Oxidative Depolymerization of Lignin Using Supported Niobium Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
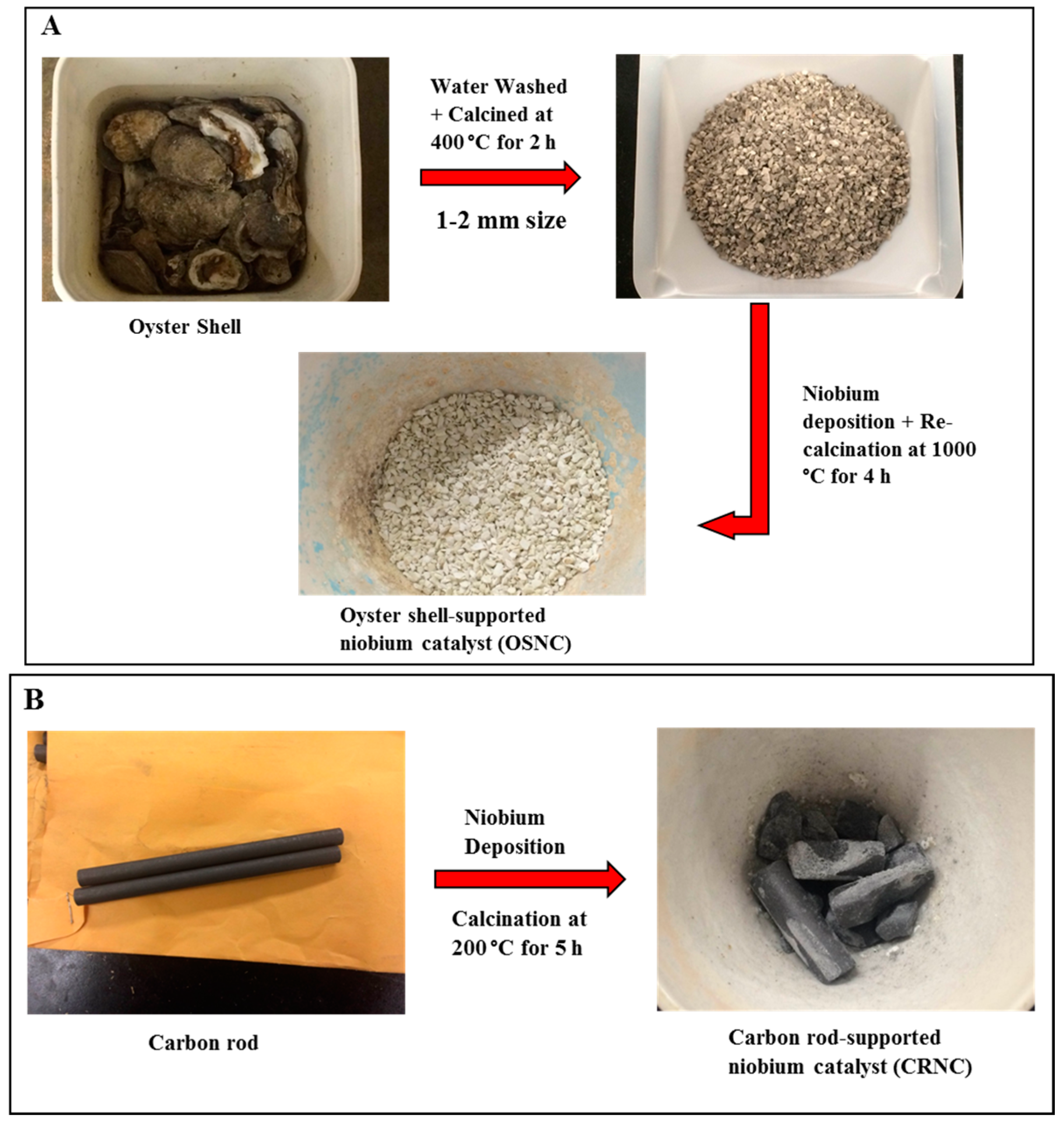
2.2. Catalyst Synthesis
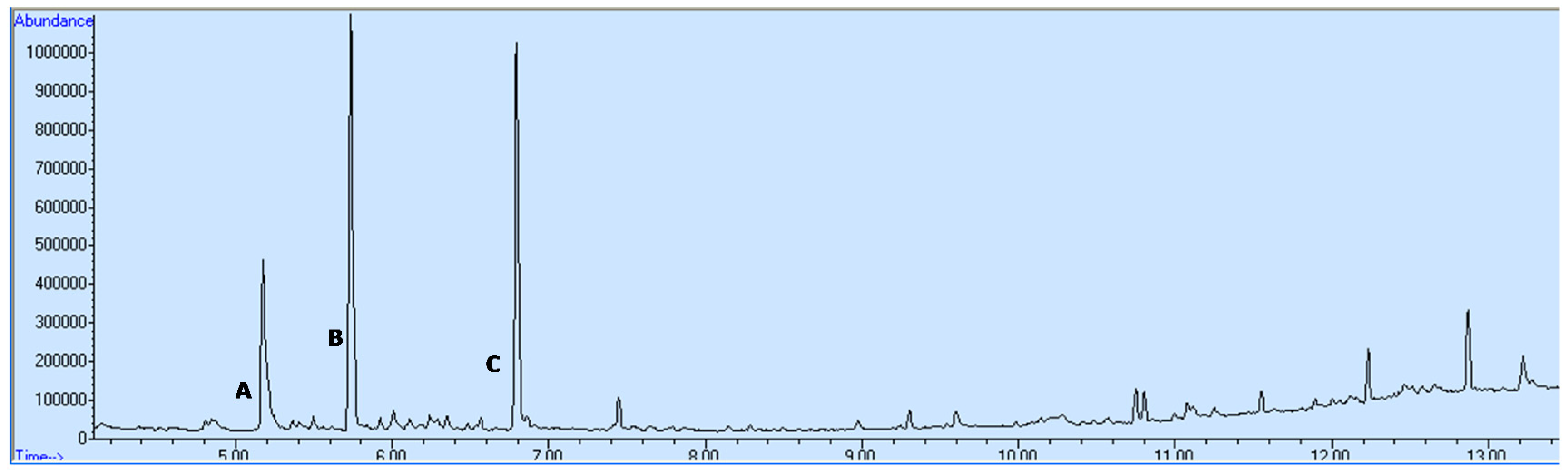
2.3. Catalyst Testing and Analytical Methods
3. Results and Discussion
3.1. Catalyst Testing and Oxidation Products
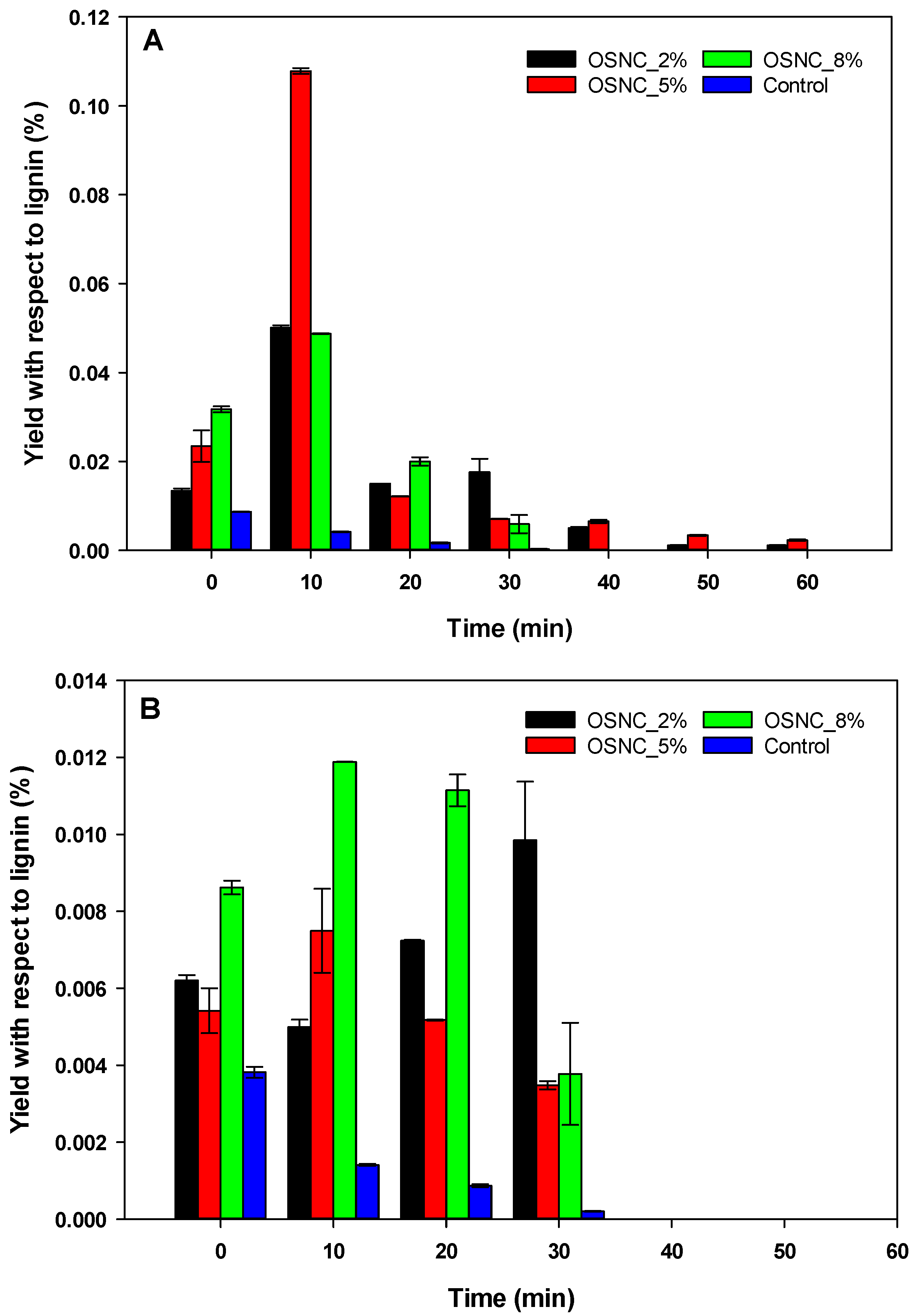
3.2. Oyster Shell-Supported Niobium Catalyst
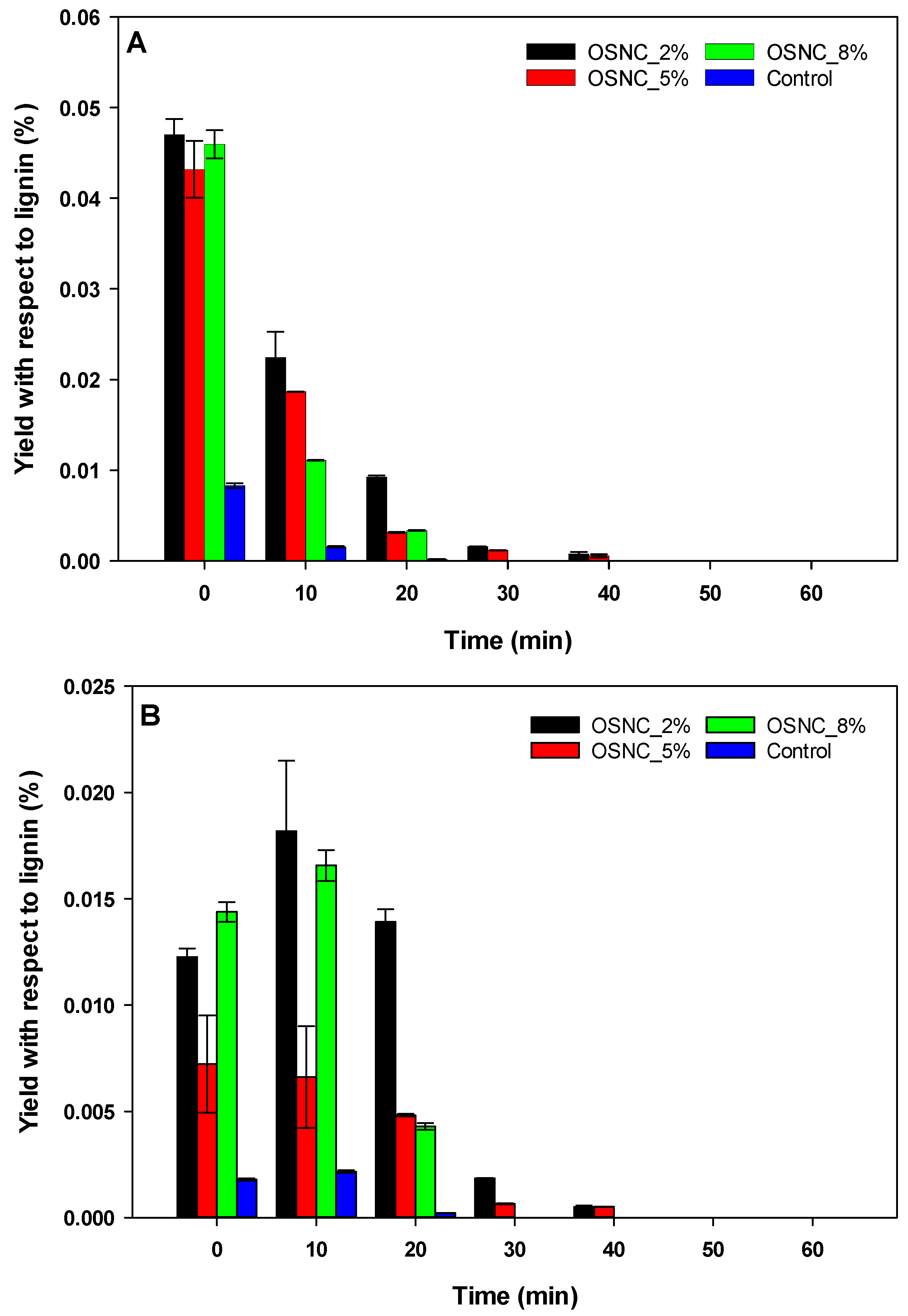
3.3. Carbon Rod Supported Niobium Catalyst
4. Future Directions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An efficient and economical process for lignin depolymerization in biomass-derived solvent tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefin. 2011, 5, 548–561. [Google Scholar] [CrossRef]
- Sanyoto, B.; Dwiatmoko, A.A.; Choi, J.-W.; Ha, J.-M.; Suh, D.J.; Kim, C.S.; Lim, J.-C. Catalytic depolymerization of alkali lignin using supported Pt nanoparticle catalysts. J. Nanosci. Nanotechnol. 2016, 16, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Dhepe, P.L. Solid base catalyzed depolymerization of lignin into low molecular weight products. Green Chem. 2017, 19, 778–788. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Patti, A.F.; Saito, K. Lignin depolymerization with phenol via redistribution mechanism in ionic liquids. ACS Sustain. Chem. Eng. 2014, 2, 2159–2164. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Tomani, P.; Axegård, P.; Berglin, N.; Lovell, A.; Nordgren, D. Integration of lignin removal into a kraft pulp mill and use of lignin as a biofuel. Cellul. Chem. Technol. 2011, 45, 533. [Google Scholar]
- Wu, W.; Dutta, T.; Varman, A.M.; Eudes, A.; Manalansan, B.; Loqué, D.; Singh, S. Lignin valorization: Two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci. Rep. 2017, 7, 8420. [Google Scholar] [CrossRef] [PubMed]
- Azarpira, A.; Ralph, J.; Lu, F. Catalytic alkaline oxidation of lignin and its model compounds: A pathway to aromatic biochemicals. Bioenergy Res. 2014, 7, 78–86. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Ma, R.; Guo, M.; Zhang, X. Recent advances in oxidative valorization of lignin. Catal. Today 2017. [Google Scholar] [CrossRef]
- Crestini, C.; Caponi, M.C.; Argyropoulos, D.S.; Saladino, R. Immobilized methyltrioxo rhenium (MTO)/H2O2 systems for the oxidation of lignin and lignin model compounds. Bioorg. Med. Chem. 2006, 14, 5292–5302. [Google Scholar] [CrossRef] [PubMed]
- Hermans, I.; Spier, E.S.; Neuenschwander, U.; Turrà, N.; Baiker, A. Selective oxidation catalysis: Opportunities and challenges. Top. Catal. 2009, 52, 1162–1174. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.; Lima Filho, N.M.; Abreu, C.A. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Wachs, I.E.; Briand, L.E.; Jehng, J.-M.; Burcham, L.; Gao, X. Molecular structure and reactivity of the Group V metal oxides. Catal. Today 2000, 57, 323–330. [Google Scholar] [CrossRef]
- Rooke, J.; Barakat, T.; Siffert, S.; Su, B.-L. Total catalytic oxidation of toluene using pd impregnated on hierarchically porous Nb2O5 and Ta2O5 supports. Catal. Today 2012, 192, 183–188. [Google Scholar] [CrossRef]
- Paulis, M.; Martın, M.; Soria, D.; Dıaz, A.; Odriozola, J.; Montes, M. Preparation and characterization of niobium oxide for the catalytic aldol condensation of acetone. Appl. Catal. A Gen. 1999, 180, 411–420. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic application of niobium compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Ziolek, M. Niobium-containing catalysts—The state of the art. Catal. Today 2003, 78, 47–64. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Wachs, I. Niobium oxalate: A new precursor for the preparation of supported niobium oxide catalysts. Am. Chem. Soc. Div. Pet. Chem. 1989, 34, 546–550. [Google Scholar]
- Nakajima, K.; Baba, Y.; Noma, R.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Nb2O5·nH2O as a heterogeneous catalyst with water-tolerant lewis acid sites. J. Am. Chem. Soc. 2011, 133, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.N.; Tang, S.Y.; Zhao, H.T.; Weiske, T.; Schlangen, M.; Schwarz, H. Thermal ethane activation by bare [V2O5]+ and [Nb2O5]+ cluster cations: On the origin of their different reactivities. Chem. Eur. J. 2014, 20, 6672–6677. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Bisio, C.; Psaro, R.; Marchese, L.; Guidotti, M. Niobium (V) saponite clay for the catalytic oxidative abatement of chemical warfare agents. Angew. Chem. Int. Ed. Engl. 2014, 15, 10095–10098. [Google Scholar] [CrossRef] [PubMed]
- Oysters in the U.S. Available online: http://www.nmfs.noaa.gov/aquaculture/homepage_stories/05_national_oyster_day_2015.html (accessed on 11th November, 2017).
- Lorio, W.J.; Malone, S. The Cultivation of American Oysters (Crassostrea virginica); Southern Regional Aquaculture Center: Stoneville, MS, USA, 1994. [Google Scholar]
- Jairam, S.; Kolar, P.; Sharma-Shivappa, R.; Osborne, J.A.; Davis, J.P. Ki-impregnated oyster shell as a solid catalyst for soybean oil transesterification. Bioresour. Technol. 2012, 104, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Cai, L.; Jiang, Y.; Han, Q. Eco-efficient, green, and scalable synthesis of 1,2,3-triazoles catalyzed by Cu(I) catalyst on waste oyster shell powders. ACS Sustain. Chem. Eng. 2014, 2, 765–771. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Villar, J.; Caperos, A.; Garcia-Ochoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci. Technol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Prado, R.; Vriamont, C.; Erdocia, X.; Labidi, J.; Hallett, J.P.; Welton, T. Oxidative depolymerization of lignin using a novel polyoxometalate-protic ionic liquid system. ACS Sustain. Chem. Eng. 2016, 4, 6031–6036. [Google Scholar] [CrossRef]
- Rodrigues Pinto, P.C.; Borges da Silva, E.A.; Rodrigues, A.E. Insights into oxidative conversion of lignin to high-added-value phenolic aldehydes. Ind. Eng. Chem. Res. 2010, 50, 741–748. [Google Scholar] [CrossRef]
- Das, L.; Kolar, P.; Osborne, J.; Sharma-Shivappa, R.; Classen, J. Selective oxidation of lignin into aromatic aldehydes using niobium oxalate. Am. Soc. Agric. Biol. Eng. 2016, 59, 727–735. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y. Oxidative cracking of precipitated hardwood lignin by hydrogen peroxide. Appl. Biochem. Biotechnol. 2000, 84, 153–162. [Google Scholar] [CrossRef]
- Kadla, J.F.; Chang, H. The Reactions of Peroxides with Lignin and Lignin Model Compounds; ACS Publications: Columbia, DC, USA, 2001. [Google Scholar]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sales, F.G.; Maranhão, L.C.; Lima Filho, N.M.; Abreu, C.A. Experimental evaluation and continuous catalytic process for fine aldehyde production from lignin. Chem. Eng. Sci. 2007, 62, 5386–5391. [Google Scholar] [CrossRef]
- Mathias, A.; Rodrigues, A. Production of vanillin by oxidation of pine kraft lignins with oxygen. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1995, 49, 273–278. [Google Scholar] [CrossRef]
- Dier, T.K.; Rauber, D.; Durneata, D.; Hempelmann, R.; Volmer, D.A. Sustainable electrochemical depolymerization of lignin in reusable ionic liquids. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, X.; Guan, N.; Li, L. Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: Role of graphene support. Appl. Catal. B Environ. 2013, 136, 177–185. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; He, Y.; Wang, J.; Kondo, J.N.; Tatsumi, T. Selective oxidation of alcohols to aldehydes/ketones over copper oxide-supported gold catalysts. J. Catal. 2013, 299, 10–19. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Wang, Y.; Chen, Y.; Li, Y.-X. Synthesis of mesoporous cemno materials and catalytic application for selective oxidation of benzyl alcohol by molecular oxygen. Catal. Lett. 2017, 147, 328–334. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, Á.; Rodrigues, A. Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 1996, 35, 28–36. [Google Scholar] [CrossRef]
- Araújo, J.D.; Grande, C.A.; Rodrigues, A.E. Vanillin production from lignin oxidation in a batch reactor. Chem. Eng. Res. Des. 2010, 88, 1024–1032. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic oxidation of lignin in solvent systems for production of renewable chemicals: A review. Polymers 2017, 9, 240. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew.Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Demesa, A.G.; Laari, A.; Turunen, I.; Sillanpää, M. Alkaline partial wet oxidation of lignin for the production of carboxylic acids. Chem. Eng. Technol. 2015, 38, 2270–2278. [Google Scholar] [CrossRef]
- Kindsigo, M.; Hautaniemi, M.; Kallas, J. Wet oxidation of recalcitrant lignin water solutions: Experimental and reaction kinetics. Environ. Chem. Lett. 2009, 7, 155. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Araújo, J.D.; Grande, C.A.; Rodrigues, A.E. Structured packed bubble column reactor for continuous production of vanillin from kraft lignin oxidation. Catal. Today 2009, 147, S330–S335. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin production from lignin and its use as a renewable chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Maziero, P.; de Oliveira Neto, M.; Machado, D.; Batista, T.; Cavalheiro, C.C.S.; Neumann, M.G.; Craievich, A.F.; de Moraes Rocha, G.J.; Polikarpov, I.; Goncalves, A.R. Structural features of lignin obtained at different alkaline oxidation conditions from sugarcane bagasse. Ind. Crops Prod. 2012, 35, 61–69. [Google Scholar] [CrossRef]
- Da Silva, E.B.; Zabkova, M.; Araújo, J.; Cateto, C.; Barreiro, M.; Belgacem, M.; Rodrigues, A. An integrated process to produce vanillin and lignin-based polyurethanes from kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Behling, R.; Valange, S.; Chatel, G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: What results? What limitations? What trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Grasselli, R.K. Genesis of site isolation and phase cooperation in selective oxidation catalysis. Top. Catal. 2001, 15, 93–101. [Google Scholar] [CrossRef]
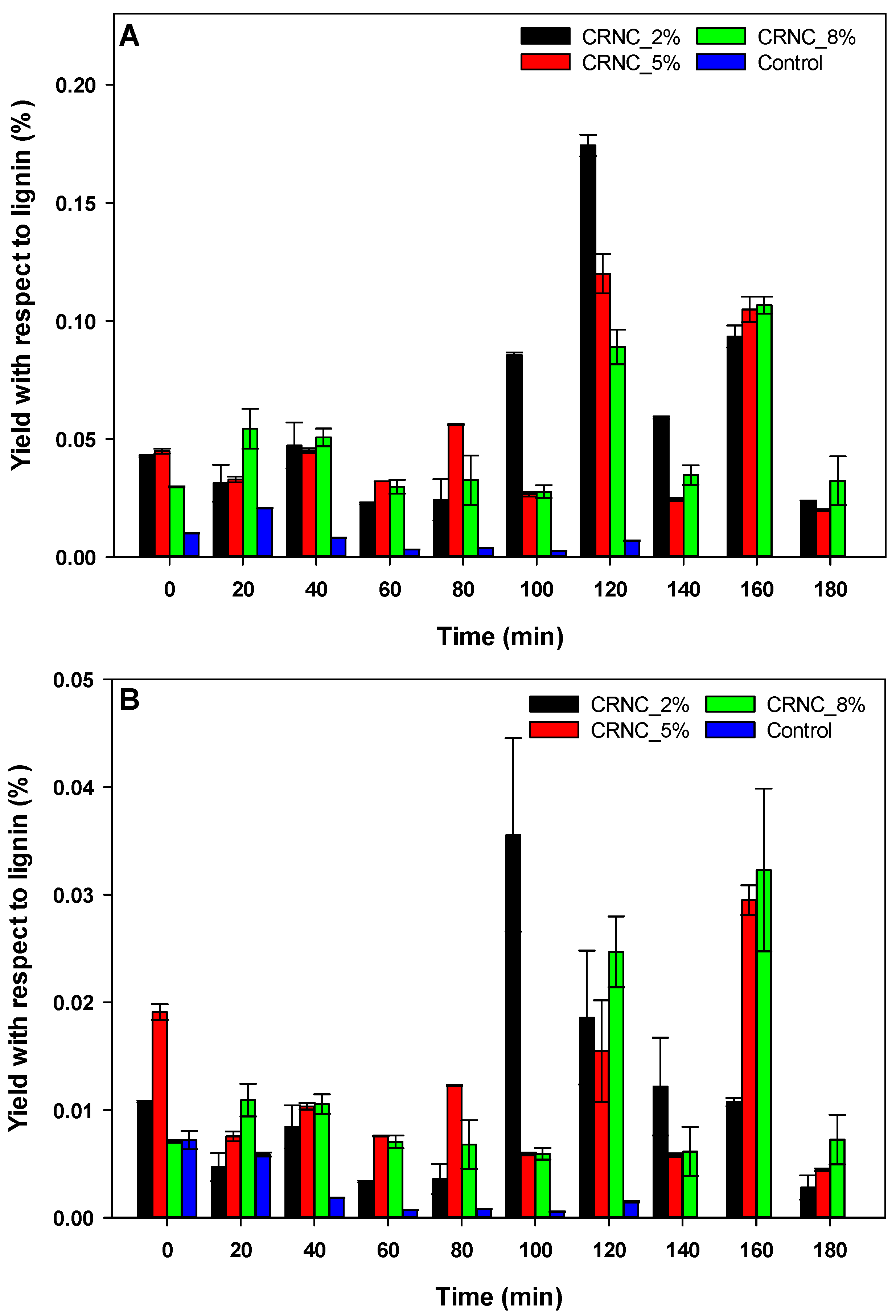
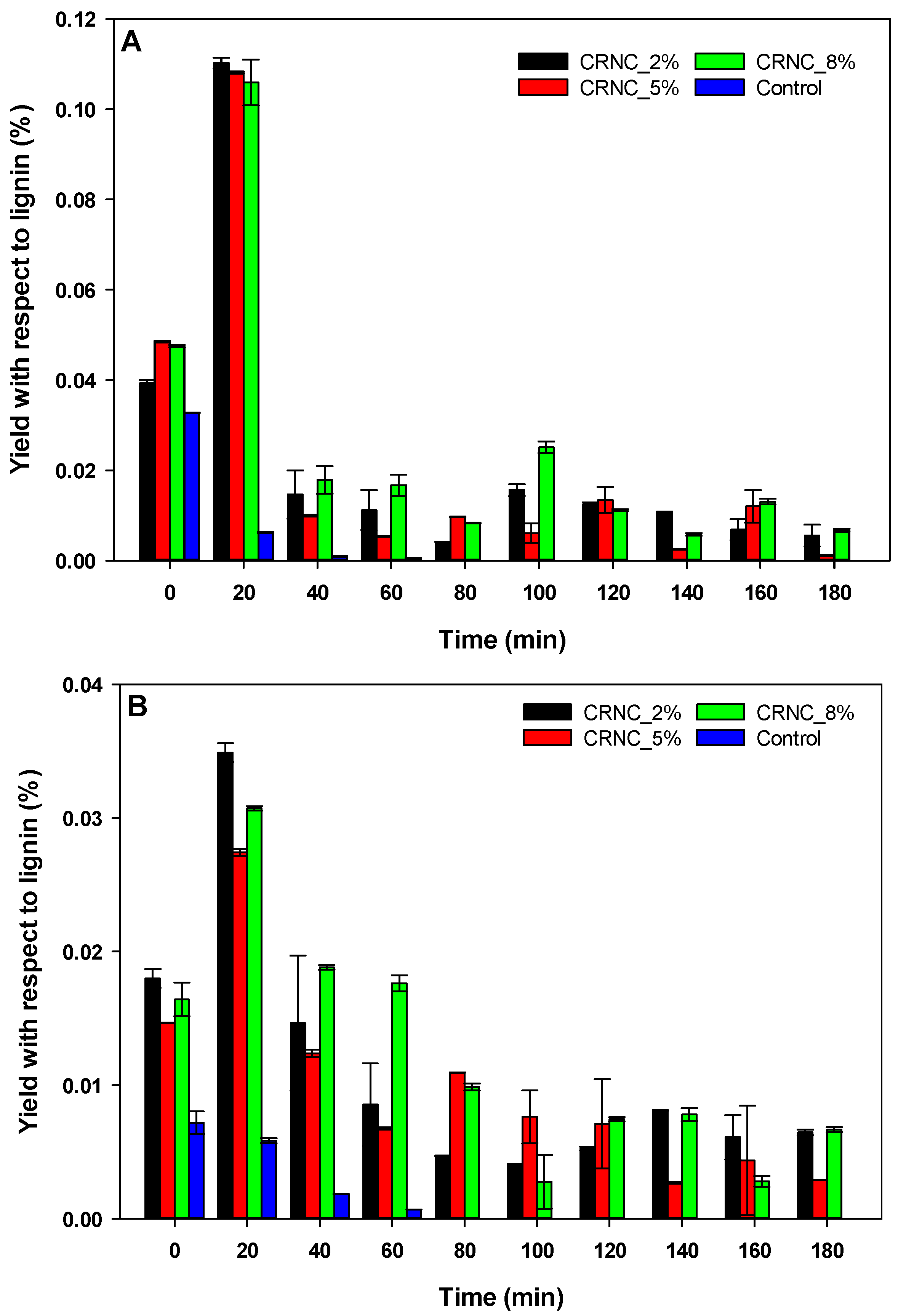
| Sample | Niobium Loading | |||||
|---|---|---|---|---|---|---|
| 2% Niobium Loading | 5% Niobium Loading | 8% Niobium Loading | ||||
| Vanillin Yield (%) | Acetovanillone Yield (%) | Vanillin Yield (%) | Acetovanillone Yield (%) | Vanillin Yield (%) | Acetovanillone Yield (%) | |
| OSNC | 0.05 | 0.01 | 0.1 | 0.01 | 0.05 | 0.01 |
| CRNC | 0.17 | 0.03 | 0.1 | 0.02 | 0.09 | 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, L.; Kolar, P.; Sharma-Shivappa, R.; Classen, J.J.; Osborne, J.A. Oxidative Depolymerization of Lignin Using Supported Niobium Catalysts. ChemEngineering 2017, 1, 17. https://doi.org/10.3390/chemengineering1020017
Das L, Kolar P, Sharma-Shivappa R, Classen JJ, Osborne JA. Oxidative Depolymerization of Lignin Using Supported Niobium Catalysts. ChemEngineering. 2017; 1(2):17. https://doi.org/10.3390/chemengineering1020017
Chicago/Turabian StyleDas, Lalitendu, Praveen Kolar, Ratna Sharma-Shivappa, John J. Classen, and Jason A. Osborne. 2017. "Oxidative Depolymerization of Lignin Using Supported Niobium Catalysts" ChemEngineering 1, no. 2: 17. https://doi.org/10.3390/chemengineering1020017
APA StyleDas, L., Kolar, P., Sharma-Shivappa, R., Classen, J. J., & Osborne, J. A. (2017). Oxidative Depolymerization of Lignin Using Supported Niobium Catalysts. ChemEngineering, 1(2), 17. https://doi.org/10.3390/chemengineering1020017






