Demonstration of the Use of 3D X-ray Tomography to Compare the Uniformity of Catalyst Coatings in Open-Cell Foams
Abstract
:1. Introduction
2. Material and Methods
2.1. Open Cell Foams
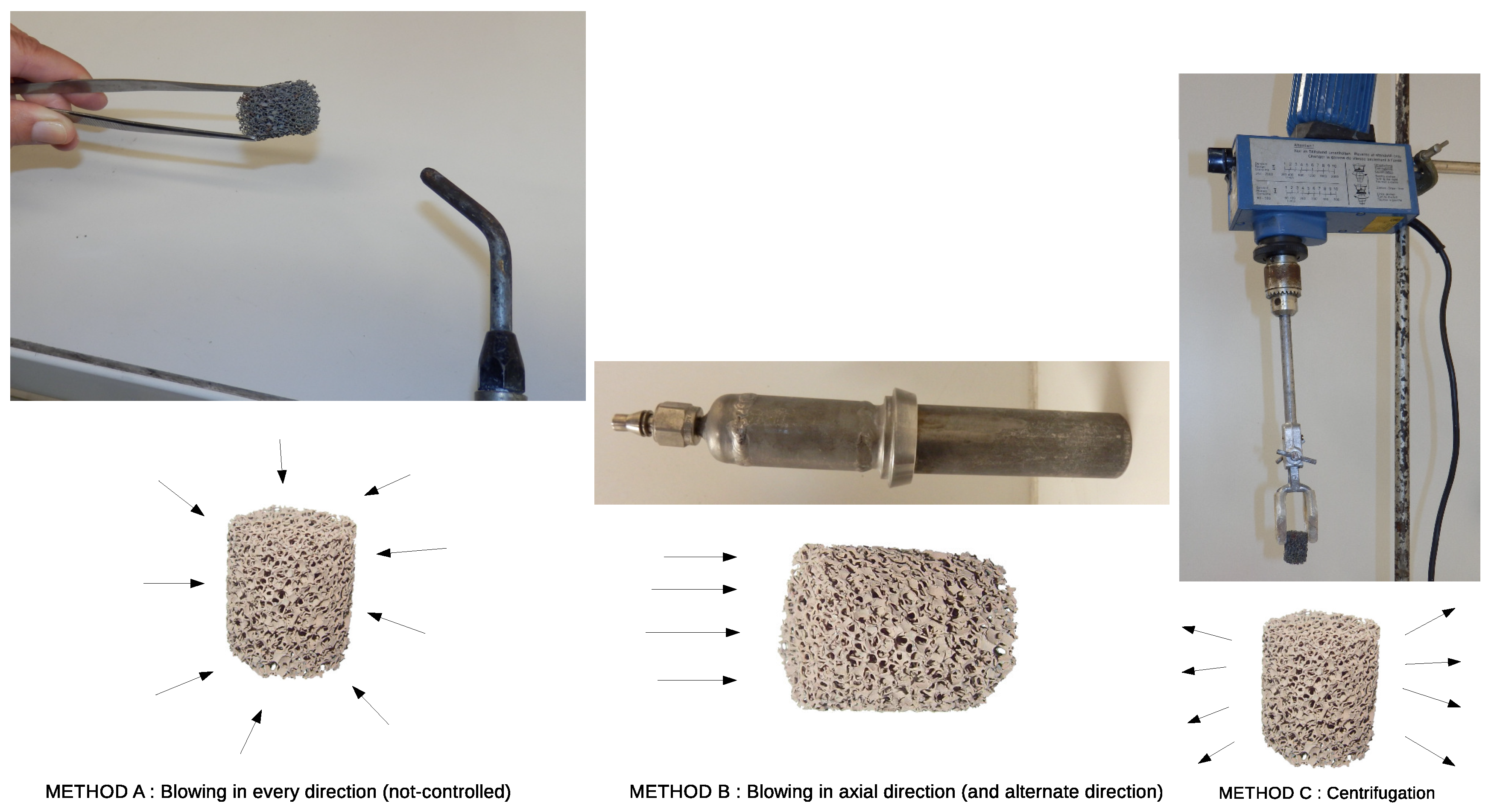
2.2. Coating Protocols
- The bismuth molybdenum oxide suspension is prepared by ball-milling the catalyst (15 g) in a mixture of water (12.5 g), Ludox AS40 (12.5 g) and PEG 200 (0.15 g) during 45 min at 300 rpm.
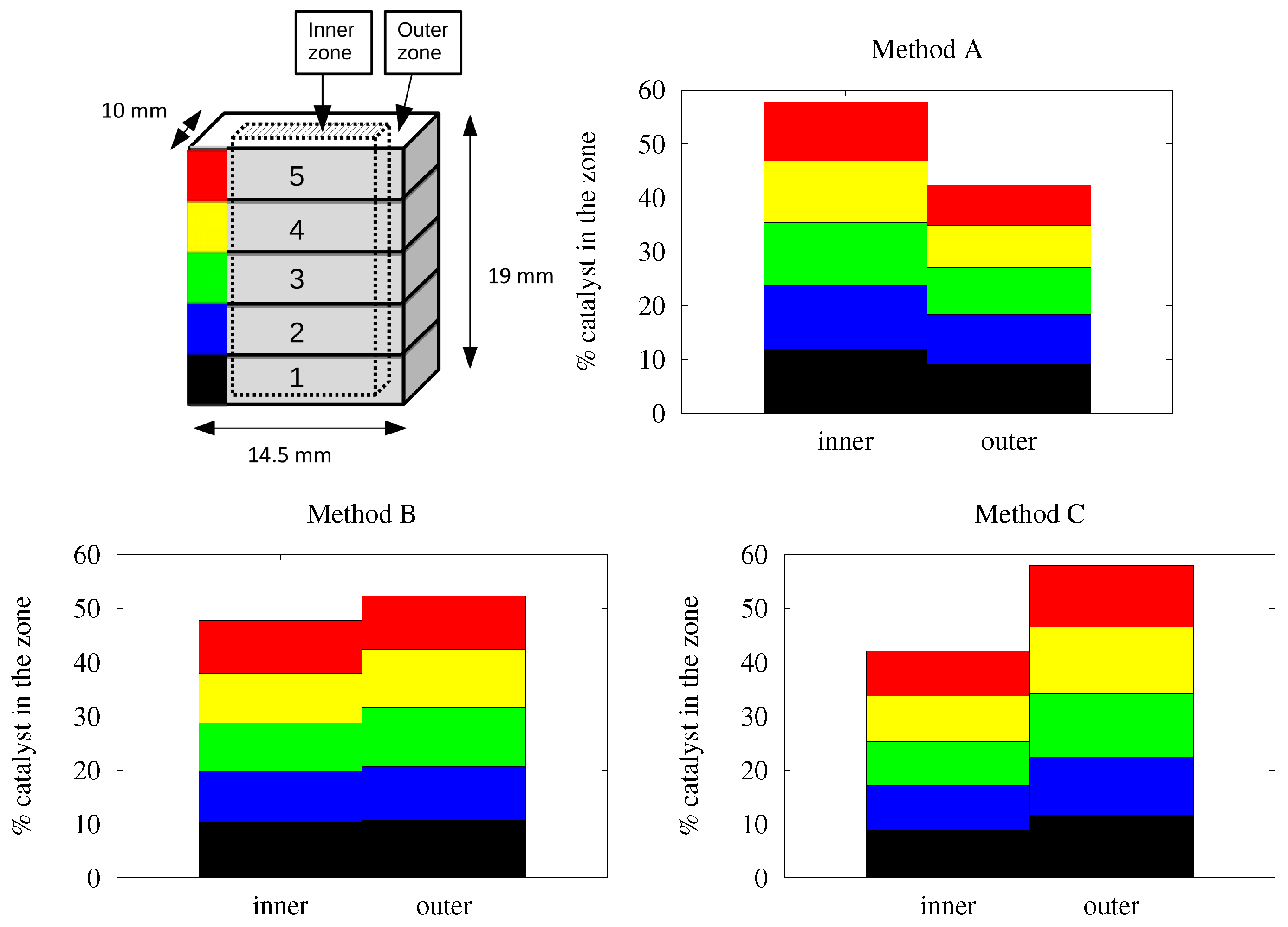
- blowing air manually in every direction (axially and radially) with a flow-rate of 30 to 45 L/min with the object at 6–8 cm from the air gun (diameter of the air gun = 3 mm). It has to be mentioned that this method is not reproducible at all and is totally dependent of the experimenter, despite it being the most widely used method (Method A).
- immobilizing the foam in a tube and blowing with air axially, with a flow-rate of 30 to 45 L/min in a cylinder of 2 cm diameter, the object being at 10 cm from the air gun (Method B).
- spin-drying/centrifuging at 400 to 800 rpm (Method C).
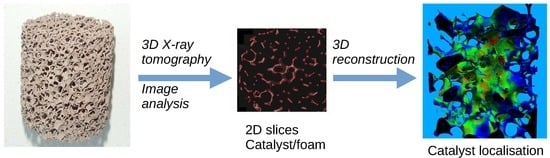
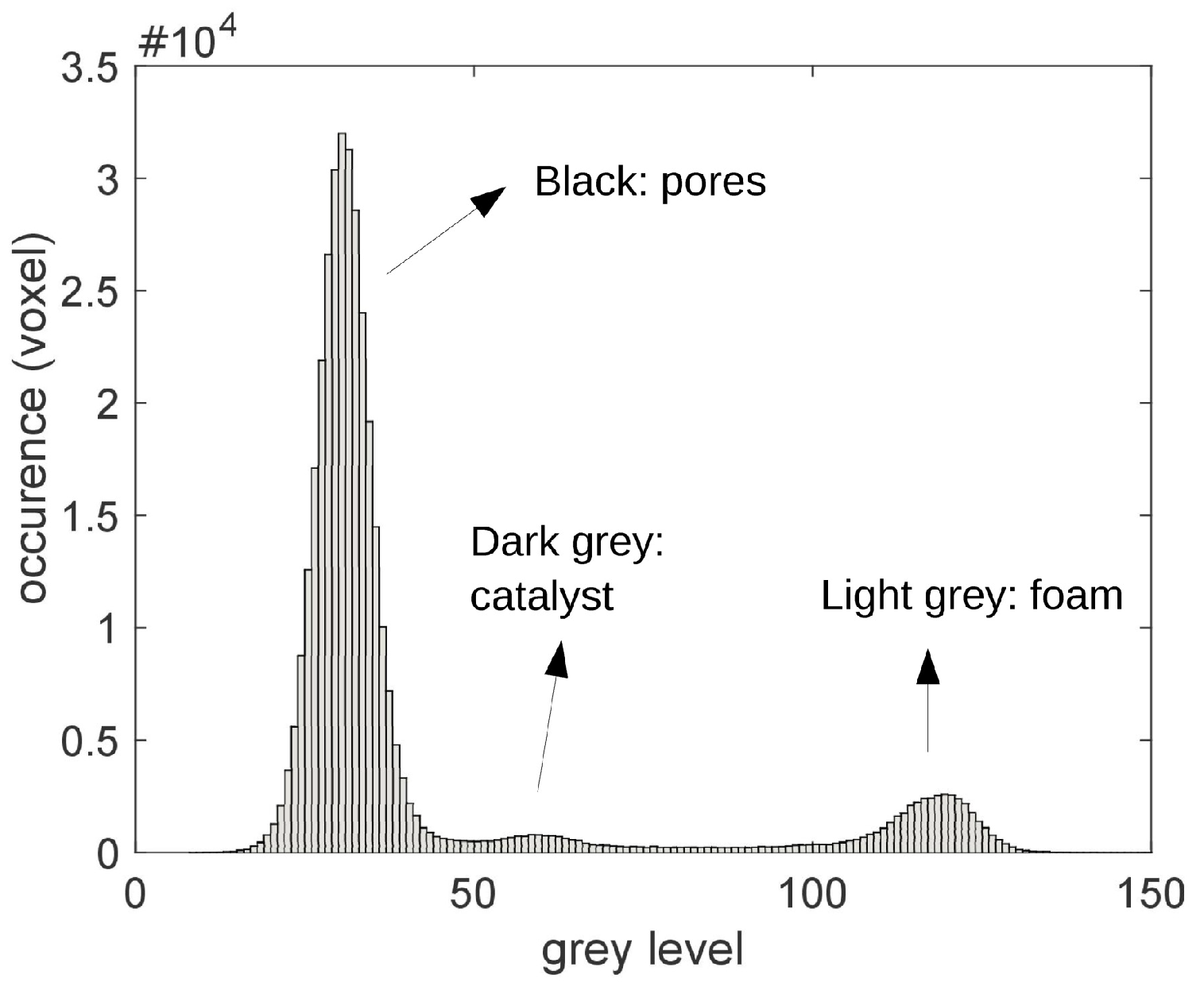
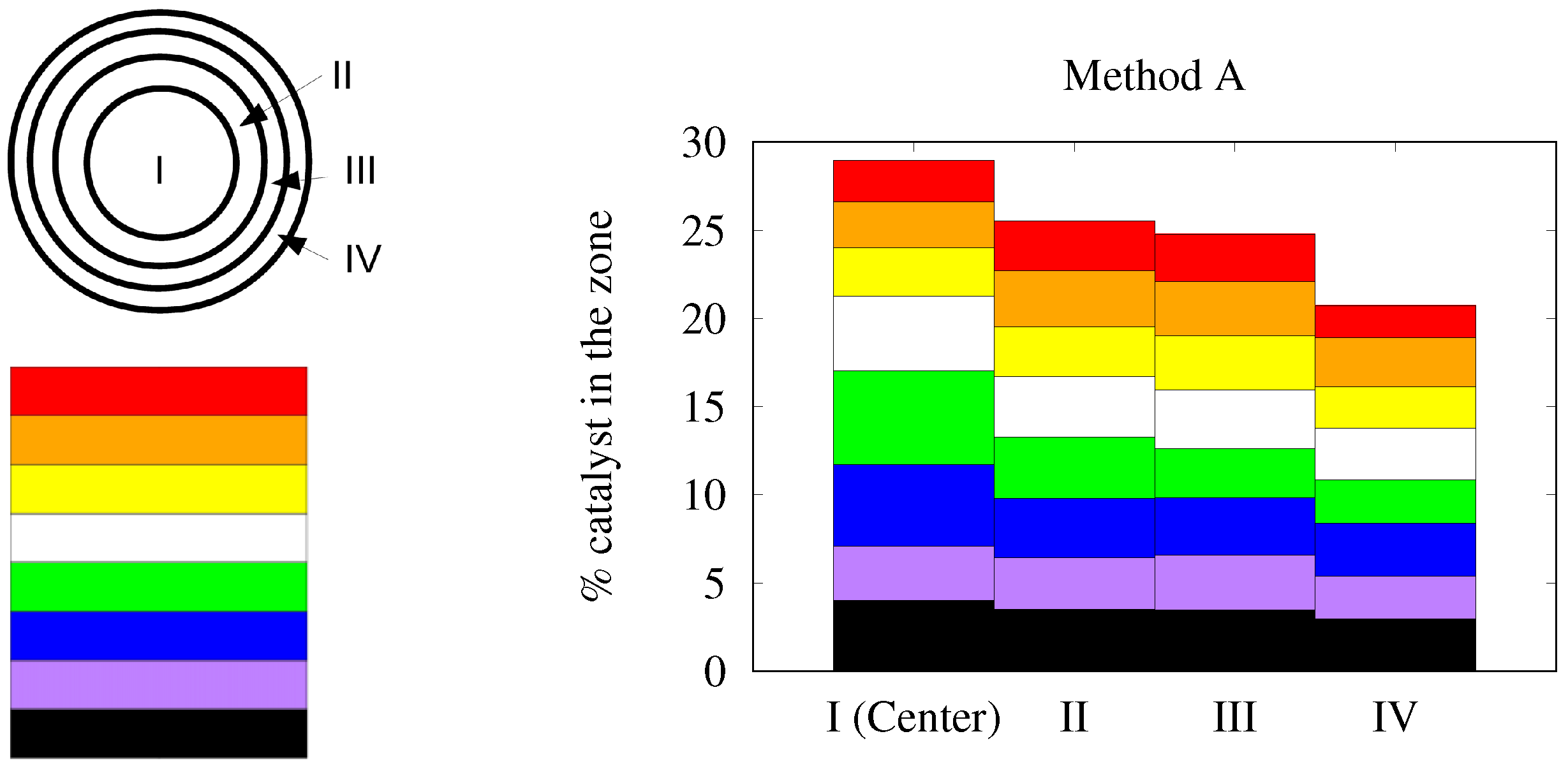
2.3. Characterization of the Catalyst Layer Distribution
3. Results and Discussion
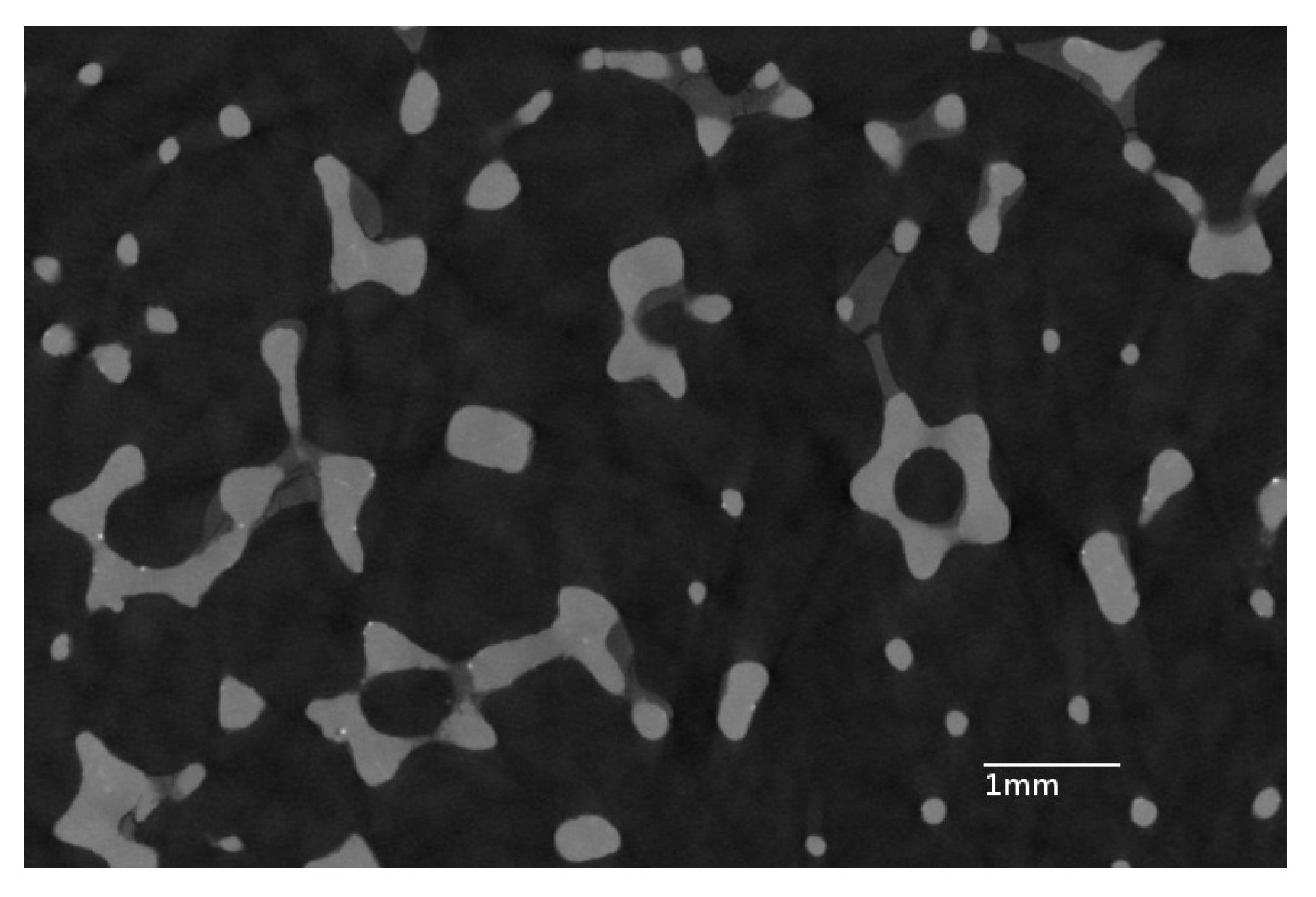
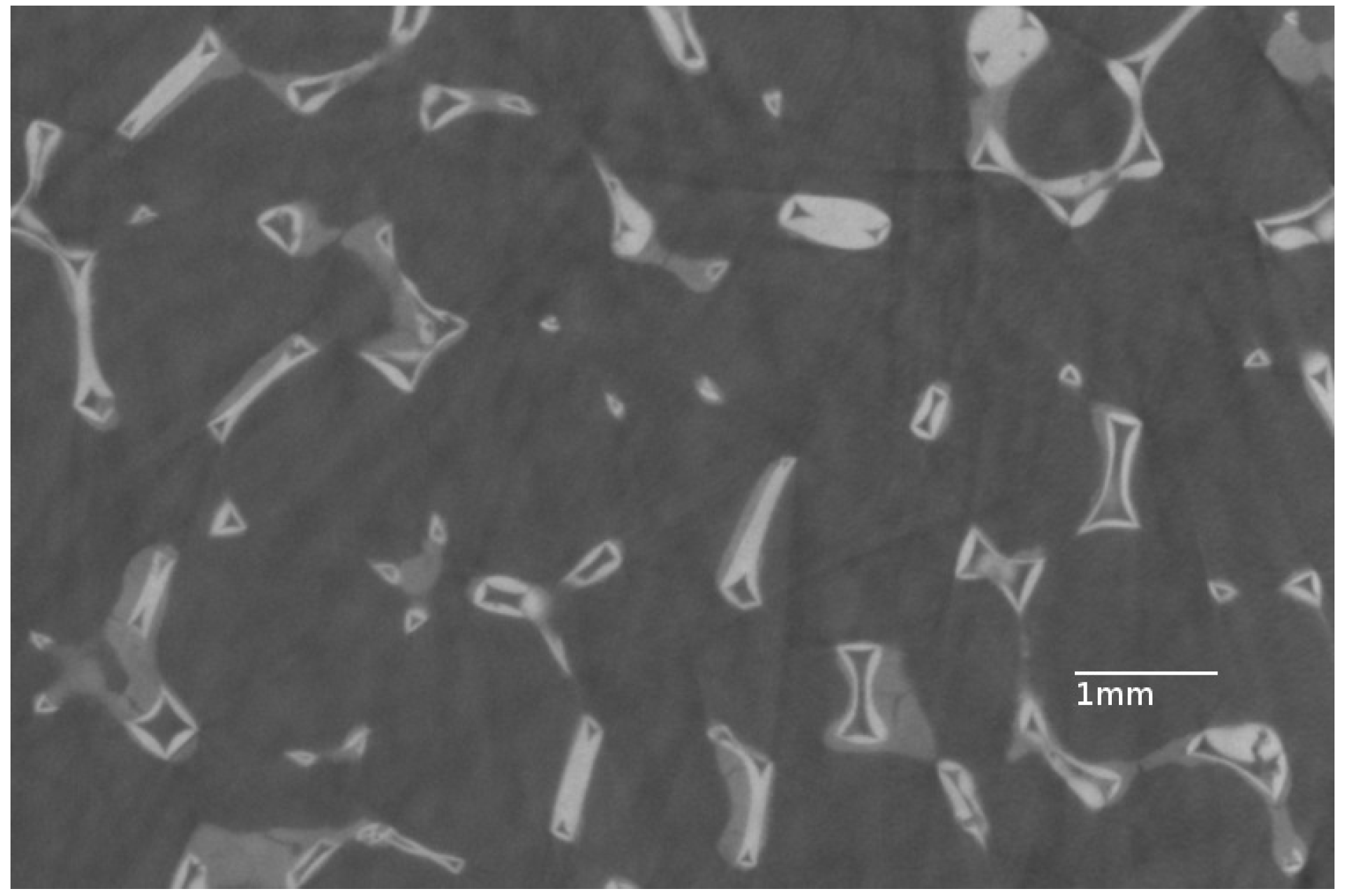
3.1. Properties of the Bare Foam
3.2. Preliminary Experiments—Alumina Layer on Aluminum Parallelepipedic Foam
3.3. Different Couples Catalyst-Foam in Cylindrical Geometry
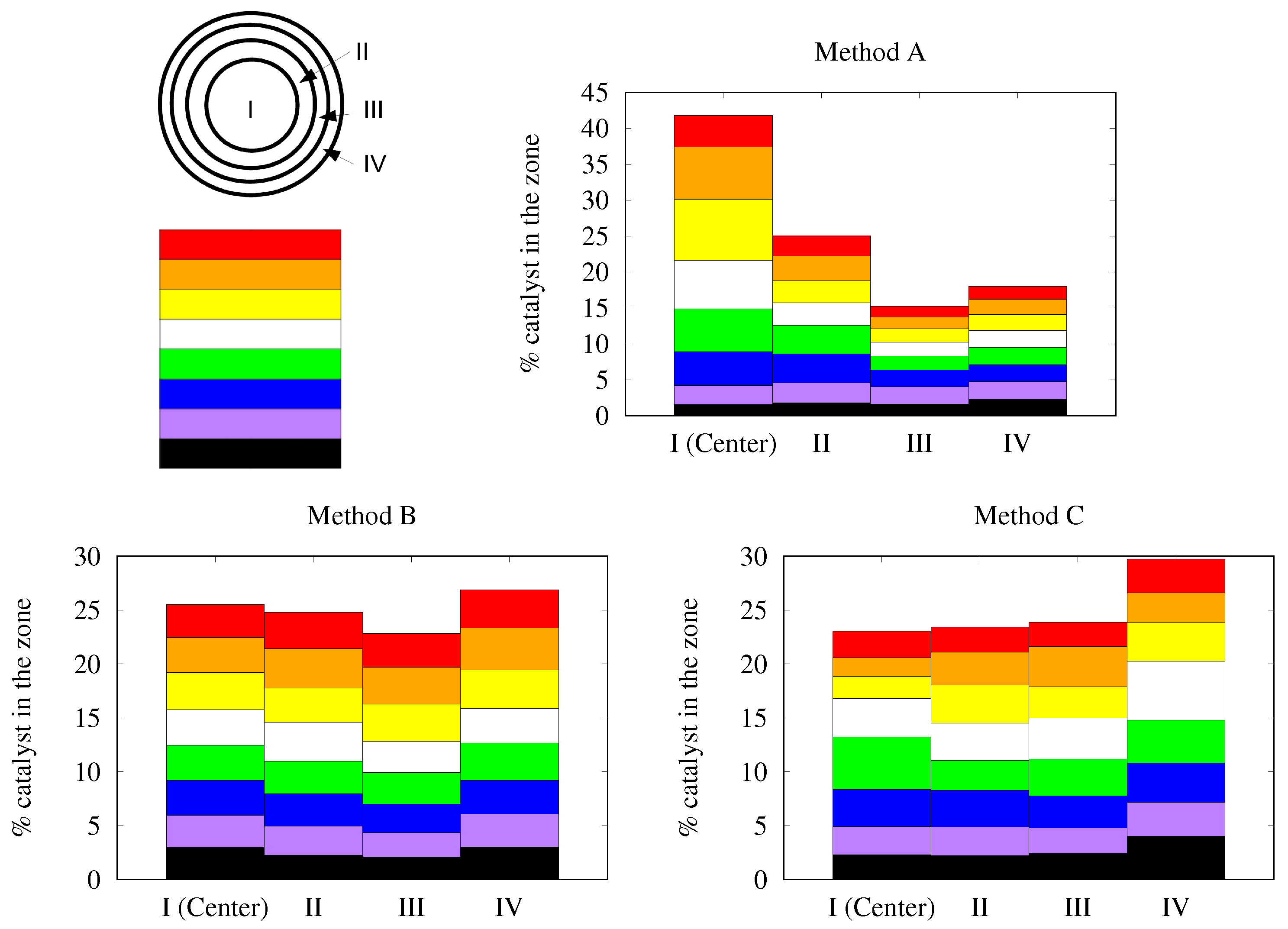
3.3.1. Alumina/FeCrAl
3.3.2. Alumina/SiC
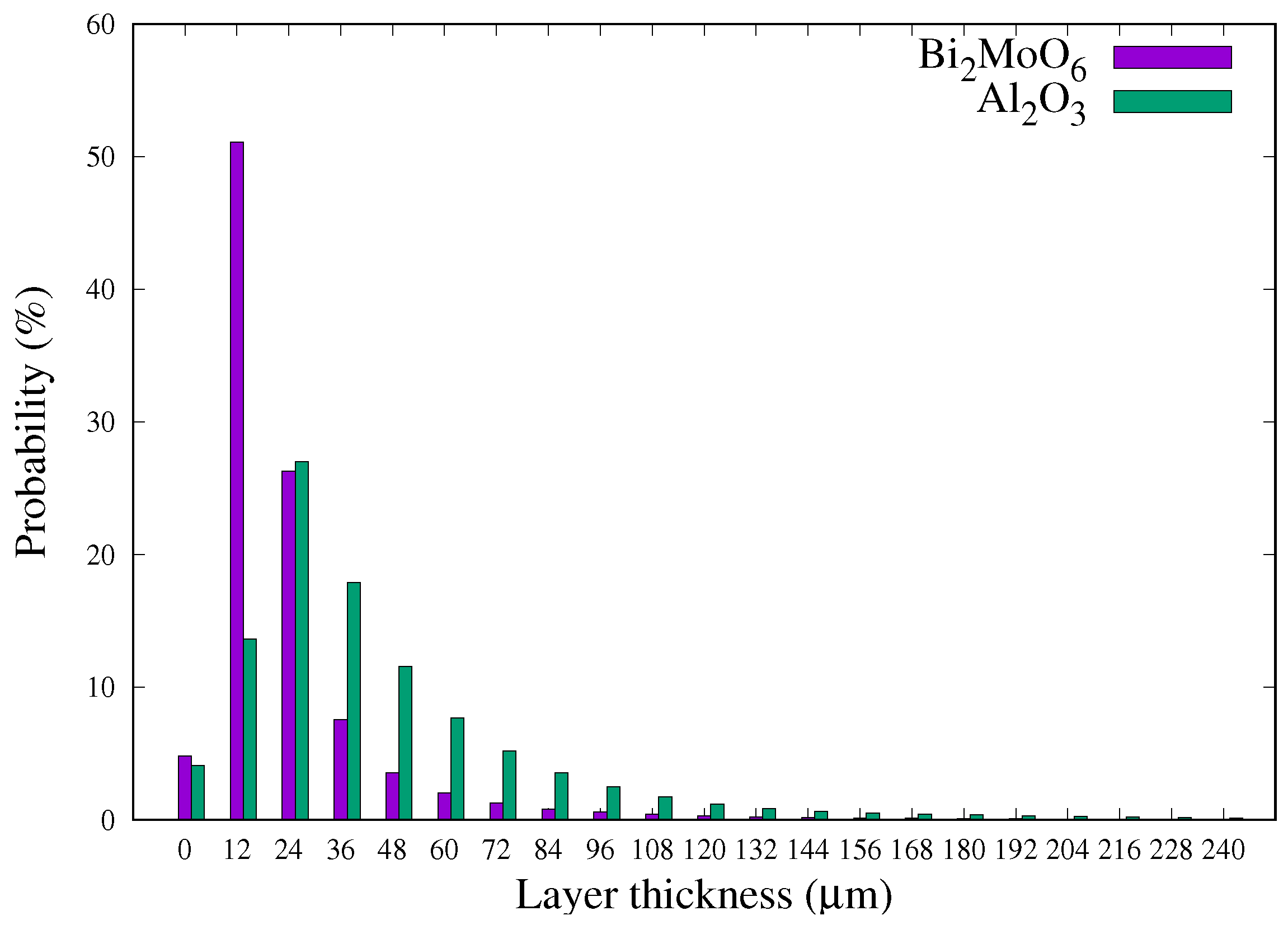
3.3.3. Bi2MoO6/FeCrAl
3.3.4. Perspective Use of Contrast Agent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez, M.J.F.; Gonzalez Bello, O.J.; Montes, M.; Tonetto, G.M.; Damiani, D.E. Pd/Al2O3-cordierite and Pd/Al2O3-Fecralloy monolithic catalysts for the hydrogenation of sunflower oil. Catal. Commun. 2009, 10, 1446–1449. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Fishwick, R.P.; Natividad, R.; Kulkarni, R.; McGuire, P.A.; Wood, J.; Winterbottom, J.M.; Stitt, E.H. Selective hydrogenation reactions: A comparative study of monolith CDC, stirred tank and trickle bed reactors. Catal. Today 2007, 128, 108–114. [Google Scholar] [CrossRef]
- Tourvieille, J.N.; Philippe, R.; de Bellefon, C. Milli-channel with metal foams under an applied gas–liquid periodic flow: External mass transfer performance and pressure drop. Chem. Eng. J. 2015, 267, 332–346. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Chai, R.; Zhao, G.; Cao, F.; Liu, Y.; Lu, Y. Metal-foam-structured Ni–Al2O3 catalysts: Wet chemical etching preparation and syngas methanation performance. Appl. Catal. A Gen. 2016, 510, 216–226. [Google Scholar] [CrossRef]
- Truong-Phuoc, L.; Truong-Huu, T.; Nguyen-Dinh, L.; Baaziz, W.; Romero, T.; Edouard, D.; Begin, D.; Janowska, I.; Pham-Huu, C. Silicon carbide foam decorated with carbon nanofibers as catalytic stirrer in liquid-phase hydrogenation reactions. Appl. Catal. A Gen. 2014, 469, 81–88. [Google Scholar] [CrossRef]
- Tschentscher, R.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Glucose Oxidation in Slurry Reactors and Rotating Foam Reactors. Ind. Eng. Chem. Res. 2012, 51, 1620–1634. [Google Scholar] [CrossRef]
- Benamara, N.; Assoua, D.; Jaffeux, L.; Vanoye, L.; Simescu-Lazar, F.; Zanota, M.L.; Bornette, F.; Meille, V.; Pitault, I. A New Concept of Stirred Multiphase Reactor Using a Stationary Catalytic Foam. Processes 2018, 6, 117. [Google Scholar] [CrossRef]
- Leon, M.A.; Tschentscher, R.; Nijhuis, T.A.; van der Schaaf, J.; Schouten, J.C. Rotating Foam Stirrer Reactor: Effect of Catalyst Coating Characteristics on Reactor Performance. Ind. Eng. Chem. Res. 2011, 50, 3184–3193. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/gamma-Al2O3 deposition on metallic foams. Appl. Catal. B 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S.; Venvik, H.J.; Myrstad, R. Washcoating and chemical testing of a commercial Cu/ZnO/Al2O3 catalyst for the methanol synthesis over copper open-cell foams. Appl. Catal. A Gen. 2014, 481, 96–103. [Google Scholar] [CrossRef]
- Simescu-Lazar, F.; Chaieb, T.; Pallier, S.; Veyre, L.; Philippe, R.; Meille, V. Direct coating of carbon-supported catalysts on monoliths and foams—Singular behaviour of Pd/MWCNT. Appl. Catal. A Gen. 2015, 508, 45–51. [Google Scholar] [CrossRef]
- Bianchi, E.; Heidig, T.; Visconti, C.G.; Groppi, G.; Freund, H.; Tronconi, E. An appraisal of the heat transfer properties of metallic open-cell foams for strongly exo-/endo-thermic catalytic processes in tubular reactors. Chem. Eng. J. 2012, 198–199, 512–528. [Google Scholar] [CrossRef]
- Bianchi, E.; Heidig, T.; Visconti, C.G.; Groppi, G.; Freund, H.; Tronconi, E. Heat transfer properties of metal foam supports for structured catalysts: Wall heat transfer coefficient. Catal. Today 2013, 216, 121–134. [Google Scholar] [CrossRef]
- Klumpp, M.; Inayat, A.; Schwerdtfeger, J.; Körner, C.; Singer, R.F.; Freund, H.; Schwieger, W. Periodic open cellular structures with ideal cubic cell geometry: Effect of porosity and cell orientation on pressure drop behavior. Chem. Eng. J. 2014, 242, 364–378. [Google Scholar] [CrossRef]
- Adam, M.; Kocanis, S.; Fey, T.; Wilhelm, M.; Grathwohl, G. Hierarchically Ordered Foams Derived from Polysiloxanes with Catalytically Active Coatings. J. Eur. Ceram. Soc. 2014, 34, 1715–1725. [Google Scholar] [CrossRef]
- Leveque, J.; Philippe, R.; Zanota, M.L.; Meille, V.; Sarrazin, F.; Baussaron, L.; de Bellefon, C. Hydrodynamics and mass transfer in a tubular reactor containing foam packings for intensification of G-L-S catalytic reactions in co-current up-flow configuration. Chem. Eng. Res. Des. 2016, 109, 686–697. [Google Scholar] [CrossRef]
- Zalucky, J.; Claußnitzer, T.; Schubert, M.; Lange, R.; Hampel, U. Pulse flow in solid foam packed reactors: Analysis of morphology and key characteristics. Chem. Eng. J. 2017, 307, 339–352. [Google Scholar] [CrossRef]
- Novak, V.; Koci, P.; Gregor, T.; Choi, J.; Stepanek, F.; Marek, M. Effect of cavities and cracks on diffusivity in coated catalyst layer. Catal. Today 2013, 216, 142–149. [Google Scholar] [CrossRef]
- Lali, F.; Böttcher, G.; Schöneich, P.; Haase, S.; Hempel, S.; Lange, R. Preparation and characterization of Pd/Al2O3 catalysts on aluminum foam supports for multiphase hydrogenation reactions in rotating foam reactors. Chem. Eng. Res. Des. 2015, 94, 365–374. [Google Scholar] [CrossRef]
- Lali, F.; Gärtner, S.; Haase, S.; Lange, R. Preparation Method for Ruthenium Catalysts Supported by Carbon-Coated Aluminum Foams. Chem. Eng. Technol. 2015, 38, 1353–1361. [Google Scholar] [CrossRef]
- Ou, X.; Xu, S.; Warnett, J.M.; Holmes, S.M.; Zaheer, A.; Garforth, A.A.; Williams, M.A.; Jiao, Y.; Fan, X. Creating Hierarchies Promptly: Microwave-Accelerated Synthesis of ZSM-5 Zeolites on Macrocellular Silicon Carbide (SiC) Foams. Chem. Eng. J. 2017, 312, 1–9. [Google Scholar] [CrossRef]
- Eslamian, M. Inorganic and Organic Solution-Processed Thin Film Devices. Nano-Micro Lett. 2016, 9, 3. [Google Scholar] [CrossRef]
- Meille, V.; Pallier, S.; Santa Cruz Bustamante, G.V.; Roumanie, M.; Reymond, J. Deposition of γ-Al2O3 layers on structured supports for the design of new catalytic reactors. Appl. Catal. A 2005, 286, 232–238. [Google Scholar] [CrossRef]
- Meille, V.; Pallier, S.; Rodriguez, P. Reproducibility in the preparation of alumina slurries for washcoat application—Role of temperature and particle size distribution. Colloids Surf. A 2009, 336, 104–109. [Google Scholar] [CrossRef]
- Della Torre, A.; Montenegro, G.; Tabor, G.R.; Wears, M.L. CFD Characterization of Flow Regimes inside Open Cell Foam Substrates. Int. J. Heat Fluid Flow 2014, 50, 72–82. [Google Scholar] [CrossRef]

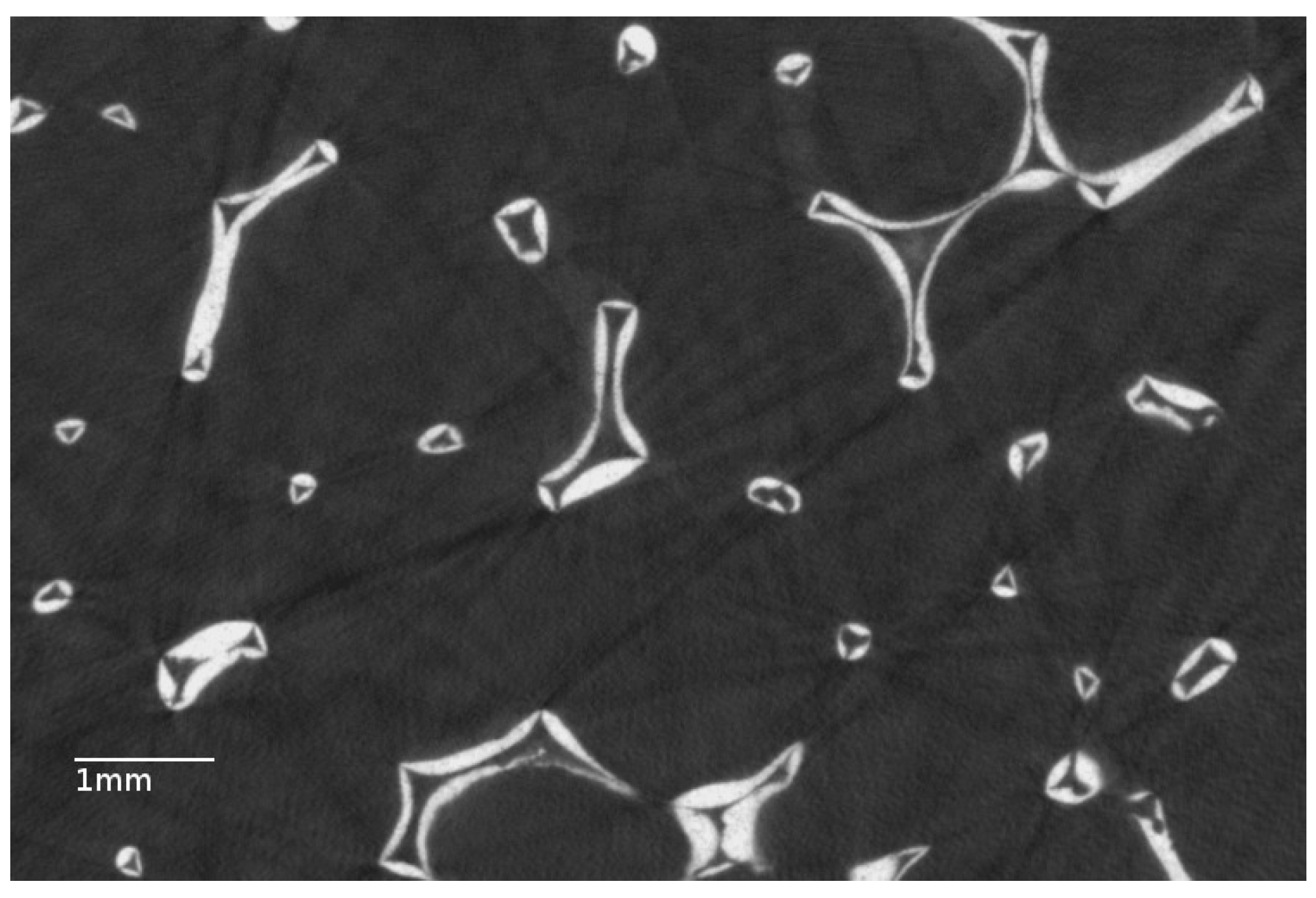
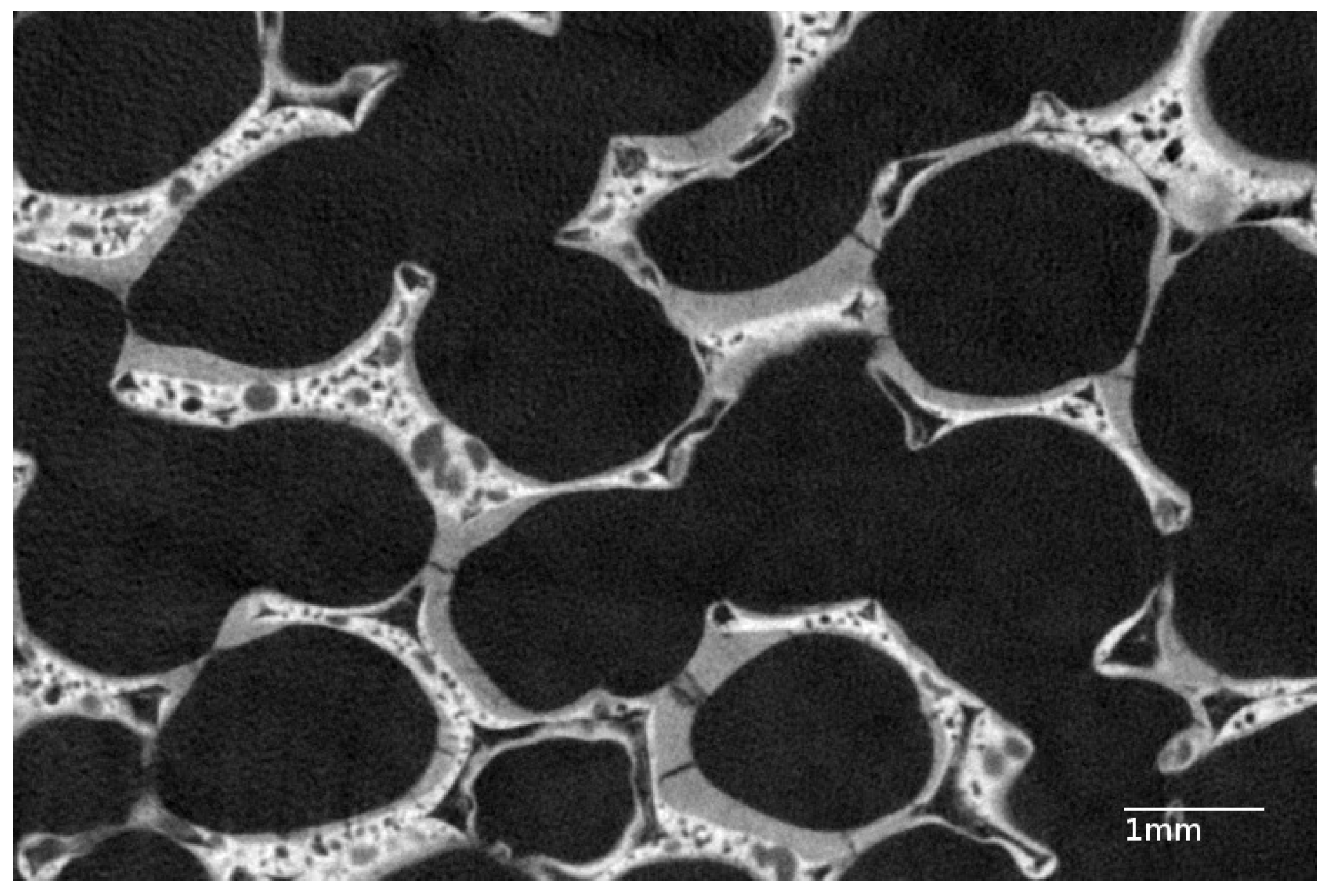
| Material | Void Fraction % | Specific Surface Area m/m | Cell Size m |
|---|---|---|---|
| Al−40PPI * | 90 | 1500 | 1400 |
| FeCrAl−40PPI | 91 | 1460 | 1350 |
| SiC−50PPI | 89 | 2270 | 1100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanota, M.-L.; Pallier, S.; Dousse, A.; Lachambre, J.; Meille, V. Demonstration of the Use of 3D X-ray Tomography to Compare the Uniformity of Catalyst Coatings in Open-Cell Foams. ChemEngineering 2018, 2, 52. https://doi.org/10.3390/chemengineering2040052
Zanota M-L, Pallier S, Dousse A, Lachambre J, Meille V. Demonstration of the Use of 3D X-ray Tomography to Compare the Uniformity of Catalyst Coatings in Open-Cell Foams. ChemEngineering. 2018; 2(4):52. https://doi.org/10.3390/chemengineering2040052
Chicago/Turabian StyleZanota, Marie-Line, Stéphanie Pallier, Anaïs Dousse, Joël Lachambre, and Valérie Meille. 2018. "Demonstration of the Use of 3D X-ray Tomography to Compare the Uniformity of Catalyst Coatings in Open-Cell Foams" ChemEngineering 2, no. 4: 52. https://doi.org/10.3390/chemengineering2040052
APA StyleZanota, M.-L., Pallier, S., Dousse, A., Lachambre, J., & Meille, V. (2018). Demonstration of the Use of 3D X-ray Tomography to Compare the Uniformity of Catalyst Coatings in Open-Cell Foams. ChemEngineering, 2(4), 52. https://doi.org/10.3390/chemengineering2040052