Abstract
Camellia sinensis (green tea) is used in traditional medicine to treat a wide range of ailments. In the present study, the insulin-releasing and glucose-lowering effects of the ethanol extract of Camellia sinensis (EECS), along with molecular mechanism/s of action, were investigated in vitro and in vivo. The insulin secretion was measured using clonal pancreatic BRIN BD11 β cells, and mouse islets. In vitro models examined the additional glucose-lowering properties of EECS, and 3T3L1 adipocytes were used to assess glucose uptake and insulin action. Non-toxic doses of EECS increased insulin secretion in a concentration-dependent manner, and this regulatory effect was similar to that of glucagon-like peptide 1 (GLP-1). The insulin release was further enhanced when combined with isobutylmethylxanthine (IBMX), tolbutamide or 30 mM KCl, but was decreased in the presence of verapamil, diazoxide and Ca2+ chelation. EECS also depolarized the β-cell membrane and elevated intracellular Ca2+, suggesting the involvement of a KATP-dependent pathway. Furthermore, EECS increased glucose uptake and insulin action in 3T3-L1 cells and inhibited dipeptidyl peptidase IV (DPP-IV) enzyme activity, starch digestion and protein glycation in vitro. Oral administration of EECS improved glucose tolerance and plasma insulin as well as inhibited plasma DPP-IV and increased active GLP-1 (7–36) levels in high-fat-diet-fed rats. Flavonoids and other phytochemicals present in EECS could be responsible for these effects. Further research on the mechanism of action of EECS compounds could lead to the development of cost-effective treatments for type 2 diabetes.
1. Introduction
There has been a significant rise in the demand for herbal medicines in recent years, especially in developing countries, because of their availability, affordability and relative lack of adverse effects compared to conventional drugs. Countries within the Southeast Asian subcontinent have a long history of using plants in traditional medicine to treat a wide range of ailments. The recent use of medicinal plants for the treatment of diabetes has gained prominence [1,2].
Diabetes mellitus (DM), a chronic condition involving defective metabolism of carbohydrates, lipids and proteins, is one of the most prevalent non-communicable diseases around the world [3]. The number of diabetes patients is rising at an alarming rate, with approximately 537 million people currently living with diabetes. This number is expected to exceed 783 million in the next 25 years. DM can be classified into three types—type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and gestational diabetes, with 10% of DM cases being type 1 and 90% of all cases being type 2 [4,5]. T1DM, which is more prevalent in children and adolescents, is characterized as an autoimmune attack on pancreatic β cells that results in complete deficiency of insulin [5]. T2DM, which is defined as inadequate insulin production, impaired insulin signalling or both, primarily affects individuals over the age of 40 [6]. Insulin resistance, obesity, chronic inflammation, oxidative stress and hyperglycaemia form the basic pathophysiology of T2DM. In addition to these, genetic factors and lifestyle choices also contribute to the prevalence of diabetes [6]. Gestational diabetes is typically diagnosed in women who are at high risk of developing T2DM due to factors such as obesity and physical inactivity [5]. Obesity-associated insulin resistance is a major contributor to the development of T2DM. The excess accumulation of fat in adipose tissues produces non-esterified fatty acids and pro-inflammatory cytokines, which result in insulin resistance and pancreatic β-cell destruction, ultimately leading to T2DM [7]. Obesity also deteriorates type 2 diabetic complications, including cardiovascular diseases (CVDs) and chronic kidney disease (CKD) [8,9].
The main treatment for T2DM includes adherence to a specific diet to control body weight along with synthetic oral drugs. Current therapies for T2DM include different classes of drugs, such as metformin, sulphonylureas, thiazolidinediones, GLP-1 mimetics, DPP-IV inhibitors and sodium-glucose cotransporter-2 (SGLT2) inhibitors [10]. Among these, DPP-IV inhibitors have gained popularity in recent years due to their ability to improve glycaemic control by reducing glucagon release and enhancing insulin secretion [11]. DPP-IV inhibitors work by inactivating the dipeptidyl peptidase IV (DPP-IV) enzyme, which is responsible for suppressing the activity of incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) by cleaving in the N terminal and producing GIP (3–42) and GLP-1 (9–36) [12]. Following nutritional consumption, these hormones are produced from the intestine and bind to specific receptors on pancreatic β cells. This interaction promotes insulin secretion from β cells by activating the cyclic adenosine monophosphate (cAMP) pathway. Thus, the use of DPP-IV inhibitors to manage post-prandial hyperglycaemia is a popular approach [13]. However, synthetic DPP-IV inhibitors and other anti-hyperglycaemic agents are often expensive, have limited availability in poorer regions and come with various adverse effects. Alternative approaches to treat T2DM, such as herbal therapy and dietary supplements, have received increased attention recently, especially in lower-income nations [14].
Camellia sinensis, commonly known as green tea, is a medicinal plant traditionally used to treat various health conditions, including diabetes, arthritis, bacterial infections and hyperlipidaemia [15]. C. sinensis has been demonstrated to decrease total and low-density lipoprotein (LDL) cholesterol as well as triacylglyceride levels, and increase high-density lipoprotein (HDL) cholesterol, thus lowering the risk of developing CVD [16,17]. Previous reports also showed that C. sinensis can inhibit DPP-IV enzyme activity and has blood-glucose-lowering and insulin secretory properties [6,18,19,20]. Recent studies have reported that C. sinensis contains phytochemicals such as epicatechin, isoquercitrin, rutin, catechin, epicatechin gallate, quercetin, kaempferol, epigallocatechin gallate, ellagic acid, myricetin and gallic acid [6,21,22,23]. Among these, kaempferol, rutin, isoquercitrin, epicatechin, quercetin, gallic acid and catechin have been previously observed to have DPP-IV enzyme inhibitory properties [24,25,26]. Additionally, previous studies have shown that kaempferol, rutin, isoquercitrin, epicatechin, quercetin, ellagic acid and epigallocatechin gallate can lower blood glucose levels, enhance insulin secretion and improve β-cell function [27,28,29,30,31,32,33]. However, there is little information available on the mode of action responsible for the antidiabetic activity of green tea. In this study, in vitro and in vivo methods were used to evaluate the anti-hyperglycaemic effects of the ethanol extract of C. sinensis (EECS) leaves and unravel their possible mechanism/s of action.
2. Materials and Methods
2.1. Collection and Extraction
The leaves of C. sinensis were collected from Jahangirnagar University, Dhaka, Bangladesh, authenticated by a botanical taxonomist at Bangladesh National Herbarium (Mirpur, Dhaka) and assigned the accession number 43,207. The leaves were thoroughly rinsed, air-dried and powdered. The plant material (200 g) was soaked in 1 L of 80% (v/v) ethanol and kept on a shaker at 900 g for 48–72 h at room temperature. Following filtration using filter paper (Whatman no. 1), the extract was dried under reduced pressure at <40 °C to afford a sticky residue that was freeze-dried (Savant Speed vac, New York, NY, USA), and then stored at 4 °C until further studies [34].
2.2. In Vitro Studies on Insulin Release
Insulin-secreting clonal pancreatic BRIN-BD11 β cells, generated via electrofusion of primary β cells obtained from rat pancreatic islets (New England Deaconess Hospital) with immortal RINm5F cells [35,36] and isolated mouse islets [37], were employed to assess the insulin secretory effects of the ethanol extract of C. sinensis (EECS). Using collagenase P obtained from Clostridium histolyticum, islets from mice pancreas were extracted, and cultured in a CO2 incubator at 37 °C for 48 h [38]. EECS was incubated in the presence or absence of known insulin secretagogues at 37 °C for 20 and 60 min at various glucose concentrations (5.6 and 16.7 mM) [30]. Samples for the insulin radioimmunoassay were aliquoted and stored at −20 °C until further analysis [39]. To measure the insulin content of the islet cells, an acid–ethanol extraction method was implemented [38].
2.3. Membrane Potential and Intracellular Calcium Ions Concentration
The effects of EECS on the membrane potential and intracellular calcium [Ca2+]i in BRIN-BD11 cells were evaluated using a FLIPR Membrane Potential and Calcium Assay Kit (Molecular Devices, Sunnyvale, CA, USA) [33]. BRIN-BD11 cells were seeded into microplates with ninety-six wells and allowed to stand for 18 h in a CO2 incubator at 37 °C. A Krebs-Ringer Bicarbonate (KRB) buffer solution (100 μL each well) was added, and the mixture was incubated at 37 °C for 10 min. Depolarizing concentrations of 30 mM KCl and 10 mM alanine were used as the positive controls. A Flex Station 3 fluorometer was used to detect the variations in signal intensity caused by EECS [6]. Variations in signal intensity were detected at excitation, emission and cut-off wavelengths of 530 nm, 565 nm and 550 nm, respectively, for membrane potential and 485 nm, 525 nm and 515 nm, respectively, for intracellular calcium [35].
2.4. Assay for Cellular Glucose Uptake
The effects of EECS on cellular glucose uptake were investigated using differentiated 3T3L1 cells [40]. The 3T3-L1 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Dulbecco’s modified eagle media (DMEM) was made by adding penicillin (50 U/mL), streptomycin (50 μL/mL) and foetal bovine serum (10% v/v) to DMEM. The differentiated 3T3-L1 cells were incubated with serum-free DMEM for 2 h at 37 °C, at an atmosphere of 5% CO2. The DMEM medium was discarded after incubation. The cells were further incubated in Krebs-Ringer Bicarbonate (KRB) buffer for 30 min at 37 °C, in 5% CO2 and 95% air. The cells were treated with 50 µL of EECS (200 µg/mL) at 37 °C for 30 min with or without 100 nM insulin, and then 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) (50 nM) was added. After allowing the solution to stand for 5 min, the cells were washed with ice-cold PBS. Coverslips were placed over the slides, and the corners were securely sealed. Images of the four corners of the coverslips were captured using a microscope (10× magnification), and the fluorescence intensity was measured as described previously [41].
2.5. Insulin Glycation
The effects of EECS on in vitro insulin glycation were assessed as previously described [42]. A solution (1 mL) was prepared by mixing D-glucose (246.5 mM), human insulin (1 mg/mL), sodium phosphate buffer (10 mM, pH 7.4) and NaBH3CN (0.0853 gm/mL), with or without EECS (50–200 µg/mL). After 24 h of incubation at 37 °C, the reaction was stopped with 30 µL of 0.5 M acetic acid. The glycated and non-glycated insulin were separated by loading 200 μL of reaction mixture into a (250 × 4.6 mm) Vydac (C-18) analytical column (The Separations Group, California, USA), and then elution was performed at a flow rate of 1 mL/min. The mobile phase consisted of two solvents—solvent A (0.12% (v/v) TFA/H2O) and solvent B (0.1% (v/v) TFA in 70% acetonitrile + 29.9% H2O). To separate the glycated and non-glycated insulin, a linear gradient of 0–35% (v/v) acetonitrile for 10 min, followed by 35–56% (v/v) acetonitrile for 20 min and finally 56–70% acetonitrile for 5 min was established. At 214 and 208 nm, elution profiles were detected using RP-HPLC. The insulin glycation inhibitor aminoguanidine was used as a positive control [43].
2.6. In Vitro Dipeptidyl Peptidase-IV Enzyme Activity
The in vitro dipeptidyl peptidase-IV (DPP-IV) enzyme activity was measured using a fluorometer according to the procedures described previously [44]. Tris-HCl (100 mM) buffer was made by mixing 0.2 M Tris-HCl and 0.1 M NaCl. The pH of this buffer was balanced to 8.0 by adding a 100 mM Tris-base as required. The test reagents, DPP-IV enzyme (8 mU/mL) and Gly-Pro-AMC (200 µM), were dissolved in the buffer and incubated in 96-well black-walled, clear-bottomed microplates (Greiner) with or without EECS (40–5000 µg/mL). The fluorescence intensity was measured using a Flex Station 3 (Molecular Devices, San Jose, CA, USA) with a 2.5 nm slit width and excitation and emission wavelengths of 370 nm and 440 nm, respectively. The standard drug sitagliptin was used as a positive control [34].
2.7. In Vitro Digestion of Starch
This in vitro assay was performed to determine the effects of EECS on starch digestion using a previously published protocol [45]. Briefly, heat-stable α-amylase from Bacillus leicheniformis (40 µL of 0.01%) (Sigma-Aldrich, St. Louis, MO, USA) was added to a starch solution (2 mg/mL; 100 mg in 50 mL water), with or without EECS (62.5–1000 µg/mL) and incubated at 80 °C for 20 min. The diluted solution was then treated with amyloglucosidase from Rhizopus mold (30 µL of 0.1%) (Sigma-Aldrich, St. Louis, MO, USA) at 60 °C for 30 min. The samples were kept at 4 °C until analysis and the glucose release was measured using the liquid glucose oxidase-phenol amino phenazone (GOD/PAP) (Randox GL 2623) method [42]. The α-glucosidase inhibitor acarbose was used as a positive control.
2.8. In Vitro Glucose Diffusion
The in vitro glucose diffusion and absorption was evaluated using a cellulose ester dialysis tube (CEDT) (20 cm × 7.5 mm, Spectra/Por®CE layer, MWCO: 2000, Spectrum, Breda, The Netherlands) containing 2 mL of 0.9% NaCl and 220 mM glucose with or without EECS (0.2–25 mg/mL) [35]. The ends were sealed tightly, and the CEDT was placed inside 50 mL Falcon conical tubes (Orange Scientific, Orange, CA, USA) containing 0.9% NaCl (45 mL). Samples were removed from the orbital shaker after 24 h at 37 °C for glucose analysis, as described before [34].
2.9. Animals
Sprague Dawley male rats (Envigo UK, around 200–250 g, 6–8 weeks old) were fed a high-fat diet (HFF) (20% protein, 45% fat and 35 % carbohydrate: 26.15 KJ/g total energy percent) (Special Diet Service, Essex, UK) for 6–8 weeks prior to the start of the studies. Age-matched rats fed a standard rodent diet (10% fat, 30% protein and 60% carbohydrate: 12.99 KJ/g total energy) (Trouw Nutrition, Cheshire, UK) were used as normal controls. In total, 108 rats, including normal and high-fat-diet-fed rats, were used in this study. The animals were housed in an environment under controlled temperature and humidity (25 ± 0.5 °C and 65–70 %). The animal housing was equipped with an automatic 12 h light-on/off mechanism that maintained a day–night circadian rhythm. The Ulster University’s Animal Welfare and Ethical Review Board (AWERB) granted approval for experiments to be conducted on animals in May 2018, and the experiments were carried out under project/personal license numbers PIL1822 and PPL 2804 issued by the UK Home Office in May 2016 and February 2017, respectively. All experiments were carried out in compliance with UK Act 1986 and EU Directive 2010/63EU. All precautions were taken to guarantee that no animals would be harmed during the study.
2.10. Acute Oral Glucose Tolerance Test
The effects of EECS on oral glucose tolerance were evaluated in high-fat-diet (HFF)-fed rats. The rats were fasted overnight, and blood samples were collected using tail vein bleeding. Blood samples were taken at specific time intervals prior to (0 min) and after (30, 60, 120 and 180 min) oral administration of glucose (18 mmol/kg body weight) with/without EECS (250 mg/5 mL/kg). After centrifuging the blood for 5 min at 12,000 rpm at 4°C, the plasma was collected and kept at −20 °C until the insulin assay was performed. Blood glucose levels were monitored using Ascencia Contour glucose meters (Bayer, Newbury, UK), and insulin levels were assessed using a dextran–charcoal radioimmunoassay [46].
2.11. In Vivo Dipeptidyl Peptidase-IV Enzyme Activity
The effects of EECS on DPP-IV enzyme activity were evaluated in the plasma of animals using a fluorometric assay [30]. HFF rats were fasted overnight, and blood samples were obtained at a specific time interval before (0 min) and after (30, 60, 120 and 180) oral administration of EECS (250 mg/5 mL/kg), DPP-IV inhibitors sitagliptin (10 µmoL/5 mL/kg) and vildagliptin (10 µmoL/5 mL/kg) or saline control. Centrifugation was performed to collect plasma serum. In 96-well microplates, plasma samples (10 μL) were incubated with 40 μL of Tris-HCl (100 mM) buffer (pH 7.4) and 50 μL of Gly-Pro-AMC (200 μM) substrate at 37 °C for 30 min. When the DPP-IV enzyme in the blood serum hydrolyzed the fluorogenic substrate bonds (H-Gly-Pro) that were conjugated to the AMC group (H-Gly-Pro-AMC), the fluorescent 7-Amino-4-Methyl Coumarin (AMC) was produced. FlexStation 3 was used to measure the fluorescence changes as stated above in the in vitro DPP-IV enzyme activity section. Active GLP-1 (7–36) levels were quantified in plasma samples obtained at 30 min using a GLP-1 (Active) ELISA Kit (EGLP-35K, Merck Millipore, Dorset, UK).
2.12. Feeding Test
The effects of EECS on food intake were observed in HFF rats. Rats were starved for 12 h before the experiment was carried out. The food intake was measured at 0, 30, 60, 90, 120, 150 and 180 min before or after oral administration of saline (5 mL/kg), EECS (250 and 500 mg/5 mL/kg) and glibenclamide (100 mg/5 mL/kg), respectively. The standard drug glibenclamide was used as a positive control.
2.13. Metabolic Studies
Metabolic studies in HFF rats were performed using metabolic cages to measure food and fluid intake, stools and urine. The rats underwent a 24 h adaption period followed by a 12 h fasting period. EECS (250 and 500 mg/5 mL/kg) was fed to the treatment groups while the positive control group was fed glibenclamide (100 mg/5 mL/kg), and the HFF control group was given saline (5 mL/kg) individually. The food and fluid intake of each group was measured and the amount of stool and urine excreted out recorded. These four factors were measured at 1 h intervals over a period of 6 h initially, followed by 2 h intervals over the next 6 h and then after 24 h.
2.14. Gut Motility
Gastrointestinal motility was measured using a BaSO4 milk solution (10% BaSO4 w/v in 0.5% Na-CMC), as previously described [47]. Rats were starved for 20 h. One hour before consuming the BaSO4 solution, the treatment groups received EECS (250 and 500 mg/5 mL/kg), bisacodyl (10 mg/5 mL/kg) and loperamide (5 mg/5 mL/kg). The animals were euthanized 15 min after receiving the BaSO4 milk solution, and the whole intestine was isolated. The distance travelled by BaSO4 was measured and calculated as a percentage of a total length of the small intestine (from the pylorus to the ileocecal junction).
2.15. Statistical Analysis
Graph Pad prism 5 was used to analyze and interpret the raw data. The unpaired Student’s t-test (non-parametric, with two-tailed p values) and one-way ANOVA with Bonferroni post hoc tests were used to analyze the data. All values are expressed as mean ± SEM with a hypothetical statistical significance limit of p < 0.05.
2.16. Phytochemical Screening
Phytochemical screening of EECS was conducted to demonstrate the presence or absence of flavonoids, alkaloids, saponins, tannins, glycosides, reducing sugar and steroids, as described previously [48]. To test for alkaloids, 2 mL of EECS was acidified using hydrochloric acid (HCl), and 1 mL of Dragendroff’s reagent was added to test for the appearance of a red colour, suggesting the presence of alkaloids. For tannins, a few drops of 10% lead acetate were added to 2 mL of EECS, and this caused the formation of a white sediment, suggesting the presence of tannins. Flavonoid testing was conducted by adding 1.5 mL of methanol to 4 mL of EECS and then heating this mixture; when metal magnesium and a few drops of HCL were added to this, a pink colour appeared, indicating the presence of flavonoids. To test for saponins, 1 mL of EECS was added to 9 mL of distilled water, and this resulted in the formation of a stable foam, indicating the presence of saponins. To test for steroids, 2 mL of EECS was mixed with 10 mL of chloroform, 1 mL of acetic anhydride and 2 mL of sulphuric acid to visualize a bluish-green colour, which indicates the presence of steroids. For glycoside testing, 1 mL of EECS was mixed with a few drops of glacial acetic acid, ferric chloride and concentrated sulphuric acid to test for the appearance of a bluish-green colour, indicating the presence of glycosides. To test for reducing sugar, 1 mL of EECS, 1 mL of water and few drops of Fehling’s reagent were mixed together and heated, and we looked for the appearance of a red-brick colour indicating the presence of reducing sugars [48].
3. Results
3.1. EECS and Insulin Release from BRIN BD11 Cells
Figure 1A,B illustrate the effects of EECS on insulin release from BRIN-BD11 cells in a concentration (1.6–5000 µg/mL)-dependent manner. At 5.6 mM glucose, the basal rate of insulin release from BRIN-BD11 cells was 1.16 ± 0.10 ng/106 cells/20 min. Using alanine (10 mM) as a positive control, the rate increased to 6.65 ± 0.29 ng/106 cells/20 min (Figure 1A; p < 0.001). At 5.6 mM glucose, EECS stimulated insulin release from 1.62 ± 0.18 to 6.09 ± 0.34 ng/106 cells/20 min (Figure 1A; p < 0.05–0.001) in a dose-dependent manner (1.6–5000 µg/mL). The basal insulin rate at 16.7 mM glucose was 2.04 ± 0.14 ng/106 cells/20 min and with the positive control, KCl (30 mM), the rate was increased to 8.50 ± 0.42 ng/106 cells/20 min (Figure 1B; p < 0.001). At 16.7 mM glucose, EECS induced insulin release from 2.80 ± 0.41 to 7.02 ± 0.44 ng/106 cells/20 min (Figure 1B; p < 0.05–0.001) at 8–5000 µg/mL. No significant effect of EECS on lactate dehydrogenase release was found at concentrations ranging from 1.6 to 200 µg/mL.
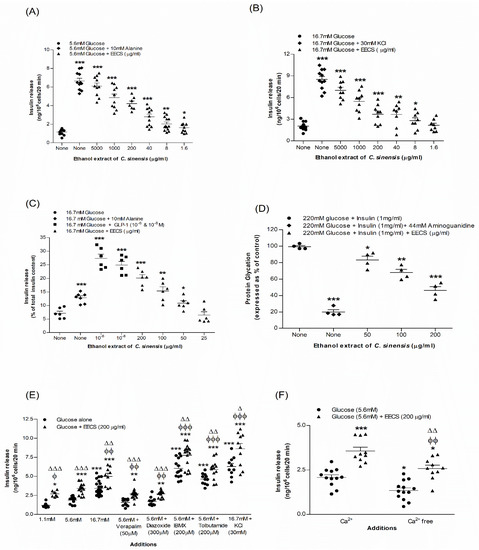
Figure 1.
Effects of EECS on insulin secretion from (A,B) clonal pancreatic BRIN-BD11 β cells and (C) islets of Langerhans, (D) glycation of protein (E), secretion of insulin with known stimulators/inhibitors and (F) plus/minus extracellular calcium from BRIN-BD11 cells. Values n = 4–8 for insulin secretion and glycation of protein are mean ± SEM. *, **, *** p < 0.05–0.001 compared to control. ϕ, ϕϕ, ϕϕϕ p < 0.05–0.001 compared to 5.6 mM glucose with EECS. Δ, ΔΔ, ΔΔΔ p < 0.05–0.001 compared to respective incubation without EECS. EECS, ethanol extract of C. sinensis.
3.2. EECS and Insulin Release from Isolated Mouse Islets
Figure 1C shows the effects of EECS on insulin release from the isolated mouse islets. At 16.7 mM glucose, the basal rate of insulin release from isolated mouse islets was 7.15 ± 0.78 ng/106 cells/20 min (Figure 1C). EECS stimulated insulin release from 10.85 ± 0.88 to 20.12 ± 1.24 ng/106 cells/20 min (Figure 1C; p < 0.05–0.001) in a concentration-dependent manner (50–200 µg/mL). The positive controls alanine (10 mM) and GLP-1 (10−6 and 10−8 M) showed a significant (p < 0.001) increase in insulin release from 12.85 ± 0.70 to 27.35 ± 1.55 (Figure 1C). However, in comparison to alanine, the insulin release caused by GLP-1 was more potent (p < 0.001).
3.3. EECS and Known Modulators/Inhibitors of Insulin Release
EECS significantly enhanced insulin release in the presence of insulin modulators, including glucose (p < 0.001), isobutylmethylxanthine (IBMX; p < 0.001) and tolbutamide (p < 0.001) (Figure 1E). In the presence of a depolarizing concentration of KCl, EECS also induced a substantial increase in insulin release (p < 0.001; Figure 1E). Diazoxide, verapamil and Ca2+-free conditions attenuated, but did not completely abolish, this effect (p < 0.01; Figure 1E,F).
3.4. EECS and Cell Membrane Depolarization and [Ca2+]i Concentration
The effects of EECS on membrane potential and intracellular calcium concentrations ([ Ca2+]i) were evaluated using BRIN-BD11 cells (Figure 2A,B). At a concentration of 200 μg/mL, EECS significantly depolarized the cell membrane (p < 0.001; Figure 2A) and increased intracellular calcium ion concentration (p < 0.001; Figure 2B). The positive controls KCl (30 mM) and alanine (10 mM) showed a greater response on the membrane potential (p < 0.001; Figure 2A) and intracellular calcium (p < 0.001; Figure 2B), respectively.
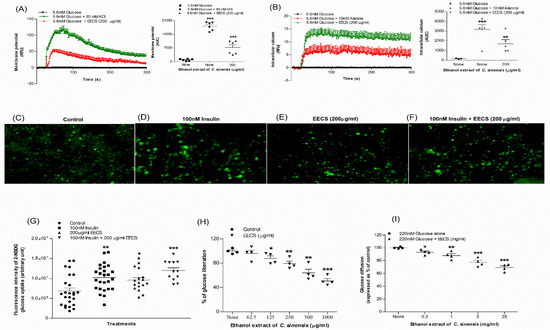
Figure 2.
Effects of EECS on (A) membrane potential and (B) intracellular calcium in clonal pancreatic BRIN BD11 β cells and (C–G) glucose uptake, (H) starch digestion and (I) glucose diffusion in vitro. Changes in fluorescence intensity in differentiated 3T3L1 adipocyte incubated with EECS (E) minus or (F) plus 100 nM insulin. Magnification of 10x was used to capture the images. Values n = 6 for membrane potential and intracellular calcium, n = 4 for uptake of glucose, digestion of starch and diffusion of glucose are mean ± SEM. *, **, *** p < 0.05–0.001 compared to control.
3.5. EECS and Insulin Glycation
3.6. EECS and Glucose Uptake
The uptake of glucose in 3T3L1 adipocyte cells was assessed using fluorescent 2-NBDG (2-(N-(7-Nitrobenz-2-oxazol-4-yl) Amino)-2-Deoxyglucose). The microscopic fluorescence intensity of 2-NBDG uptake is shown in Figure 2C–F. In differentiated 3T3L1 adipocyte cells, EECS (200 μg/mL) improved glucose uptake with/without 100 nM insulin (p < 0.05; p < 0.001; Figure 2G). Glucose uptake was also considerably enhanced by 100 nM insulin alone (p < 0.01; Figure 2G).
3.7. EECS and Starch Digestion
EECS decreased starch digestion by 12–54% (p < 0.05–0.001; Figure 2H) in a dose-dependent manner (125–1000 µg/mL). The positive control acarbose (1000 µg/mL) inhibited starch digestion by 79% (data not shown).
3.8. EECS and In Vitro Glucose Diffusion
3.9. EECS and In Vitro Dipeptidyl Peptidase-IV Enzyme Activity
The effects of EECS on in vitro DPP-IV enzyme activity are illustrated in Figure 3A. In the presence of the DPP-IV enzyme, EECS (40–5000 μg/mL) showed a significant (p < 0.05–0.001) decrease (16–72%) in AMC liberation from Gly-Pro-AMC (Figure 3A). The DPP-IV inhibitor sitagliptin significantly (p < 0.001) attenuated (98%) AMC liberation from Gly-Pro-AMC (data not shown).
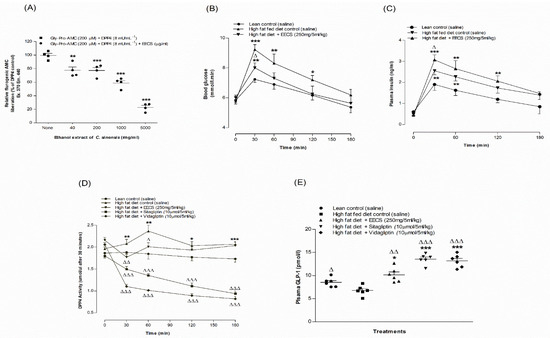
Figure 3.
Effects of EECS on (A) DPP-IV enzyme in vitro, (B) glucose tolerance, (C) plasma insulin, (D) DPP-IV and (E) active GLP-1 (7–36) in high-fat-diet-fed rats. In vivo parameters were evaluated before and after oral administration of glucose alone (18 mmol/kg body weight, control) or with EECS (250 mg/5 mL/kg body weight), sitagliptin and vidagliptin (both at 10 μmol/5 mL/kg, body weight). Plasma active GLP-1 (7–36) levels were measured 30 min following treatment. Values n = 4 for in vitro DPP-IV enzyme activity and n = 6 for in vivo parameters are mean ± SEM. *, **, *** p < 0.05–0.001 compared to control and Δ, ΔΔ, ΔΔΔ p < 0.05–0.001 compared to high-fat-diet-fed control rats.
3.10. EECS and Oral Glucose Tolerance and Plasma Insulin Levels
Oral gavage of EECS (250 mg/5 mL/kg) in combination with glucose (18 mmoL/5 mL/kg body weight) significantly improved oral glucose tolerance at 30 and 60 min in HFF rats (p < 0.05; Figure 3B) compared to the control. EECS (250 mg/5 mL/kg) also significantly ameliorated plasma insulin levels at 30 min in HFF rats (p < 0.05; Figure 3C).
3.11. EECS and Plasma DPP-IV Enzyme Activity and Active GLP-1 (7–36) Levels
Oral gavage of EECS (250 mg/ 5 mL/kg, body weight) significantly decreased plasma DPP-IV enzyme activity at 30 and 60 min (p < 0.05–0.01; Figure 3D) compared to HFF rats. Interestingly, there was a consistent reduction in plasma DPP-IV enzyme activity in the presence of sitagliptin (10 µmoL/5 mL/kg) and vidagliptin (10 µmoL/5 mL/kg) in a time-dependent manner (p < 0.001; Figure 3D). Oral administration of EECS (250 mg/5 mL/kg body weight) elevated plasma active GLP-1 (7–36) levels in the circulation by 32% (p < 0.01; Figure 3E), and this was increased to 82–90% (p < 0.001; Figure 3E) with sitagliptin (10 µmoL/5 mL/kg) and vidagliptin (10 µmoL/5 mL/kg), respectively.
3.12. EECS and Feeding Test
EECS, at 500 mg/5 mL/kg, consistently reduced the food intake at most of the time points (p < 0.05; p < 0.001; Figure 4A), whereas at 250 mg/5 mL/kg, it significantly decreased the food intake only at 30 and 60 min (p < 0.001; Figure 4A). The sulfonylurea glibenclamide also substantially decreased the food intake in a time-dependent manner (p < 0.01–0.001; Figure 4A).
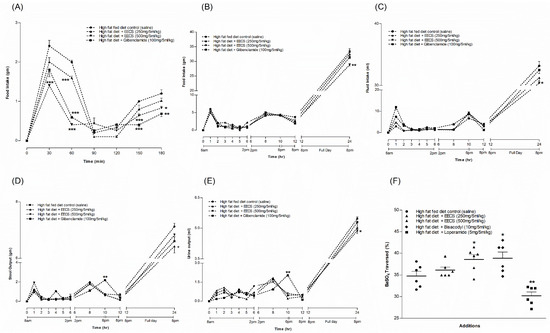
Figure 4.
Effects of EECS on (A) food intake during feeding test and (B) food intake, (C) fluid intake, (D) stool and (E) urine output after 24 h of metabolic study. (F) BaSO4 traversed. Values n = 6 for feeding test and metabolic parameters are mean ± SEM. *, **, *** p < 0.05–0.001 compared to high-fat-diet-fed control rats.
3.13. EECS and Metabolic Parameters
EECS (250 and 500 mg/5 mL/kg, b.w.) decreased food consumption at night (between 6 and 8 pm) and after 24 h (p < 0.01; Figure 4B) compared to HFF rats alone. EECS (250 and 500 mg/5 mL/kg, b.w.) also reduced fluid intake at night (between 6 pm and 8 pm) and after 24 h (p < 0.05; Figure 4C) compared to HFF rats alone. EECS also attenuated stool and urine output at night (between 6 and 8 pm) and after 24 h (p < 0.05; Figure 4D,E), which was consistent with food and fluid intake. The positive control, glibenclamide, improved these parameters in comparison to HFF rats alone (Figure 4B–E). Glibenclamide also significantly improved the frequency of stool and urine output at night (between 6 and 8 pm; p < 0.01; Figure 4D,E).
3.14. EECS and Gastrointestinal Motility
EECS (500 mg/5 mL/kg) significantly improved gastrointestinal motility (p < 0.05; Figure 4F). However, at 250 mg/5 mL/kg, it showed no significant improvement in gut motility (Figure 4F). The antidiarrheal drug loperamide (5 mg/5 mL/kg) decreased gut motility ((p < 0.01; Figure 4F), while the stimulant laxative bisacodyl (10 mg/5 mL/kg) increased gut motility (p < 0.05; Figure 4F).
3.15. EECS and Phytochemical Screening
To establish the presence of possible antidiabetic phytochemicals, further investigation was carried out. EECS was found to contain alkaloids, flavonoids, saponins and tannins (Table 1).

Table 1.
Phytochemical screening of ethanol extract of C. sinensis.
4. Discussion
Diabetes is one of the most widespread and devastating metabolic illnesses in the world, affecting millions of people [49]. Although T2DM can be managed using oral anti-hyperglycaemic agents, it often requires the use of synthetic insulin in the long term. Many oral antidiabetic drugs, such as sulfonylureas, biguanides, glinides, glycosidase and DPP-4 inhibitors, have adverse side-effects and/or are expensive. Moreover, the long-term use of insulin increases insulin receptor sensitivity and causes insulin resistance [35,50]. Natural products have become an important source of safer and more economical anti-hyperglycaemic drugs. Medicinal plants and their constituents (e.g., flavonoids) have been reported previously to exhibit antidiabetic properties, including insulin-releasing and glucose-lowering activities, inhibiting α-amylase and α-glucosidase and protecting and improving the function clonal pancreatic β cells [51,52]. The regular consumption of dietary fibres from plants has also been reported to reduce the incidence of diabetes [53].
Camellia sinensis has been reported to possess remarkable pharmacological activities in traditional medicine against various ailments including diabetes [54]. It was recently reported to have antidiabetic properties, and previous studies have demonstrated that C. sinensis lowers blood glucose levels, improves glucose tolerance and prevents hyperlipidaemia by reducing total cholesterol and LDL levels in diabetic animal models [55,56,57]. However, the exact mechanism of action of Camellia sinensis remains elusive. Our results reveal that EECS enhanced insulin secretion in a concentration-dependent manner from BRIN-BD11 cells and isolated mouse islets in response to glucose stimulation. Specific secretory pathways were targeted using insulin-releasing/inhibiting modulators to develop a better understanding of the mechanism of action of a non-toxic dose of EECS on β cells [58]. In the presence of the KATP channel opener diazoxide, the insulin-releasing activity of EECS was reduced, suggesting that C. sinensis might act via KATP, a channel-dependent pathway [59]. The voltage-dependent calcium channel blocker verapamil also reduced the insulin release mediated by EECS. These findings suggest that the mode of action of EECS involves the closing of KATP channels and the opening of L-type Ca2+ channels [60]. EECS also increased insulin release in the presence of the depolarizing concentration of KCl (30 mM) and the KATP channel blocker tolbutamide. These results support the ability of EECS to potentiate insulin secretion via other pathways, such as a direct effect on exocytosis, the phosphatidylinositol (PI3) or the adenylate cyclase/cAMP pathways [61]. The cAMP phosphodiesterase inhibitor IBMX also potentiated the insulin secretion induced by EECS, indicating a modulation of intracellular cAMP production [41]. Thus, EECS may be involved in the increase in cAMP levels in lung muscle tissue and the relaxation of smooth muscles in the airway passage [62].
Post-prandial glucose is controlled by insulin via glucose transporter 4 (GLUT4) translocation in skeletal muscle and adipose tissue [63]. Inadequate or defective signalling reduces GLUT4 translocation and leads to the development of insulin resistance [64]. In this study, we investigated the effect of EECS on glucose uptake in differentiated 3T3L1 adipocyte cells. EECS enhanced glucose uptake in the presence and absence of insulin. Previous studies have revealed that phytochemicals such as kaempferol, quercetin and gallic acid could activate the AMP-activated protein kinase (AMPK) pathway and enhance GLUT4 translocation [65,66]. The fact that C. sinensis is known to contain flavonoids, such as rutin, isoquercitrin and catechin, may explain how it can activate signalling pathways to enhance glucose transport in adipocytes with or without insulin [6,67,68].
The increased glycation of insulin has a vital role in the pathogenesis of numerous disease, such as diabetes, leading to the formation of advanced glycation end products (AGEs). AGEs accumulate in cells, resulting in impaired cell signalling, and increasing the severity of diabetes complications [69]. EECS was found to decrease insulin glycation in a dose-dependent manner. Phytochemicals isolated from C. sinensis, such as epigallocatechin gallate, isoquercitrin and rutin [6,70], have previously been observed to have anti-glycating properties [71,72,73]. Our results suggest that C. sinensis possesses potent anti-glycating activity. This is possibly due to the presence of polyphenolic compounds.
Numerous factors are involved in the pathophysiology of diabetes, including starch digestion by α-amylase and α-glucosidase and glucose absorption and diffusion in the gastrointestinal tract [73]. EECS significantly decreased starch digestion in a concentration-dependent manner. We hypothesize that rutin and isoquercitrin, known to be present in C. sinensis, might be responsible for such activity [74]. Previous studies showed that these flavonoids are effective against α-amylase and delay starch digestion [75]. EECS also demonstrated significant concentration-dependent inhibition of glucose absorption and diffusion, which is consistent with previous studies on a hot water extract of C. sinensis [6].
Obesity, a major risk factor of T2DM, is characterized by the presence of non-esterified fatty acids (NEFAs) released from adipose tissue that contribute to insulin resistance and β-cell dysfunction, causing T2DM [76]. In our study, EECS ameliorated oral glucose tolerance and plasma insulin levels in HFF obese rats. This is consistent with previous results showing that C. sinensis improves oral glucose tolerance, plasma insulin and β-cell function in HFF rats [6,77] and streptozotocin (STZ)-induced diabetic rats [48].
Our further in vivo studies using HFF rats showed that EECS reduced plasma DPP-IV enzyme activity, which is consistent with our in vitro results. EECS also enhanced active GLP-1 (7–36) levels in the circulation. GLP-1, an incretin hormone secreted by the intestine after a meal, plays a crucial role in maintaining post-prandial glucose homeostasis [78]. Thus, GLP-1 mimetics and DPP-IV inhibitors are important targets in T2DM drug discovery research. Interestingly, previous studies have revealed that flavonoids found in C. sinensis, such as rutin and isoquercitrin, have DPP-IV enzyme inhibitory activity [74,79,80].
Feeding test and metabolic studies were performed to observe the effects of EECS on different parameters, including food and fluid intake, as well as stool and urine output. EECS was found to reduce all of the four abovementioned parameters, specifically at night between 6 pm and 8 pm. Rats are most active at night and diabetic rats usually have the highest blood glucose levels at the night phase of their circadian rhythm [81]. The blood-glucose-lowering activity of EECS may be due to a reduction in food intake during that time period.
Phytochemical screening of EECS showed the presence of phytoconstituents including flavonoids, alkaloids, tannins and saponins. Recent reports also showed that C. sinensis consists of flavonoids such as rutin and isoquercitrin [67,68,82] and alkaloids [83], as well as tannins and saponins [84]. Flavonoids have previously been found to inhibit in vitro α-glucosidase activity, as well improve glucose tolerance, enhance insulin release and protect pancreatic β cells from oxidative stress damage in HFF- and STZ-induced diabetic rats [6,85,86,87]. In addition, alkaloids and saponins are known to regulate glucose homeostasis via the AMPK pathway; along with this, tannins have been observed to increase glucose uptake via the phosphatidylinositol (PI3) pathway [88,89,90]. The presence of these phytochemicals may have contributed to the insulin-releasing and glucose-lowering effects of EECS. Further investigations, particularly long-term animal studies, are required to fully understand the role of EECS in T2DM.
5. Conclusions
Our results suggest that EECS may increase the insulin secretion ability of clonal pancreatic β cells. EECS impeded glucose diffusion and absorption, insulin glycation and DPP-IV enzyme activity in vitro. In our in vivo studies, EECS improved glucose tolerance and plasma insulin levels, and decreased plasma DPP-IV enzymatic activity while elevating active GLP-1 levels in the circulation, showing that EECS may be involved in the augmentation of the GLP-1 and GIP half-life. The presence of flavonoids in C. sinensis, such as rutin and isoquercitrin, may be responsible for the insulin-releasing and glucose-lowering effects observed for EECS. Our findings validate, to a certain extent, the traditional use of Camellia sinensis as a dietary supplement for T2DM. Further studies, including the purification and identification of active compounds from EECS, may help researchers to discover new drug templates for the management of T2DM.
Author Contributions
P.A., Y.H.A.A.-W. and J.M.A.H. were equally responsible for the conception and design of the study as well as the supervision of the study; P.A., S.T.C., S.S.I. and A.T. conducted the experiments, analyzed the data, evaluated the results, and created the figures, and P.A. and S.T.C. drafted the paper, while P.A. and V.S. edited the revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Strategic Research Funding from Ulster University and a sponsored research project from Independent University, Bangladesh (IUB).
Institutional Review Board Statement
All protocols were authorized by Ulster University’s Animal Welfare and Ethical Review Board (AWERB) in May 2018 and were carried out under the UK Home Office Animal project/personal licence numbers PPL 2804 and PIL1822.
Informed Consent Statement
Not applicable.
Data Availability Statement
Due to certain restrictions, the information is unavailable to the public. The corresponding author can disclose the data obtained from this study upon request.
Acknowledgments
The authors are thankful to the Strategic Research Funding at Ulster University and the Independent University, Bangladesh, for facilitating the use of their laboratories.
Conflicts of Interest
According to the authors, there are no conflicts of interest associated with this manuscript.
References
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Scartezzini, P.; Speroni, E. Review on Some Plants of Indian Traditional Medicine with Antioxidant Activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Singh, N.; Kesherwani, R.; Tiwari, A.K.; Patel, D.K. A Review on Diabetes Mellitus. J. Pharm. Innov. 2016, 5, 36–40. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2014, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H. Anti-hyperglycaemic and insulin-releasing effects of Camellia sinensis leaves and isolation and characterization of active compounds. Brit. J. Nutr. 2020, 126, 1149–1163. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. Obesity and Kidney Disease. Can. J. Kidney Health Dis. 2017, 4, 205435811769866. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Hannan, J.M.A.; Flatt, P.R.; Abdel Wahab, Y.H.A. Anti-Hyperglycaemic Activity of H. Rosa-Sinensis Leaves Is Partly Mediated by Inhibition of Carbohydrate Digestion and Absorption, and Enhancement of Insulin Secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef]
- Vella, A. Mechanism of Action of DPP-4 Inhibitors—New Insights. J. Clin. Endocrinol. Metab. 2012, 97, 2626–2628. [Google Scholar] [CrossRef]
- Flatt, P.R. Dipeptidyl Peptidase IV (DPP IV) and Related Molecules in Type 2 Diabetes. Front. Biosci. 2008, 13, 3648. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Abdel Wahab, Y.H.A. Effects of Spirulina platensis on Insulin Secretion, Dipeptidyl Peptidase IV Activity and Both Carbohydrate Digestion and Absorption Indicate Potential as an Adjunctive Therapy for Diabetes. Brit. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green Tea (Camellia sinensis) and L -Theanine: Medicinal Values and Beneficial Applications in Humans—A Comprehensive Review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruoma, O.I. The Effect of Black Tea on Risk Factors of Cardiovascular Disease in a Normal Population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef]
- Hakim, I.A.; Alsaif, M.A.; Alduwaihy, M.; Al-Rubeaan, K.; Al-Nuaim, A.R.; Al-Attas, O.S. Tea Consumption and the Prevalence of Coronary Heart Disease in Saudi Adults: Results from a Saudi National Study. Prev. Med. 2003, 36, 64–70. [Google Scholar] [CrossRef]
- Fu, Q.-Y.; Li, Q.-S.; Lin, X.-M.; Qiao, R.-Y.; Yang, R.; Li, X.-M.; Dong, Z.-B.; Xiang, L.-P.; Zheng, X.-Q.; Lu, J.-L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef]
- Ekayanti, M.; Sauriasari, R.; Elya, B. Dipeptidyl Peptidase IV Inhibitory Activity of Fraction from White Tea Ethanolic Extract (Camellia sinensis (L.) Kuntze) Ex Vivo. Pharmacogn. J. 2017, 10, 190–193. [Google Scholar] [CrossRef]
- Fujimura, Y.; Watanabe, M.; Morikawa-Ichinose, T.; Fujino, K.; Yamamoto, M.; Nishioka, S.; Inoue, C.; Ogawa, F.; Yonekura, M.; Nakasone, A.; et al. Metabolic Profiling for Evaluating the Dipeptidyl Peptidase-IV Inhibitory Potency of Diverse Green Tea Cultivars and Determining Bioactivity-Related Ingredients and Combinations. J. Agric. Food Chem. 2022, 70, 6455–6466. [Google Scholar] [CrossRef]
- Nag, A.; Dhull, N.; Gupta, A. Evaluation of Tea (Camellia sinensis L.) Phytochemicals as Multi-Disease Modulators, a Multidimensional in Silico Strategy with the Combinations of Network Pharmacology, Pharmacophore Analysis, Statistics and Molecular Docking. Mol. Divers. 2022, 26, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Barreira, S.; Moutinho, C.; Silva, A.M.N.; Neves, J.; Seo, E.-J.; Hegazy, M.-E.F.; Efferth, T.; Gomes, L.R. Phytochemical Characterization and Biological Activities of Green Tea (Camellia sinensis) Produced in the Azores, Portugal. Phytomed. Plus 2021, 1, 100001. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and Citrus Phenolic Compounds Inhibit Dipeptidyl Peptidase IV: Implications in Diabetes Management. Evid. Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef] [PubMed]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Altyar, A.E.; Al-Azizi, M.M.; Ashour, M.L. A Comprehensive Insight on the Health Benefits and Phytoconstituents of Camellia sinensis and Recent Approaches for Its Quality Control. Antioxidants 2019, 8, 455. [Google Scholar] [CrossRef]
- Huang, P.-K.; Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Lee, D.-N.; Weng, C.-F. Natural Phenolic Compounds Potentiate Hypoglycemia via Inhibition of Dipeptidyl Peptidase IV. Sci. Rep. 2019, 9, 15585. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H. Effects of 22 Traditional Anti-Diabetic Medicinal Plants on DPP-IV Enzyme Activity and Glucose Homeostasis in High-Fat Fed Obese Diabetic Rats. Biosci. Rep. 2021, 41, BSR20203824. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the Treatment of Diabetes: A Comprehensive and Latest Review. Front. Endocrinol. 2022, 13, 990299. [Google Scholar] [CrossRef]
- Rakhmat, I.I.; Yuslianti, E.R.; Koswara, T. Flavonoid-Rutin Effect to Blood Glucose Level and Pancreas Regeneration in Diabetic Rats. In Proceedings of the 2th Annual Scientific Meeting, Medical Faculty, Universitas Jenderal Achmad Yani, International Symposium on “Emergency Preparedness and Disaster Response during COVID 19 Pandemic” (ASMC 2021), Virtual, 10–12 May 2021; pp. 64–66. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.; Li, W.-N.; Fu, X.-Q. Hypoglycemic Effect and Mechanism of Isoquercitrin as an Inhibitor of Dipeptidyl Peptidase-4 in Type 2 Diabetic Mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An Effective Polyphenol in Alleviating Diabetes and Diabetic Complications. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic Uses of Epicatechin in Diabetes and Cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Elbandrawy, M.M.; Sweef, O.; Elgamal, D.; Mohamed, T.M.; EhabTousson; Elgharabawy, R.M. Ellagic Acid Regulates Hyperglycemic State through Modulation of Pancreatic IL-6 and TNF- α Immunoexpression. Saudi J. Biol. Sci. 2022, 29, 3871–3880. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin Gallate Supplementation Alleviates Diabetes in Rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Seidel, V.; Abdel-Wahab, Y.H.A. In vitro and in vivo antihyperglycemic activity of the ethanol extract of Heritiera fomes bark and characterization of pharmacologically active phytomolecules. J. Pharm. Pharmacol. 2022, 3, 415–425. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef]
- McClenaghan, N.H.; Barnett, C.R.; O’Harte, F.P.M.; Flatt, P.R. Mechanisms of Amino Acid-Induced Insulin Secretion from the Glucose-Responsive BRIN-BD11 Pancreatic B-Cell Line. J. Endocrinol. 1996, 151, 349–357. [Google Scholar] [CrossRef]
- Ojo, O.O.; Srinivasan, D.K.; Owolabi, B.O.; Vasu, S.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Esculentin-2CHa-Related Peptides Modulate Islet Cell Function and Improve Glucose Tolerance in Mice with Diet-Induced Obesity and Insulin Resistance. PLoS ONE 2015, 10, e0141549. [Google Scholar] [CrossRef]
- Flatt, P.R.; Bailey, C.J. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 1981, 20, 573–577. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Flatt, P.R.; Conlon, J.M. Insulin releasing properties of the Temporin family of antimicrobial peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Antihyperglycemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. Brit. J. Nutri. 2012, 107, 1316–1323. [Google Scholar] [CrossRef][Green Version]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H. Insulinotropic and antidiabetic properties of Eucalyptus citriodora leaves and isolation of bioactive phytomolecules. J. Pharm. Pharmacol. 2021, 73, 1049–1061. [Google Scholar] [CrossRef]
- O’Harte, F.P.M.; Højrup, P.; Barnett, C.R.; Flatt, P.R. Identification of the Site of Glycation of Human Insulin. Peptides 1996, 17, 1323–1330. [Google Scholar] [CrossRef]
- Duffy, N.A.; Green, B.D.; Irwin, N.; Gault, V.A.; McKillop, A.M.; O’Harte, F.P.M.; Flatt, P.R. Effects of Antidiabetic Drugs on Dipeptidyl Peptidase IV Activity: Nateglinide Is an Inhibitor of DPP IV and Augments the Antidiabetic Activity of Glucagon-like Peptide-1. Eur. J. Pharmacol. 2007, 568, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.; Ojo, O.; Flatt, P.; AbdelWahab, Y. Antidiabetic actions of aqueous bark extract of Swertia chirayita on insulin secretion, cellular glucose uptake and protein glycation. J. Exp. Integrat. Med. 2014, 4, 268. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H.A. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutri. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S. Nigella sativa Stimulates Insulin Secretion from Isolated Rat Islets and Inhibits the Digestion and Absorption of (CH2O)N in the Gut. Biosci. Rep. 2019, 39, BSR20190723. [Google Scholar] [CrossRef]
- Ansari, P.; Badhan, S.S.; Azam, S.; Sultana, N.; Anwar, S.; Mohamed Abdurahman, M.S.; Hannan, J.M.A. Evaluation of Antinociceptive and Anti-Inflammatory Properties of Methanolic Crude Extract of Lophopetalum javanicum (Bark). J. Basic Clin. Physiol. Pharmacol. 2016, 27, 379–385. [Google Scholar] [CrossRef]
- Tabish, S.A. Is Diabetes Becoming the Biggest Epidemic of the Twenty-First Century? Int. J. Health Sci. 2007, 1, V–VIII. [Google Scholar]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal Plants of India with Anti-Diabetic Potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Patel, D.; Prasad, S.; Kumar, R.; Hemalatha, S. An Overview on Antidiabetic Medicinal Plants Having Insulin Mimetic Property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Dietary Fibre and Incidence of Type 2 Diabetes in Eight European Countries: The EPIC-InterAct Study and a Meta-Analysis of Prospective Studies. Diabetologia 2015, 58, 1394–1408. [CrossRef]
- Chopade, V.V.; Phatak, A.A.; Upaganlawar, A.B.; Tankar, A.A. Green Tea (Camellia sinensis): Chemistry, Traditional, Medicinal Uses and Its Pharmacological Activities- a Review. Pharmacogn. Rev. 2008, 2, 157–162. [Google Scholar]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Mohamad Shahi, M. Green Tea (Camellia sinensis) Supplementation to Diabetic Rats Improves Serum and Hepatic Oxidative Stress Markers. Iran. J. Pharm. Res. 2013, 12, 109–114. [Google Scholar]
- Islam, M.S. Effects of the Aqueous Extract of White Tea (Camellia sinensis) in a Streptozotocin-Induced Diabetes Model of Rats. Phytomedicine 2011, 19, 25–31. [Google Scholar] [CrossRef]
- Ferreira, M.C.L.; Lima, L.N.; Cota, L.H.T.; Costa, M.B.; Orsi, P.M.E.; Espíndola, R.P.; Albanez, A.V.; Rosa, B.B.; Carvalho, M.G.S.; Garcia, J.A.D. Effect of Camellia sinensis Teas on Left Ventricular Hypertrophy and Insulin Resistance in Dyslipidemic Mice. Braz. J. Med. Biol. 2020, 53, e9303. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef]
- Lambadiari, V.; Triantafyllou, K.; Dimitriadis, G.D. Insulin Action in Muscle and Adipose Tissue in Type 2 Diabetes: The Significance of Blood Flow. World J. Diabetes 2015, 6, 626. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rorsman, P. KATP Channels and Islet Hormone Secretion: New Insights and Controversies. Nat. Rev. Endocrinol. 2013, 9, 660–669. [Google Scholar] [CrossRef]
- Park, J.E.; Han, J.S. A Portulaca oleracea L. extract promotes insulin secretion via a K+ATP channel dependent pathway in INS-1 pancreatic β-cells. Nutr. Res. Pract. 2018, 12, 183–190. [Google Scholar] [CrossRef]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. CAMP Regulation of Airway Smooth Muscle Function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-T.; Song, Z.; Zhang, W.-C.; Jiao, B.; Yu, Z.-B. Impaired Translocation of GLUT4 Results in Insulin Resistance of Atrophic Soleus Muscle. Biomed Res. Int. 2015, 2015, 291987. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and Quercetin Isolated from Euonymus Alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Sayem, A.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef]
- Gonbad, R.A.; Afzan, A.; Karimi, E.; Sinniah, U.R.; Swamy, M.K. Phytoconstituents and Antioxidant Properties among Commercial Tea (Camellia sinensis L.) Clones of Iran. Electron. J. Biotechnol. 2015, 18, 433–438. [Google Scholar] [CrossRef]
- Sukito, A.; Tachibana, S. Isolation of Hyperoside and Isoquercitrin from Camellia sasanqua as Antioxidant Agents. Pak. J. Biol. Sci. 2014, 17, 999–1006. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Setyawan, E.I.; Setyowati, E.P.; Rohman, A.; Nugroho, A.K. Simultaneous Determination of Epigallocatechin gallate, Catechin, and Caffeine from Green Tea Leaves (Camellia sinensis L.) Extract by RP-HPLC. Res. J. Pharm. Technol. 2020, 13, 1489. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Hu, X.; Pan, J.; Liao, Y.; Ding, H. Inhibitory Effect of Epicatechin Gallate on Protein Glycation. Int. Food Res. J. 2019, 122, 230–240. [Google Scholar] [CrossRef]
- Jang, D.; Kim, J.; Kim, J.; Yoo, J.; Kim, Y.; Kim, J. Effects of Compounds Isolated from the Fruits ofRumex Japonicuson the Protein Glycation. Chem. Biodivers. 2008, 5, 2718–2723. [Google Scholar] [CrossRef]
- Dias, D.T.M.; Palermo, K.R.; Motta, B.P.; Kaga, A.K.; Lima, T.F.O.; Brunetti, I.L.; Baviera, A.M. Rutin Inhibits the in Vitroformation of Advanced Glycation Products and Protein Oxidation More Efficiently than Quercetin. Rev. Cienc. Farm. Basica Apl. 2021, 42, e718. [Google Scholar] [CrossRef]
- Falla, N.M.; Demasi, S.; Caser, M.; Scariot, V. Phytochemical Profile and Antioxidant Properties of Italian Green Tea, a New High Quality Niche Product. Horticulturae 2021, 7, 91. [Google Scholar] [CrossRef]
- Gromova, L.V.; Fetissov, S.O.; Gruzdkov, A.A. Mechanisms of Glucose Absorption in the Small Intestine in Health and Metabolic Diseases and Their Role in Appetite Regulation. Nutrients 2021, 13, 2474. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Interactions of Flavonoids with α-Amylase and Starch Slowing down Its Digestion. Food Funct. 2018, 9, 677–687. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid. Based Complementary Altern. Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef]
- McKillop, A.M.; Duffy, N.A.; Lindsay, J.R.; Green, B.D.; Patterson, S.; O’Harte, F.P.M.; Bell, P.M.; Flatt, P.R. Insulinotropic Actions of Nateglinide in Type 2 Diabetic Patients and Effects on Dipeptidyl Peptidase-IV Activity and Glucose-Dependent Insulinotropic Polypeptide Degradation. Eur. J. Endocrinol. 2009, 161, 877–885. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z.; Liu, J.; Jiao, Z. Inhibition of Dipeptidyl Peptidase-4 by Flavonoids: Structure–Activity Relationship, Kinetics and Interaction Mechanism. Front. Nutr. 2022, 9, 892426. [Google Scholar] [CrossRef]
- Golic, M.; Kräker, K.; Fischer, C.; Alenina, N.; Haase, N.; Herse, F.; Schütte, T.; Henrich, W.; Müller, D.N.; Busjahn, A.; et al. Continuous Blood Glucose Monitoring Reveals Enormous Circadian Variations in Pregnant Diabetic Rats. Front. Endocrinol. 2018, 9, 271. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Wang, D.; Yu, F.; Zhang, N.; Song, C.; Granato, D. Analytical Strategy Coupled to Chemometrics to Differentiate Camellia sinensis Tea Types Based on Phenolic Composition, Alkaloids, and Amino Acids. J. Food Sci. 2020, 85, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teucrium Poliumon β-Cell Destruction in a Model of Streptozotocin-Induced Diabetes. Planta Medica 2009, 75, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a Bioflavonoid Improves Glucose Homeostasis in Streptozotocin-Induced Diabetic Tissues by Altering Glycolytic and Gluconeogenic Enzymes. Fundam. Clin. Pharmacol. 2009, 24, 357–364. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic Enzyme Inhibitory and Antiglycation Potential of Rutin. Future J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Muhammad, I.; Rahman, N.; Gul-E-Nayab; Nishan, U.; Shah, M. Antidiabetic Activities of Alkaloids Isolated from Medicinal Plants. Braz. J. Pharm. Sci. 2021, 57. [Google Scholar] [CrossRef]
- Li, Y.; Park, J.; Wu, Y.; Cui, J.; Jia, N.; Xi, M.; Wen, A. Identification of AMPK Activator from Twelve Pure Compounds Isolated from Aralia taibaiensis: Implication in Antihyperglycemic and Hypolipidemic Activities. Korean J. Physiol. Pharmacol. 2017, 21, 279–286. [Google Scholar] [CrossRef]
- Muthusamy, V.S.; Anand, S.; Sangeetha, K.N.; Sujatha, S.; Arun, B.; Lakshmi, B.S. Tannins Present in Cichorium Intybus Enhance Glucose Uptake and Inhibit Adipogenesis in 3T3-L1 Adipocytes through PTP1B Inhibition. Chem. Biol. Interact. 2008, 174, 69–78. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).