Recent Advances in Supported Metal Catalysts for Syngas Production from Methane
Abstract
1. Introduction
2. Syngas Production from Methane
2.1. Dry Reforming of Methane (DRM)
2.1.1. Effects of the Support on the Catalytic Activity in DRM
2.1.2. Bimetallic Catalysts
2.2. Steam Reforming of Methane (SRM)
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Abatzoglou, N.; Fauteux-Lefebvre, C. Review of catalytic syngas production through steam or dry reforming and partial oxidation of studied liquid compounds. Wiley Interdiscip. Rev. Energy Environ. 2016, 2, 169–187. [Google Scholar] [CrossRef]
- Wilhelm, D.J.; Simbeck, D.R.; Karp, A.D.; Dickenson, R.L. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001, 71, 139–148. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Yusuf, R.O.; Noor, Z.Z.; Abba, A.H.; Hassan, M.A.A.; Din, M.F.M. Methane emission by sectors: A comprehensive review of emission sources and mitigation methods. Renew. Sustain. Energy Rev. 2012, 16, 5059–5070. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Aloise, A.; Antonucci, P.L.; Giordano, G.; Nagy, J.B. Effect of support surface on methane dry-reforming catalyst preparation. Catal. Today 2013, 218, 18–29. [Google Scholar] [CrossRef]
- Mo, W.; Ma, F.; Liu, Y.; Liu, J.; Zhong, M.; Nulahong, A. Preparation of porous Al2O3 by template method and its application in Ni-based catalyst for CH4/CO2 reforming to produce syngas. Int. J. Hydrogen Energy 2015, 40, 16147–16158. [Google Scholar] [CrossRef]
- Bang, S.; Hong, E.; Baek, S.W.; Shin, C.-H. Effect of acidity on Ni catalysts supported on P-modified Al2O3 for dry reforming of methane. Catal. Today 2018, 303, 100–105. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Zhang, R.-J.; Xia, G.-F.; Li, M.-F.; Wu, Y.; Nie, H.; Li, D.-D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M. Syngas production via dry reforming of methane over Ni-based nanocatalyst over various supports of clinoptilolite, ceria and alumina. J. Nat. Gas Sci. Eng. 2015, 23, 16–25. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis gas production over highly active and stable nanostructured NiMgOAl2O3 catalysts in dry reforming of methane: Effects of Ni contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Titus, J.; Goepel, M.; Schunk, S.A.; Wilde, N.; Gläser, R. The role of acid/base properties in Ni/MgO-ZrO2-based catalysts for dry reforming of methane. Catal. Commun. 2017, 100, 76–80. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni-Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Maleki, M.; Rahemi, N. Sequential impregnation vs. sol-gel synthesized Ni/Al2O3-CeO2 nanocatalyst for dry reforming of methane: Effect of synthesis method and support promotion. Mol. Catal. 2017, 431, 39–48. [Google Scholar] [CrossRef]
- Han, J.; Zhan, Y.; Street, J.; To, F.; Yu, F. Natural gas reforming of carbon dioxide for syngas over Ni-Ce-Al catalysts. Int. J. Hydrogen Energy 2017, 42, 18364–18374. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno; Rashid, U.; Zainal, Z. CeO2-SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Appl. Catal. A Gen. 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. CeO2 Promoted Ni-MgO-Al2O3 nanocatalysts for carbon dioxide reforming of methane. J. CO2 Util. 2018, 24, 128–138. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Calvino, J.J.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Arias, D.C.; Pérez-Omil, J.A.; Hernández-Garrido, J.C.; González-Leal, J.M.; Cauqui, M.A.; Yeste, M.P. Highly stable ceria-zirconia-yttria supported Ni catalysts for syngas production by CO2 reforming of methane. Appl. Surf. Sci. 2017, 426, 864–873. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, Y.; Han, J.; Li, Y.; Yu, F. Highly active and stable Ni-based bimodal pore catalyst for dry reforming of methane. Appl. Catal. A Gen. 2015, 491, 116–126. [Google Scholar] [CrossRef]
- Li, B.; Su, W.; Lin, X.; Wang, X. Catalytic performance and characterization of Neodymium-containing mesoporous silica supported nickel catalysts for methane reforming to syngas. Int. J. Hydrogen Energy 2017, 42, 12197–12209. [Google Scholar] [CrossRef]
- Yabe, T.; Mitarai, K.; Oshima, K.; Ogo, S.; Sekine, Y. Low-temperature dry reforming of methane to produce syngas in an electric field over La-doped Ni/ZrO2 catalysts. Fuel Process. Technol. 2017, 158, 96–103. [Google Scholar] [CrossRef]
- Movasati, A.; Alavi, S.M.; Mazloom, G. CO2 reforming of methane over Ni/ZnAl2O4 catalysts: Influence of Ce addition on activity and stability. Int. J. Hydrogen Energy 2017, 42, 16436–16448. [Google Scholar] [CrossRef]
- Mei, D.H.; Liu, S.Y.; Tu, X. CO2 reforming with methane for syngas production using a dielectric barrier discharge plasma coupled with Ni/γ-Al2O3 catalysts: Process optimization through response surface methodology. J. CO2 Util. 2017, 21, 314–326. [Google Scholar] [CrossRef]
- Meric, G.G.; Arbag, H.; Degirmenci, L. Coke minimization via SiC formation in dry reforming of methane conducted in the presence of Ni-based core-shell microsphere catalysts. Int. J. Hydrogen Energy 2017, 42, 16579–16588. [Google Scholar] [CrossRef]
- Benrabaa, R.; Barama, A.; Boukhlouf, H.; Guerrero-Caballero, J.; Rubbens, A.; Bordes-Richard, E.; Löfberg, A.; Vannier, R.-N. Physico-chemical properties and syngas production via dry reforming of methane over NiAl2O4 catalyst. Int. J. Hydrogen Energy 2017, 42, 12989–12996. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Nguyen-Huy, C.; Setiabudi, H.D.; Abidin, S.Z.; Truong, Q.D.; Vo, D.-V.N. Syngas production from methane dry reforming over Ni/SBA-15 catalyst: Effect of operating parameters. Int. J. Hydrogen Energy 2017, 42, 11283–11294. [Google Scholar] [CrossRef]
- Albarazi, A.; Beaunier, P.; da Costa, P. Hydrogen and syngas production by methane dry reforming on SBA-15 supported nickel catalysts: On the effect of promotion by Ce0.75Zr0.25O2 mixed oxide. Int. J. Hydrogen Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ayodele, B.V.; Cheng, C.K.; Khan, M.R. Artificial neural network modeling of hydrogen-rich syngas production from methane dry reforming over novel Ni/CaFe2O4 catalysts. Int. J. Hydrogen Energy 2016, 41, 11119–11130. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Jiao, Y. Dry reforming of methane towards CO-rich hydrogen production over robust supported Ni catalyst on hierarchically structured monoclinic zirconia nanosheets. Int. J. Hydrogen Energy 2016, 41, 17907–17921. [Google Scholar] [CrossRef]
- Estephane, J.; Aouad, S.; Hany, S.; el Khoury, B.; Gennequin, C.; el Zakhem, H.; el Nakat, J.; Aboukaïs, A.; Aad, E.A. CO2 reforming of methane over Ni-Co/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrogen Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Albarazi, A.; Gálvez, M.E.; da Costa, P. Synthesis strategies of ceria-zirconia doped Ni/SBA-15 catalysts for methane dry reforming. Catal. Commun. 2015, 59, 108–112. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Rahmani, F.; Karimipour, S. Syngas production via dry reforming of CH4 over Co- and Cu-promoted Ni/Al2O3-ZrO2 nanocatalysts synthesized via sequential impregnation and sol-gel methods. J. Nat. Gas Sci. Eng. 2014, 21, 993–1004. [Google Scholar] [CrossRef]
- Mahoney, E.G.; Pusel, J.M.; Stagg-Williams, S.M.; Faraji, S. The effects of Pt addition to supported Ni catalysts on dry (CO2) reforming of methane to syngas. J. CO2 Util. 2014, 6, 40–44. [Google Scholar] [CrossRef]
- Abasaeed, A.E.; Al-Fatesh, A.S.; Naeem, M.A.; Ibrahim, A.A.; Fakeeha, A.H. Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2015, 40, 6818–6826. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst: Effects of reactants partial pressure. J. Nat. Gas Sci. Eng. 2015, 27, 1016–1023. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 198–207. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Hossain, S.S.; Lam, S.S.; Osazuwa, O.U.; Khan, M.R.; Cheng, C.K. Syngas production from CO2 reforming of methane over neodymium sesquioxide supported cobalt catalyst. J. Nat. Gas Sci. Eng. 2016, 34, 873–885. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Lam, S.S.; Cheng, C.K. Production of CO-rich hydrogen from methane dry reforming over lanthania-supported cobalt catalyst: Kinetic and mechanistic studies. Int. J. Hydrogen Energy 2016, 41, 4603–4615. [Google Scholar] [CrossRef]
- Mirzaei, F.; Rezaei, M.; Meshkani, F.; Fattah, Z. Carbon dioxide reforming of methane for syngas production over Co-MgO mixed oxide nanocatalysts. J. Ind. Eng. Chem. 2015, 21, 662–667. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, P.; Xu, Y.; Qu, J. Characterization of Ca-promoted Co/AC catalyst for CO2-CH4 reforming to syngas production. J. CO2 Util. 2017, 18, 326–334. [Google Scholar] [CrossRef]
- José-Alonso, D.S.; Illán-Gómez, M.J.; Román-Martínez, M.C. Low metal content Co and Ni alumina supported catalysts for the CO2 reforming of methane. Int. J. Hydrogen Energy 2013, 38, 2230–2239. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhang, J.; Zhao, Y.; Li, H.; Li, H.X.; Wu, K.; Xu, G.Q.; Chen, W. Tuning the metal-support interaction in catalysts for highly efficient methane dry reforming reaction. Appl. Catal. B Environ. 2016, 180, 511–520. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Lu, M.; Lin, X.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ru catalysts supported on Mg-Al oxides: A highly dispersed and stable Ru/Mg(Al)O catalyst. Appl. Catal. B Environ. 2017, 200, 566–577. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Mancino, G. Effect of phosphorous addition to Rh-supported catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 23587–23598. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.; Kumar, S.; Jilani, S. Comparative modeling study of catalytic membrane reactor configurations for syngas production by CO2 reforming of methane. J. CO2 Util. 2017, 20, 336–346. [Google Scholar] [CrossRef]
- De Vasconcelos, B.R.; Zhao, L.; Sharrock, P.; Nzihou, A.; Minh, D.P. Catalytic transformation of carbon dioxide and methane into syngas over ruthenium and platinum supported hydroxyapatites. Appl. Surf. Sci. 2016, 390, 141–156. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Hydrogen Production from Oxygenated Hydrocarbons: Review of Catalyst Development, Reaction Mechanism and Reactor Modeling; Hydrogen Production Technologies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–76. [Google Scholar]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Peng, H.; Li, C.; Zhou, W.; Yuan, P.; et al. Ni-Co/Al2O3 Bimetallic Catalysts for CH4 Steam Reforming: Elucidating the Role of Co for Improving Coke Resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Siang, T.J.; Singh, S.; Omoregbe, O.; Bach, L.G.; Phuc, N.H.H.; Vo, D.-V.N. Hydrogen production from CH4 dry reforming over bimetallic Ni-Co/Al2O3 catalyst. J. Energy Inst. 2017, in press. [Google Scholar] [CrossRef]
- Huo, J.; Jing, J.; Li, W. Reduction time effect on structure and performance of Ni-Co/MgO catalyst for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2014, 39, 21015–21023. [Google Scholar] [CrossRef]
- Mahboob, S.; Haghighi, M.; Rahmani, F. Sonochemically preparation and characterization of bimetallic Ni-Co/Al2O3-ZrO2 nanocatalyst: Effects of ultrasound irradiation time and power on catalytic properties and activity in dry reforming of CH4. Ultrason. Sonochem. 2017, 38, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Djinović, P.; Pintar, A. Stable and selective syngas production from dry CH4-CO2 streams over supported bimetallic transition metal catalysts. Appl. Catal. B Environ. 2017, 206, 675–682. [Google Scholar] [CrossRef]
- Xin, J.; Cui, H.; Cheng, Z.; Zhou, Z. Bimetallic Ni-Co/SBA-15 catalysts prepared by urea co-precipitation for dry reforming of methane. Appl. Catal. A Gene. 2018, 554, 95–104. [Google Scholar] [CrossRef]
- Erdogan, B.; Arbag, H.; Yasyerli, N. SBA-15 supported mesoporous Ni and Co catalysts with high coke resistance for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 1396–1405. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Müller, C.; Copéret, C. Supported Bimetallic NiFe Nanoparticles through Colloid Synthesis for Improved Dry Reforming Performance. ACS Catal. 2017, 7, 6942–6948. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and Dynamics Increase the Performance of NiFe Dry Reforming Catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, A.I.; Caglayan, B.S.; Aksoylu, A.E. A study on characterization and methane dry reforming performance of Co-Ce/ZrO2 catalyst. Appl. Catal. B Environ. 2015, 168, 164–174. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Abdollahifar, M. Sono-dispersion of bimetallic Ni-Co over zeolite Y used in conversion of greenhouse gases CH4/CO2 to high valued syngas. J. Nat. Gas Sci. Eng. 2015, 23, 547–558. [Google Scholar] [CrossRef]
- Khavarian, M.; Chai, S.-P.; Mohamed, A.R. The effects of process parameters on carbon dioxide reforming of methane over Co-Mo-MgO/MWCNTs nanocomposite catalysts. Fuel 2015, 158, 129–138. [Google Scholar] [CrossRef]
- Aw, M.S.; Črnivec, I.G.O.; Pintar, A. Tunable ceria-zirconia support for nickel-cobalt catalyst in the enhancement of methane dry reforming with carbon dioxide. Catal. Commun. 2014, 52, 10–15. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Allahyari, S.; Jafari, M.F. Syngas production from reforming of greenhouse gases CH4/CO2 over Ni-Cu/Al2O3 nanocatalyst: Impregnated vs. plasma-treated catalyst. Energy Convers. Manag. 2014, 84, 50–59. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, J.; Peng, X.; Tong, D.; Hu, C. Comparative study on the promotion effect of Mn and Zr on the stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrogen Energy 2013, 38, 7268–7279. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Naeem, M.A.; Khan, W.U.; Al-Fatesh, A.S. Syngas production via CO2 reforming of methane using Co-Sr-Al catalyst. J. Ind. Eng. Chem. 2014, 20, 549–557. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wang, Z.; Zhou, X.; Wang, Z.; Liu, C.-J. Steam reforming of methane over Ni/SiO2 catalyst with enhanced coke resistance at low steam to methane ratio. Catal. Today 2015, 256, 130–136. [Google Scholar] [CrossRef]
- Kho, E.T.; Scott, J.; Amal, R. Ni/TiO2 for low temperature steam reforming of methane. Chem. Eng. Sci. 2016, 140, 161–170. [Google Scholar] [CrossRef]
- Bej, B.; Pradhan, N.C.; Neogi, S. Production of hydrogen by steam reforming of methane over alumina supported nano-NiO/SiO2 catalyst. Catal. Today 2013, 207, 28–35. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. Catalytic performance of highly dispersed Ni/TiO2 for dry and steam reforming of methane. RSC Adv. 2014, 4, 4817–4826. [Google Scholar] [CrossRef]
- Katheria, S.; Gupta, A.; Deo, G.; Kunzru, D. Effect of calcination temperature on stability and activity of Ni/MgAl2O4 catalyst for steam reforming of methane at high pressure condition. Int. J. Hydrogen Energy 2016, 41, 14123–14132. [Google Scholar] [CrossRef]
- Silveira, E.B.; Rabelo-Neto, R.C.; Noronha, F.B. Steam reforming of toluene, methane and mixtures over Ni/ZrO2 catalysts. Catal. Today 2017, 289, 289–301. [Google Scholar] [CrossRef]
- Lim, Z.-Y.; Wu, C.; Wang, W.G.; Choy, K.-L.; Yin, H. Porosity effect on ZrO2 hollow shells and hydrothermal stability for catalytic steam reforming of methane. J. Mater. Chem. A 2016, 4, 153–159. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, X.; Guo, Y.; Chen, M.; Liu, W.; Xu, X.; Peng, H.; Gao, Z.; Wang, X.; Li, C. Highly active and stable Ni/Y2Zr2O7 catalysts for methane steam reforming: On the nature and effective preparation method of the pyrochlore support. Int. J. Hydrogen Energy 2016, 41, 11141–11153. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Zhang, X.; Liu, W.; Liu, H.; Qiu, J.; Yeung, K.L. New synthesis strategies for Ni/Al2O3-Sil-1 core-shell catalysts for steam reforming of methane. Catal. Today 2014, 236, 34–40. [Google Scholar] [CrossRef]
- Andrade, M.L.; Almeida, L.; Rangel, M.D.C.; Pompeo, F.; Nichio, N. Ni-Catalysts Supported on Gd-Doped Ceria for Solid Oxide Fuel Cells in Methane Steam Reforming. Chem. Eng. Technol. 2014, 37, 343–348. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yang, E.-H.; Lim, S.-S.; Jung, J.S.; Lee, J.-S.; Hong, G.H.; Noh, Y.-S.; Lee, K.Y.; Moon, D.J. Hydrogen production by steam reforming of methane over mixed Ni/MgAl + CrFe3O4 catalysts. Int. J. Hydrogen Energy 2015, 40, 11848–11854. [Google Scholar] [CrossRef]
- Yang, X.; Da, J.; Yu, H.; Wang, H. Characterization and performance evaluation of Ni-based catalysts with Ce promoter for methane and hydrocarbons steam reforming process. Fuel 2016, 179, 353–361. [Google Scholar] [CrossRef]
- Ali, S.; Al-Marri, M.J.; Abdelmoneim, A.G.; Kumar, A.; Khader, M.M. Catalytic evaluation of nickel nanoparticles in methane steam reforming. Int. J. Hydrogen Energy 2016, 41, 22876–22885. [Google Scholar] [CrossRef]
- Mundhwa, M.; Thurgood, C.P. Methane steam reforming at low steam to carbon ratios over alumina and yttria-stabilized-zirconia supported nickel-spinel catalyst: Experimental study and optimization of microkinetic model. Fuel Process. Technol. 2017, 168, 27–39. [Google Scholar] [CrossRef]
- Roy, P.S.; Park, C.S.; Raju, A.S.K.; Kim, K. Steam-biogas reforming over a metal-foam-coated (Pd-Rh)/(CeZrO2-Al2O3) catalyst compared with pellet type alumina-supported Ru and Ni catalysts. J. CO2 Util. 2015, 12, 12–20. [Google Scholar] [CrossRef]
- Jaiswar, V.K.; Katheria, S.; Deo, G.; Kunzru, D. Effect of Pt doping on activity and stability of Ni/MgAl2O4 catalyst for steam reforming of methane at ambient and high pressure condition. Int. J. Hydrogen Energy 2017, 42, 18968–18976. [Google Scholar] [CrossRef]
- Mei, D.; Glezakou, V.-A.; Lebarbier, V.; Kovarik, L.; Wan, H.; Albrecht, K.O.; Gerber, M.; Rousseau, R.; Dagle, R.A. Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: A combined experimental and theoretical study. J. Catal. 2014, 316, 11–23. [Google Scholar] [CrossRef]
- De Castro, T.P.; Silveira, E.B.; Rabelo-Neto, R.C.; Borges, L.E.P.; Noronha, F.B. Study of the performance of Pt/Al2O3 and Pt/CeO2/Al2O3 catalysts for steam reforming of toluene, methane and mixtures. Catal. Today 2017. [Google Scholar] [CrossRef]
- Nieva, M.A.; Villaverde, M.M.; Monzón, A.; Garetto, T.F.; Marchi, A.J. Steam-methane reforming at low temperature on nickel-based catalysts. Chem. Eng. J. 2014, 235, 158–166. [Google Scholar] [CrossRef]
- Arcotumapathy, V.; Vo, D.-V.N.; Chesterfield, D.; Tin, C.T.; Siahvashi, A.; Lucien, F.P.; Adesina, A.A. Catalyst design for methane steam reforming. Appl. Catal. A Gen. 2014, 479, 87–102. [Google Scholar] [CrossRef]
- Rodemerck, U.; Schneider, M.; Linke, D. Improved stability of Ni/SiO2 catalysts in CO2 and steam reforming of methane by preparation via a polymer-assisted route. Catal. Commun. 2017, 102, 98–102. [Google Scholar] [CrossRef]
- Duarte, R.B.; Krumeich, F.; van Bokhoven, J.A. Structure, Activity, and Stability of Atomically Dispersed Rh in Methane Steam Reforming. ACS Catal. 2014, 4, 1279–1286. [Google Scholar] [CrossRef]
- Wu, H.; la Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.; Liotta, L. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Homsi, D.; Aouad, S.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. A highly reactive and stable Ru/Co6−xMgxAl2 catalyst for hydrogen production via methane steam reforming. Int. J. Hydrogen Energy 2014, 39, 10101–10107. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Z.; Yang, Z. Tuning the Performance of Ni-Based Catalyst by Doping Coinage Metal on Steam Reforming of Methane and Carbon-Tolerance. Fuel Cells 2014, 14, 251–258. [Google Scholar] [CrossRef]
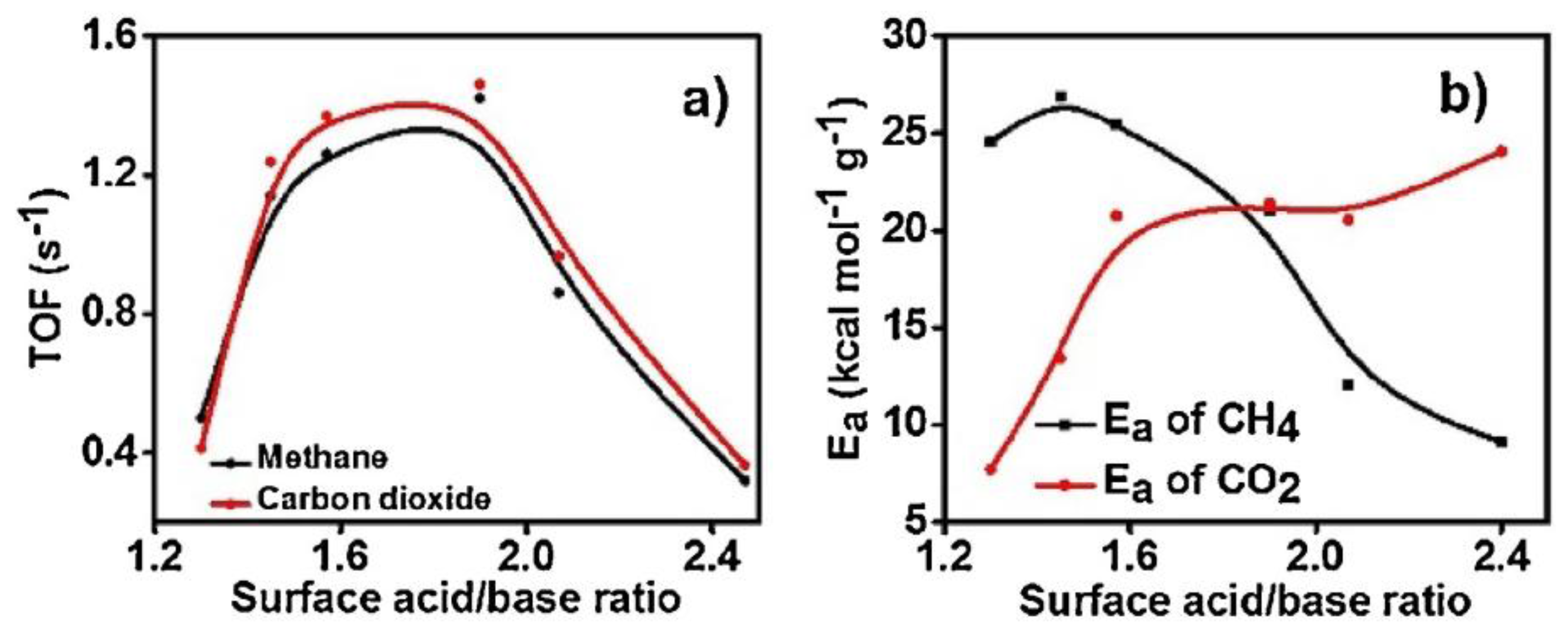
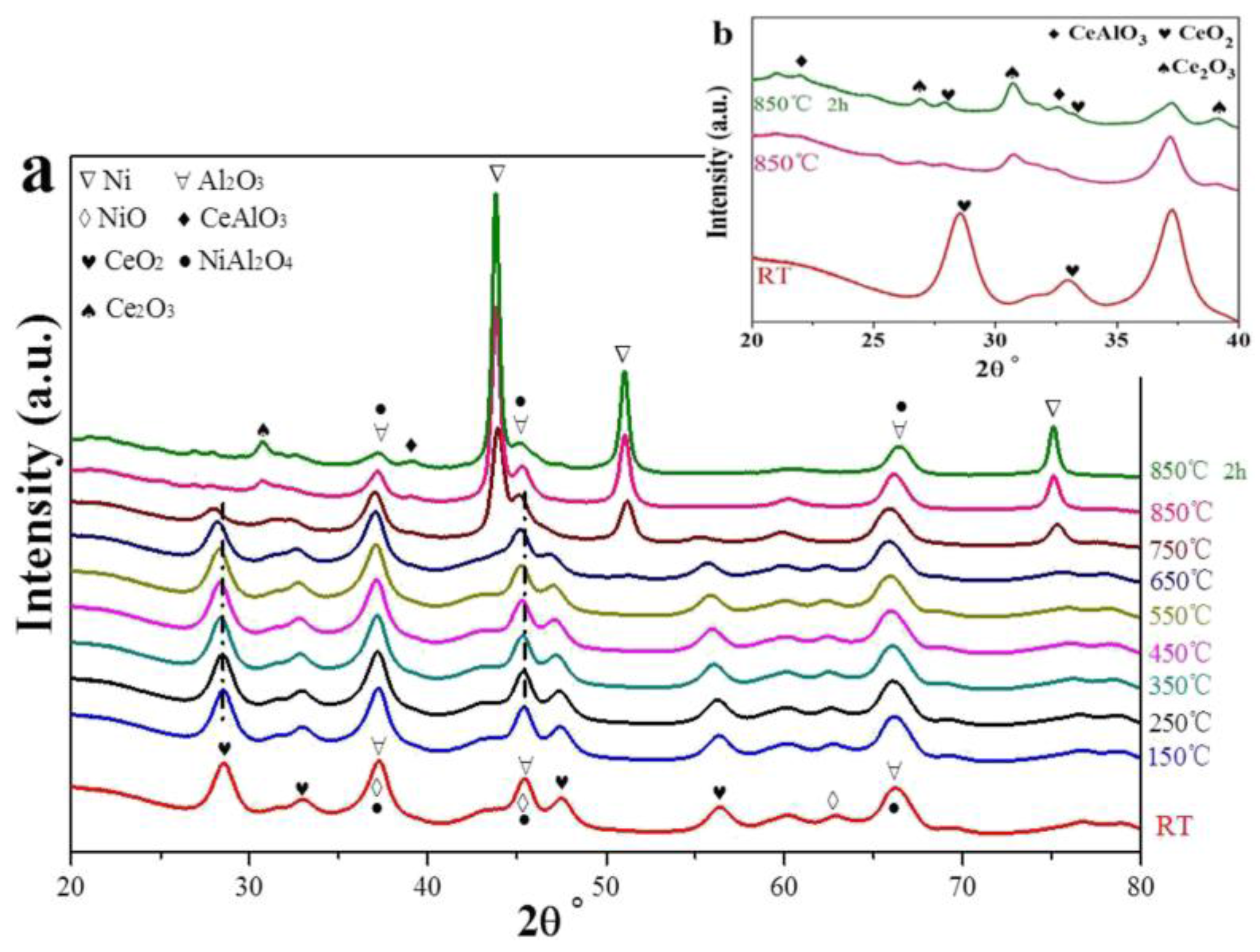
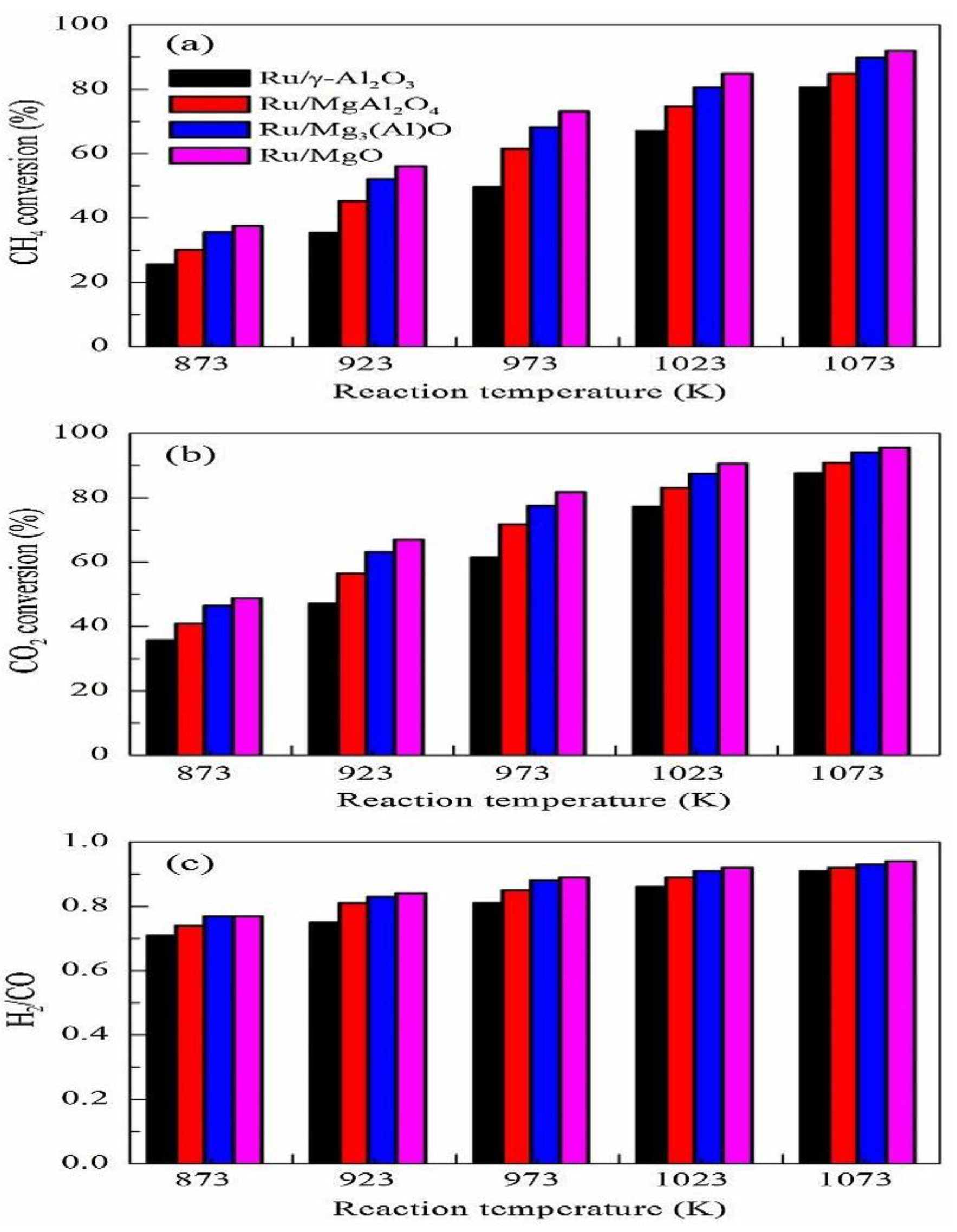

| Metal | Support | Temperature (K) | CH4 Conversion | H2/CO | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Ni | Nd-mesoporous silica | 973 | 53 | 0.75 | Mesoporous silica support was modified using Neodymium (Nd) to improve the metal dispersion and support basicity | [24] |
| Ni | La-ZrO2 | 555 | 22.8 | 0.83 | The use of electric field to improve the catalytic performance of DRM was studied, and it was concluded that the electric power accelerates the surface reaction of CH4 hence reaction can proceed at very low temperatures | [25] |
| Ce-ZrO2 | 545 | 13.2 | 0.75 | |||
| Pr-ZrO2 | 538 | 15.2 | 0.78 | |||
| Nd-ZrO2 | 498 | 12.5 | 0.88 | |||
| Y-ZrO2 | 496 | 12.4 | 0.75 | |||
| Ni | Ce-Al | 1073 | 90 | 1.1 | The reflux precipitation synthesis method was used to prepare different Ni loadings on Ce-Al support which achieved improved stability due to the existence of various Ce, Al, and Ce-Al oxides with excellent oxidative properties that burns off the deposited carbon | [19] |
| Ni | CeO2 | 1073 | 95 | 2 | Impregnation synthesis was used to prepare the catalyst to be used under chemical looping DRM conditions to achieve high H2/CO ratios due to the continuous oxidation-reduction of the catalyst | [17] |
| Ni | Ce-ZnAl2O4 | 1073 | 82 | 1 | Cerium (Ce) was used as a support promotor using co-precipitation method to improve the Ni dispersion and reduce particle size to achieve longer catalyst lifetime | [26] |
| Ni | γ-Al2O3 | NA | 30 | 1 | Dielectric plasma was used to improve the catalytic performance for DRM and the impacts of the different process conditions (Ni loading, feed composition, and discharge power) was statistically correlated | [27] |
| Ni | Si microspheres | 1023 | 70 | 0.8 | Sol-gel microencapsulation synthesis was used to prepare the catalyst, and the conversion of the carbon formed into SiC was confirmed which prevented catalyst deactivation | [28] |
| Ni | Al2O4 | 1073 | 90 | 0.81 | Co-precipitation synthesis was used to prepare catalysts with improved activity and stability attributed to the small crystallite size, high surface area, and the good metal dispersion | [29] |
| Ni | MgO-Al2O3 | 973 | 74 | 0.94 | The small metal cluster size and high surface areas were deemed responsible for the observe activity and stability. | [14] |
| Ni | Al2O3 | 1023 | 70 | 1 | Sequential impregnation versus sol-gel synthesis methods were compared for the preparation of the catalyst. The sol-gel route resulted in a small particle size with enhanced dispersion and catalytic activity | [18] |
| CeO2-Al2O3 | 1023 | 90 | 1 | |||
| Ni | γ-Al2O3 | 1023 | 68 | The excellent catalytic performance of CeO2-YSZ as a support for Ni catalyst was ascribed to its high oxidative abilities leading to an increased Ni dispersion and hence reducing carbon deposition | [22] | |
| CeO2-YSZ | 1023 | 100 | 0.75 | |||
| Ce0.15Zr0.85O2 | 1023 | 100 | ||||
| Ni | SBA-15 | 1023 | 92 | 0.98 | The optimum operating conditions were developed using SBA-15 with high surface area suitable for high Ni content impregnation | [30] |
| Ni | MgO-SBA-15 | 1073 | 96 | 1.1 | A comparison between the impregnation route versus coating for introducing MgO into the porous structure of SBA-15 was investigated | [13] |
| Ni | Ce0.75Zr0.25-SBA-15 | 1073 | 100 | 0.9 | The catalyst lifetime was increased by adding CeZr into the SBA-15 support as oxygen providcers | [31] |
| Ni | CaFe2O4 | 1073 | 90.04 | 0.98 | The support was prepared by sol-gel method and the Ni was introduced using wet impregnation technique. Predictions of the DRM was performed using Artificial Neural Network (ANN) based on metal loadings, feed composition, and reaction temperature. | [32] |
| Ni | SiO2 (core-shell) | 1023 | 86 | 0.98 | Water in oil micromulsion technique was used to prepare the catalyst with a core-shell structure which reduced Ni sintering during DRM | [33] |
| SiO2 (conventional impregnation) | 1023 | 74 | 0.65 | |||
| Ni | ZrO2 | 1123 | 97 | 1 | Monoclinic ZrO2 substrates were prepared by adjusting the catalyst synthesis conditions to vary the support surface morphology leading to higher dispersion, surface oxygen availability, and hence showing superior performance to conventional supports | [34] |
| Ni | ZSM-5 | 1073 | 66 | 0.95 | Introducing Co to Ni/ZSM-5 catalyst has significantly improved the catalyst stability due to the activity of Co in oxidation reactions | [35] |
| Ni | Ce-Zr-SBA-15 | 873 | 63 | 0.85 | The effect of Ce-Zr doping into Ni/SBA-15 prepared using different impregnation strategies was studied to gain insights into the role of the synthesis route on the catalyst activity and stability | [36] |
| Ni | SiO2 | 1023 | 88 | The catalytic performance of different metal-oxides support were tested for DRM and it was concluded that MgO modified Al2O3 possess a great stability due to the improved dispersion and the strong metal-support interaction | [11] | |
| TiO2 | 1023 | 3 | ||||
| Al2O3 | 1023 | 78 | ||||
| ZrO2 | 1023 | 88 | ||||
| MgO | 1023 | 90 | ||||
| MgO-AL2O3 | 1023 | 87 | ||||
| Ni | CeMgAl | 1073 | 96.5 | 0.8 | The reflux co-precipitation technique was used to prepare the catalyst and the effects of the calcination and reduction temperatures were evaluated | [23] |
| Ni | Al2O3-ZrO2 | 1123 | 85 | 0.95 | A comparison between catalyst preparation methods was presented | [37] |
| Ni | Al2O3 | 1073 | 93 | 1.3 | The catalytic performance of Ni supported onto two different supports was investigated | [38] |
| Ni | CeZrO2 | 1073 | 91 | 0.9 | ||
| Ni | CeO2-SiO2 | 1073 | 98 | 1.2 | The physicochemical properties of the catalyst were improved by using the mixed oxide approach | [20] |
| Ni | Al2O3 | 1073 | 94 | 0.86 | Different formulations and precursor concentrations were used for the preparation of the Al2O3 support in order to vary the support characteristics and performance in DRM | [8] |
| Ni | Silicalite | 973 | 65 | 1.23 | The role of the support surface morphology and defects on the cata;ytic performance in DRM was investigated | [7] |
| MCM-41 | 973 | 77 | 1.03 | |||
| Silica delaminated zeolite | 973 | 79 | 1.39 | |||
| Ni | clinoptilolite | 1123 | 88 | 0.94 | The impacts of the support type on the catalyst properties in DRM was analyzed. The utilization of clinoptilolite as cheap support was found promising | [12] |
| Al2O3 | 1123 | 93 | 0.97 | |||
| CeO2 | 1123 | 75 | 0.93 |
| Metal | Support | Temperature (K) | CH4 Conversion | H2/CO | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Co | Ca-AC | 1173 | 94 | The co-impregnation of Ca on the surface of AC has enhanced the Co-AC interaction, Co dispersion and induced CO2 adsorption properties resulting in an improved stability | [45] | |
| Co | Nd2O3 | 1023 | 62.7 | 0.97 | Wet impregnation method was used to prepare the catalyst with 20 wt% Co loading and a reaction mechanism was proposed and modelled | [42] |
| Co | La2O3 | 1023 | 50 | Wet impregnation technique was used to prepare the catalysts up to 20 wt % Co loading. The DRM kinetics was modelled using Langmuir-Hinshelwood mechanisms with excellent agreement | [43] | |
| Co | CeO2 | 1023 | 79.5 | 0.97 | Wet impregnation was employed as a synthesis method achieving enhanced catalytic activity due to the excellent metal disperion | [41] |
| Co | CeO2 | 1023 | 78 | The effect of the reactants partial pressure on the catalytic performance was investigated | [40] | |
| Co | Al2O3 | 973 | 61 | Catalysts with low metal loadings were investigated for DRM | [46] | |
| Co | MgO | 973 | 69.38 | 0.79 | Co-precipitation technique was used to prepare nanosized catalysts for DRM and the metal loading was optimized | [44] |
| Ir | Ce0.9Pr0.1O2 | 1025 | 62 | 0.98 | Different synthesis strategies were analyzed to control the Ir dispersion, and it was found that deposition-precipitation possesses the lowest coke deposition | [47] |
| Ru | γ-Al2O3 | 1023 | 67 | The influence of the support on the metal dispersion was analyzed and it was found that the MgAlO mixed oxide support has excellent stability due to the strong basicity of the support | [48] | |
| MgAl2O4 | 75 | |||||
| Mg3AlO | 80 | |||||
| MgO | 86 | |||||
| Rh | La2O3-γ-Al2O3 | The effect of phosphorous addition to the support was studied under different impregnation scenarios to study the impacts on catalyst activity and stability | [49] | |||
| Rh | γ-Al2O3 | 1073 | 97.5 | Numerical simulation of the catalytic DRM in a membrane reactor was performed to find the optimum operating conditions and it was found that Ni/La2O3 is the most stable catalyst at 1073 K | [50] | |
| Pt | hydroxyapatite (HAP) | 973 | 30 | 0.92 | Different synthesis methods were deployed to introduce Pt into HAP and it was concluded that the incipient wetness is the most efficient technique | [51] |
| Metal | Support | Temperature (K) | CH4 Conversion | H2/CO | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Ni-Co | CeZrO2/β-SiC | 1023 | 65 | 0.8 | Deposition precipitation was used to produce catalyst with controlled metal cluster size to reduce coking | [57] |
| Ni-Co | γ-Al2O3 | 973 | 67 | 1 | The bimetallic catalyst with small crystallite size was prepared using to improve the catalyst activity and stability. The formation of an amorphous carbon was evident explaining the catalyst prolonged lifetime | [54] |
| Ni-Co | ZrO2-Al2O3 | 1123 | 93 | 1 | The ultrasound-mediated synthesis produced catalysts with high surface area, small crystal size, and high metal dispersion resulted in the observed enhanced stability | [56] |
| Ni-Co | CeO2 | 973 | 64 | 0.80 | The effects of the catalyst calcination temperature on the catalytic activity and stability was studied, and it was certain that at higher calcination temperatures the carbon deposition was minimized | [16] |
| Co-Ce | ZrO2 | 973 | 78 | 0.67 | The oxygen storage property of the ZrO2 support was promoted by adding Ce at different ratios to reduce metal sintering and carbon formation | [62] |
| Ni-Co | ZSM-5 | 1073 | 80 | 0.98 | Introducing Co to Ni/ZSM-5 catalyst has significantly improved the catalyst stability due to the activity of Co in oxidation reactions | [47] |
| Ni-Co | Zeolite Y | 1123 | 96 | 0.93 | The utilization of ultrasonic power in the synthesis of Co-Ni bimetallic catalyst has resulted in higher metal dispersion | [63] |
| Co-Mo | MgO/MWCNTs | 1223 | 98.6 | 1.1 | The insitu growth of multiwall carbon nanotubes (MWCNTs) in the solution of mixed Co and Mo precursors was used to prepare the composite catalyst with excellent activity for syngas production | [64] |
| Ni-Co | CeO2-ZrO2 | 973 | 90 | 0.73 | The mixed support was prepared by thermal precipitation in ethylene glycol media | [65] |
| Ni-Cu | Al2O3 | 1123 | 82 | 0.83 | Plasma treatment was applied to synthesize the bimetallic nanocatalysts to improve the uniformity of metal dispersion | [66] |
| Ni-Co | MgO | 1073 | 92 | 0.98 | The impacts of the catalyst reduction duration was studied and it was concluded that the short reduction time is preferable for better stability | [55] |
| Mn-Ni | SiO2 | 1073 | 77.8 | 0.65 | The positive impact of Mn as a catalyst promotor was confirmed | [67] |
| Zr-Ni | SiO2 | 1073 | 89.3 | |||
| Co-Sr | γ-Al2O3 | 973 | 80 | 0.88 | The Sr acted as a promotor to enhance the support basicity and hence reduced carbon formation with no effects on the catalytic activity | [68] |
| Pt-Ni | Al2O3 | 1073 | 92 | 1.1 | The role of the addition of Pt to the Ni-based catalyst was analyzed using two oxide supports. The improvement in the catalyst lifetime was highly pronounced when adding Pt to Ni/CeZrO2 | [38] |
| Pt-Ni | CeZrO2 | 1073 | 94 | 0.8 | ||
| Ni-Co | Al2O3-ZrO2 | 1123 | 96 | 0.98 | The effects of synthesis method and the use of metal promotors was investigated | [37] |
| Ni-Cu | Al2O3-ZrO2 | 1123 | 93 | 0.93 |
| Catalyst | CH4 Conversion % | Conditions | Catalyst Characteristics | Remarks | Ref. |
|---|---|---|---|---|---|
| 10 wt % Ni/SiO2 | 35 | 700 °C, 3.5 h, H2O/CH4 = 0.5 | Ni particle size = 6.9 nm | The catalyst prepared using plasma was evaluated for long-term stability | [70] |
| 10 wt % Ni/TiO2 | 27 | 500 °C, H2O/CH4 = 1 | BET surface area = 42 m2/g and Ni dispersion = 2.8% | Different Ni contents were loaded on TiO2 for SRM at low temperatures | [71] |
| 10 wt % Ni/Al2O3 | 30 | 500 °C, H2O/CH4 = 1 | BET surface area = 40 m2/g and Ni dispersion = 1.3% | ||
| 10 wt % Ni/SiO2 | 10 | 500 °C, H2O/CH4 = 1 | BET surface area = 300 m2/g and Ni dispersion = 2.6% | ||
| 10 wt % Ni/SiO2 | 96 | 700 °C, H2O/CH4 = 3.5 | BET surface area = 268 m2/g and particle size = 11 nm | The sol-gel technique was used to prepare the catalyst with small particle sizes | [72] |
| 15 wt % Ni/TiO2 | 92 | 700 °C, H2O/CH4 = 1.2 | BET surface area = 62 m2/g | Ultrasound irradiation was used to enhance the Ni dispersion | [73] |
| 15 wt % Ni/MgAl2O4 | 45 | 600 °C, 5 bar, H2O/CH4 = 5 | BET surface area = 43 m2/g, Ni dispersion = 6.3%, and particle size = 10 nm | The effect of calcination temperature on the physicochemical properties was analyzed | [74] |
| 9 wt % Ni/ZrO2 | 65 | 973 K and, H2O/CH4 = 4 | BET surface area = 19 m2/g and particle size = 23 nm | Different structures of ZrO2 supports were used for SRM | [75] |
| 6 wt % Ni/ZrO2 | 30 | 650 °C and, H2O/CH4 = 2.5 | BET surface area = 16 m2/g | The textural properties of ZrO2 support were controlled to improve Ni dispersion | [76] |
| Ni/SiO2/ZrO2 | 95 | 650 °C and, H2O/CH4 = 2.5 | BET surface area = 176 m2/g | ||
| 10 wt % Ni/Y2Zr2O7 | 85 | 700 °C and, H2O/CH4 = 2 | BET surface area = 21 m2/g and particle size = 12 nm and Ni dispersion = 100% | Different synthesis approaches were used to prepare the support which showed different metal-support interactions | [77] |
| Ni/Al2O3-Silicalite | 69 | 650 °C, and H2O/CH4 = 3 | The silicalite shell was grown around Al2O3 followed by impregnation to introduce Ni into the core of the catalyst | [78] | |
| Ni/90 wt % Ce-10 wt % Gd | 73 | 700 °C, and H2O/CH4 = 3 | BET surface area = 65 m2/g and metal dispersion = 1.6% | Doping the support with Gd improved H2/CO ratio at the expense of the catalytic activity | [79] |
| Ni/MgAl | 40 | 600 °C, and H2O/CH4 = 2 | BET surface area = 97 m2/g and particle size = 14 nm | Ni-based hydrotalcite catalyst was prepared and the use of Fe and Cr promotors on the Ni dispersion was studied. | [80] |
| Fe-Ni/MgAl | 50 | 600 °C, and H2O/CH4 = 2 | BET surface area = 98 m2/g and particle size = 8 nm | ||
| Cr-Ni/MgAl | 45 | 600 °C, and H2O/CH4 = 2 | BET surface area = 104 m2/g and particle size = 8 nm | ||
| Ni/MgAl + CrFe3O4 | 75 | 600 °C, and H2O/CH4=2 | BET surface area = 13 m2/g | ||
| 13 wt % Ni-1 wt % Ce/Al2O3 | 75 | 750 °C, and H2O/CH4 = 3 | BET surface area = 26 m2/g and Ni dispersion = 8.3% | The effects of Ce as a promotor for Ni-based catalyst was investigated | [81] |
| 5 wt % Ni/SiO2-Al2O3 | 75 | 750 °C, and H2O/CH4 = 1 | BET surface area = 73 m2/g and particle size = 19 nm | Ni nanoparticles supported catalysts exhibited better dispersion, particle size, and reducibility than conventional Ni catalysts | [82] |
| 5 wt % Ni nano-particles/SiO2-Al2O3 | 98 | 750 °C, and H2O/CH4 = 1 | BET surface area = 106 m2/g and particle size = 6 nm | ||
| 5 wt % Ni/Al2O3-Y2O3-ZrO2 | 95 | 1125 K, and H2O/CH4 = 1.5 | SRM was studied at low H2O/CH4 to enhance the energy effeciency | [83] | |
| 0.09 wt % (Pd-Rh)/CeZrO2-Al2O3 | 93 | 1073 K, and H2O/CH4 = 1.5 | BET surface area = 146.8 m2/g and metal dispersion = 37.5% | The bimetallic catalyst was coated on metallic foam to reduce coke formation | [84] |
| 8 wt % Rh/Al2O3 | 88 | 1073 K, and H2O/CH4 = 1.5 | BET surface area = 6.4 m2/g | ||
| 13 wt % Ni/Al2O3 | 85 | 1073 K, and H2O/CH4 = 1.5 | BET surface area = 164 m2/g | ||
| 0.1 wt % Pt-13 wt % Ni/MgAl2O4 | 65 | 600 °C, and H2O/CH4 = 5 | BET surface area = 44 m2/g and particle size = 7.6 nm | The cata;yst reducibility was improved by doping the catalyst with different Pt loadings | [85] |
| 5 wt % Ir/MgAl2O4 | 55 | 850 °C, and H2O/CH4 = 3 | particle size = 1 nm and metal dispersion = 100% | Ir and Rh supported on MgAl2O4 were found to have high metal dispersion and hence better catalytic activity | [86] |
| 5 wt % Rh/MgAl2O4 | 41 | 850 °C, and H2O/CH4 = 3 | particle size = 2 nm and metal dispersion = 50% | ||
| 5 wt % Pt/MgAl2O4 | 49 | 850 °C, and H2O/CH4 = 3 | particle size = 3 nm and metal dispersion = 29% | ||
| 5 wt % Pd/MgAl2O4 | 31 | 850 °C, and H2O/CH4 = 3 | particle size = 16 nm and metal dispersion = 6% | ||
| 5 wt % Ru/MgAl2O4 | 3 | 850 °C, and H2O/CH4 = 3 | particle size = 20 nm and metal dispersion = 5% | ||
| 5 wt % Ni/MgAl2O4 | 4 | 850 °C, and H2O/CH4 = 3 | particle size = 7 nm and metal dispersion = 15% | ||
| 1 wt % Pt/Al2O3 | 70 | 973 K, and H2O/CH4 = 4 | BET surface area = 131 m2/g and metal dispersion = 50% | The effect of the addition of toluene to the feed stream was studied | [87] |
| 1.4 wt % Pt/CeO2-Al2O3 | 75 | 973 K, and H2O/CH4 = 4 | BET surface area = 84 m2/g and metal dispersion = 83% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamedali, M.; Henni, A.; Ibrahim, H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering 2018, 2, 9. https://doi.org/10.3390/chemengineering2010009
Mohamedali M, Henni A, Ibrahim H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering. 2018; 2(1):9. https://doi.org/10.3390/chemengineering2010009
Chicago/Turabian StyleMohamedali, Mohanned, Amr Henni, and Hussameldin Ibrahim. 2018. "Recent Advances in Supported Metal Catalysts for Syngas Production from Methane" ChemEngineering 2, no. 1: 9. https://doi.org/10.3390/chemengineering2010009
APA StyleMohamedali, M., Henni, A., & Ibrahim, H. (2018). Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering, 2(1), 9. https://doi.org/10.3390/chemengineering2010009






