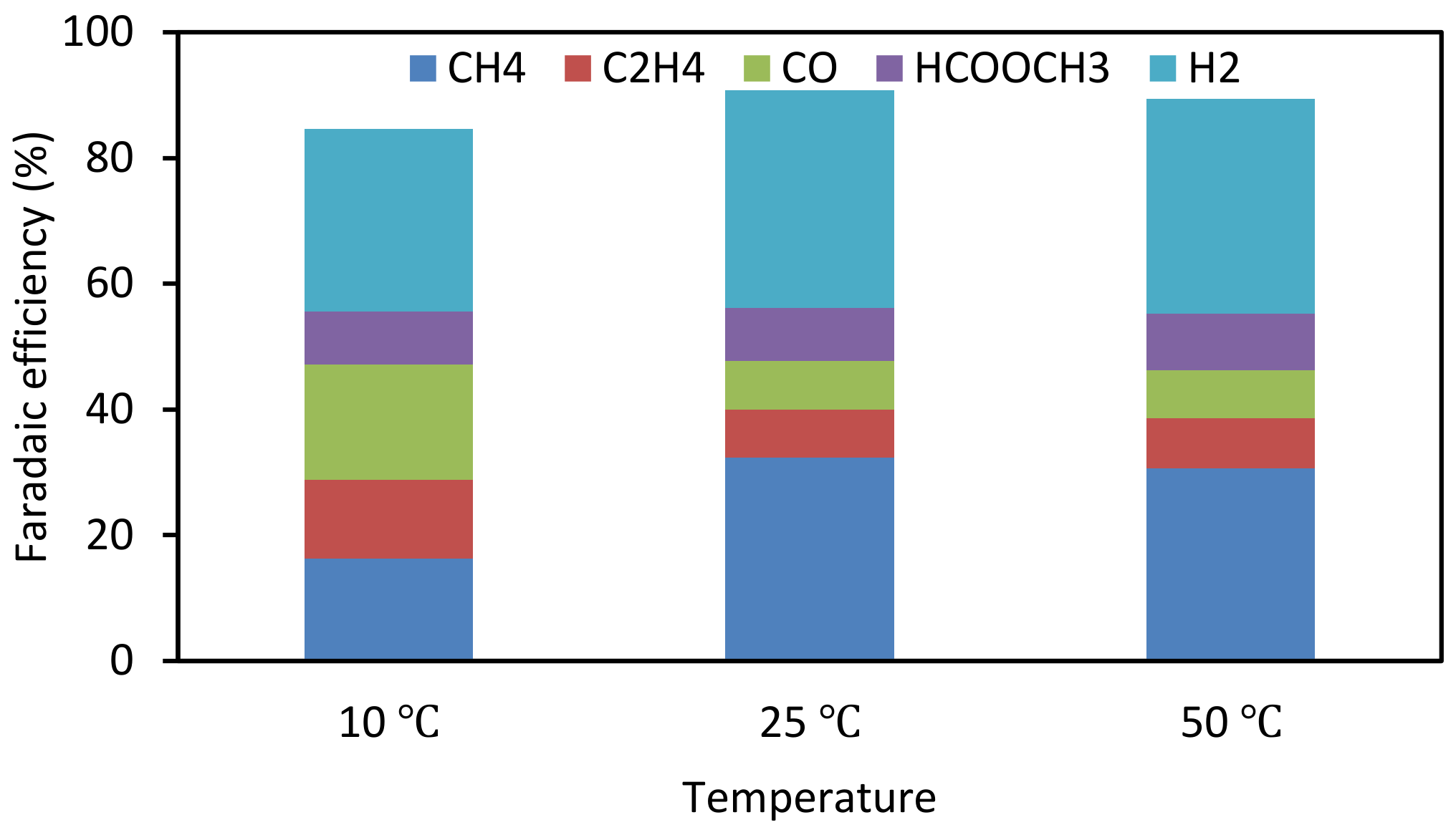
3.4. Effect of Catalyst Preparation Temperature
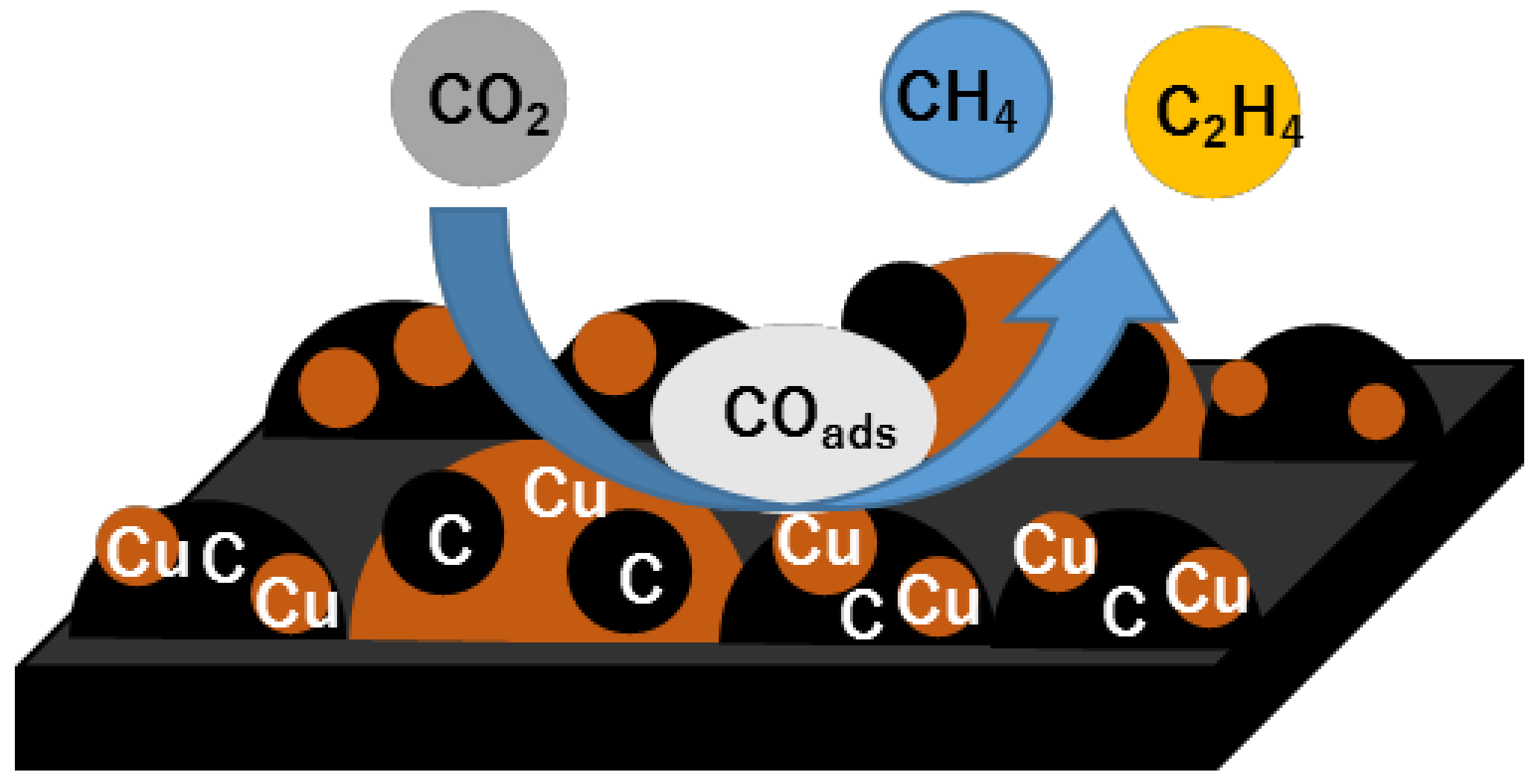
3.5. Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Santhanam, K.S.V.; Ahamed, N.N.N. Greenhouse gas sensors fabricated with new materials for climatic usage: A review. ChemEngineering 2018, 2, 38. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef]
- Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of CO2 to methane at the Cu electrode in methanol with sodium supporting salts and its comparison with other alkaline salts. Energy Fuel 2006, 20, 409–414. [Google Scholar] [CrossRef]
- Kaneco, S.; Ueno, Y.; Katsumata, H.; Suzuki, T.; Ohta, K. Photoelectrochemical reduction of CO2 at p-InP electrode in copper particle-suspended methanol. Chem. Eng. J. 2009, 148, 57–62. [Google Scholar] [CrossRef]
- Ohya, S.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Electrochemical reduction of CO2 in methanol with aid of CuO and Cu2O. Catal. Today 2009, 148, 329–334. [Google Scholar] [CrossRef]
- Murugananthan, M.; Kumaravel, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Electrochemical reduction of CO2 using Cu electrode in methanol/LiClO4 electrolyte. Int. J. Hydrog. Energy 2015, 40, 6740–6744. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photoelectrochemical reduction of CO2 in methanol with TiO2 photoanode and metal cathode. ECS Trans. 2017, 75, 31–37. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Tayyebi, E.; Hussain, J.; Abghoui, Y.; Skúlason, E. Trends of electrochemical CO2 reduction reaction on transition metal oxide catalysts. J. Phys. Chem. 2018, 122, 10078–10087. [Google Scholar] [CrossRef]
- Hochgesand, G. Rectisol and purisol. Ind. Eng. Chem. 1970, 62, 37–43. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Electrochemical behavior of the carbon black Vulcan XC-72R: Influence of the surface chemistry. Int. J. Hydrog. Energy 2018, 43, 7911–7922. [Google Scholar] [CrossRef]
- Baturina, O.A.; Lu, Q.; Padilla, M.A.; Xin, L.; Li, W.; Serov, A.; Artyushkova, K.; Atanassov, P.; Xu, F.; Epshteyn, A.; et al. CO2 electroreduction to hydrocarbons on carbon-supported Cu nanoparticles. ACS Catal. 2014, 4, 3682–3695. [Google Scholar] [CrossRef]
- Baturina, O.; Lu, Q.; Xu, F.; Purdy, A.; Dyatkin, B.; Sang, X.; Unocic, R.; Brintlinger, T.; Gogotsi, Y. Effect of nanostructured carbon support on copper electrocatalytic activity toward CO2 electroreduction to hydrocarbon fuels. Catal. Today 2017, 288, 2–10. [Google Scholar] [CrossRef]
- Shinagawa, T.; Larrazábal, G.O.; Martín, A.J.; Krumeich, F.; Pérez-Ramírez, J. Sulfur-modified copper catalysts for the electrochemical reduction of carbon dioxide to formate. ACS Catal. 2018, 8, 837–844. [Google Scholar] [CrossRef]
- Furukawa, H.; Matsuda, S.; Tanaka, S.; Shironita, S.; Umeda, M. CO2 electroreduction characteristics of Pt-Ru/C powder and Pt-Ru sputtered electrodes under acidic condition. Appl. Surf. Sci. 2018, 434, 681–686. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, D.; Yao, S.; Zhao, B.; Cai, F.; Lin, L.; Tang, P.; Zhai, P.; Wang, G.; Ma, D.; et al. Highly selective palladium-copper bimetallic electrocatalysts for the electrochemical reduction of CO2 to CO. Nano Energy 2016, 27, 35–43. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Geioushy, R.A.; Khaled, M.M.; Hakeem, A.S.; Alhooshani, K.; Basheer, C. High efficiency graphene/Cu2O electrode for the electrochemical reduction of carbon dioxide to ethanol. J. Electroanal. Chem. 2014, 716, 53–57. [Google Scholar]
- Cao, C.; Wen, Z. Cu nanoparticles decorating rGO nanohybrids as electrocatalyst toward CO2 reduction. J. CO2 Util. 2017, 22, 231–237. [Google Scholar] [CrossRef]
- Qin, T.; Qian, Y.; Zhang, F.; Lin, B.L. Cloride-derived copper electrode for efficient electrochemical reduction of CO2 to ethylene. Chin. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, S.; Wu, X.; Li, H.; Zhou, Y.; Zhu, C.; Yang, N.; Jiang, X.; Gao, J.; Bai, L.; et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas. Nat. Commun. 2017, 8, 1828. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Noble metal-free catalysts supported on carbon for CO2 electrochemical reduction. J. CO2 Util. 2017, 18, 41–52. [Google Scholar] [CrossRef]
- Montoya, J.H.; Peterson, A.A.; Nørskov, J.K. Insights into C-C coupling in CO2 electroreduction on copper electrodes. Chem. Cat. Chem. 2013, 5, 737–742. [Google Scholar]
- Boruban, C.; Esenturk, E.N. Activated carbon-supported CuO nanoparticles: A hybrid material for carbon dioxide adsorption. J. Nanopart. Res. 2018, 20, 59. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemoto, N.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes. ChemEngineering 2019, 3, 15. https://doi.org/10.3390/chemengineering3010015
Uemoto N, Furukawa M, Tateishi I, Katsumata H, Kaneco S. Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes. ChemEngineering. 2019; 3(1):15. https://doi.org/10.3390/chemengineering3010015
Chicago/Turabian StyleUemoto, Naoki, Mai Furukawa, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes" ChemEngineering 3, no. 1: 15. https://doi.org/10.3390/chemengineering3010015
APA StyleUemoto, N., Furukawa, M., Tateishi, I., Katsumata, H., & Kaneco, S. (2019). Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes. ChemEngineering, 3(1), 15. https://doi.org/10.3390/chemengineering3010015