Abstract
In cardiac surgery, patients are at risk of phrenic nerve injury, which leads to diaphragm dysfunction and acute respiratory failure. Diaphragm dysfunction (DD) is relatively frequent in cardiac surgery and particularly affects patients after coronary artery bypass graft. The onset of DD affects patients’ prognosis in term of weaning from mechanical ventilation and hospital length of stay. The authors present a narrative review about diaphragm physiology, techniques used to assess diaphragm function, and the clinical application of diaphragm ultrasound in patients undergoing cardiac surgery.
1. Introduction
Diaphragm is the most important inspiratory muscle and is innerved by the phrenic nerve. In cardiac surgery, patients are at risk of phrenic nerve injury, which leads to diaphragm dysfunction (DD) [1]. The onset of DD is a serious outcome, affecting morbidity and length of hospital stay [1,2,3,4,5]. This is particularly a pejorative burden in patients with previous pulmonary diseases. In fact, after cardiac surgery, pulmonary complications are frequent, with an incidence reaching 30% in adults [2,6]. In addition, the incidence of DD, after cardiac surgery may reach 75% [7,8,9]. Many factors are involved in the onset of respiratory complications after cardiac surgery, namely, general anesthesia, invasive mechanical ventilation, damage of the surfactant, presence of sternotomy and cardiopulmonary bypass. Diaphragm dysfunction may be related to perioperative phrenic nerve injury, either during harvesting of the internal mammary artery (IMA) or in relation to cold injury caused by local hypothermia [4,10]. Diaphragm ultrasound can be performed at the bedside to assess diaphragm function. This technique may help physicians to manage patients in the context of postoperative weaning trials and to stratify patients regarding respiratory prognosis.
This review aims to report the physiological aspects of diaphragm muscle and phrenic nerve, the radiological and non-radiological techniques used to assess diaphragm function, and the clinical applications of diaphragm ultrasound in cardiac surgery.
2. The Diaphragm
The diaphragm is the main inspiratory muscle, contributing to 60–70% of the total ventilation at rest. Respiratory system elastic recoil refers to the respiratory system’s intrinsic tendency to deflate following inflation. The diaphragm is a dome-shaped fibro-muscular structure with a central tendon and a cylindroid muscular portion, inserted within the inner rib cage and attached to the xiphisternum and the lumbar vertebrae. Physiologically, there is a rhythmic contraction of the diaphragm against elastic and resistive forces. During expiration, the diaphragm returns progressively to a relaxation state position determined by the balance between chest wall and lung recoil forces [11]. During inspiration, the diaphragm shortens and moves caudally in a piston-like manner, with an increase of the abdominal pressure and a decrease of the pleural pressure, leading to a decrease of the alveolar pressure, below atmospheric pressure. This generates airflow into the lungs against a resistance, following the principles stated by Ohm’s Law [12]. Accordingly, the transpulmonary pressure, i.e., the difference between alveolar pressure and intrapleural pressure, is a key determinant of lung volume. During inspiration, gastric pressure increases as the diaphragmatic muscle contracts, descends, and displaces the abdominal content downward and outward [13]. In the meantime, there is an outward displacement of the lower ribs, firstly due to the insertional force applied by the contraction of the fibers that exert a direct, cranially oriented force on these ribs [14]. The second mechanism, also called the “appositional force,” is due to the transmission of abdominal pressure to the lower rib cage in the apposition zone [14].
Diaphragm strength affects the inspiratory lung volume directly. In addition, with diaphragm displacement, the scalene, parasternal, and external intercostal muscles are activated during tidal breathing, preventing the downward displacement of the upper ribcage due to the negative pleural pressure caused by the diaphragm displacement [13,14,15]. In a patient breathing steadily, the expiratory process is passive, depending on the respiratory system recoil [13]. The abdominal muscles may be solicited with contraction during expiration in patients with respiratory failure, followed by relaxation providing assistance to inspiration if the volume at the end of expiration decreases below the equilibrium volume, i.e., the functional residual capacity.
The neural control of the diaphragm relies on the phrenic nerve. After originating from C3–C5 (anterior horn), it crosses the scalenus anterior muscle. The right phrenic nerve, at the apex of the thorax, crosses the right IMA, runs along the right side of the superior vena cava, then goes along the pericardium over the right cardiac cavities before reaching the diaphragm [8]. The left phrenic nerve, after crossing the scalenus muscle, runs along the left subclavian artery and behind the thoracic duct, before crossing the left IMA and descending in the thorax, closer to the pericardium, adjacent to the left ventricle, and finally reaches the diaphragm. Since the phrenic nerves are closer to the pericardium, they are vulnerable to the cooling methods used to protect the myocardium during cardiac surgery. In addition, since the phrenic nerves are closer to the IMA in the apical region of the chest, they are also vulnerable during the harvesting phase [8].
3. Traditional Techniques Used to Assess the Diaphragm Function
Historically, diaphragm function analysis relies on trans-diaphragmatic pressure measurement or fluoroscopy in radiology. The latter requires ionizing radiation and specific devices, and patients need to be transported to the radiology department, which is a major drawback for patients hospitalized in the Intensive Care Unit (ICU). Other exams can be performed at the bedside. Pulmonary functional tests (FPT) can be used to measure the maximal inspiratory pressure (MIP) [16]. MIP is an index of the inspiratory muscles strength that includes the diaphragm and the intercostal and accessory inspiratory muscles. It depends on lung volume and is classically measured at residual volume, although the recoil of the chest wall may be a confounding element. Hence, this technique is not specific to measure the diaphragm strength, considering that the ribcage muscles can generate inspiratory pressures at the airway opening without a significant diaphragm contribution. In addition, this technique is volitional and depends on the patient’s motivation, cooperation, and ability. In the context of post-cardiac surgery, it is not usual to perform this test, since the patient may be in a painful situation (i.e., sternotomy, Redon drain). The maximal sniff nasal inspiratory pressure (SNIP) is another parameter used to assess respiratory muscles’ strength at the bedside. SNIP is measured using a pressure transducer connected to a catheter localized in the nostril, at the functional residual capacity (FRC) level. SNIP is not classically recorded in ICU after surgery, as it is not reliable for intubated/tracheostomized patients. Delta pulmonary vital capacity (VC) can analyze diaphragm function. Delta VC is the change of VC from the upright to the supine position. A decrease in delta VC is an index of diaphragm dysfunction [16]. The ratio of maximal expiratory pressure (MEP) to maximal inspiratory pressure (MEP/MIP) can also be used as an index of diaphragm dysfunction. In fact, patients with diaphragm dysfunction but with preserved expiratory muscle function have a decreased MIP but a normal MEP. The MEP/MIP ratio can be used as an alternative to assess diaphragm function in patients that can tolerate the supine position.
The measurement of the trans-diaphragmatic pressure (pdi) is the reference test to assess diaphragm strength, although it is an invasive technique. It requires a gastric balloon catheter and an esophageal balloon catheter, providing the measurement of gastric and esophageal pressures, respectively. The pdi is equal to the gastric pressure minus the esophageal pressure. It can be obtained in patients during volitional maneuvers such as maximal inspiratory effort (pdi max) at FRC, during a sniff maneuver (pdi sniff) [17], or during a magnetic stimulation (non-volitional maneuver). Normal pdi values swing during these maneuvers, depending on sex, size, initial volume of the respiratory system, and body position. High values exclude diaphragm weakness. Normal values range from 100 to 150 cm H₂O [17]. The Gilbert index, which is the ratio between delta gastric pressure and delta pdi, can also be used to determine the contribution of the diaphragm during inspiration, after cardiac surgery. A high value is associated with a great contribution of the diaphragm muscle during inspiration. Twitch mouth pressure is a non-invasive and non-volitional test that can be used to assess diaphragm function in non-intubated patients [18]. This measurement requires a flanged mouthpiece connected to a pneumotachograph, which is in turn connected to a differential pressure transducer [18]. For this test, magnetic phrenic nerve stimulation is necessary. However, these techniques require expertise and are time-consuming. In addition, the stimulation of phrenic nerves cannot be performed in patients with implanted cardiac devices (pacemakers or defibrillators).
4. Diaphragm Ultrasound Technique
Diaphragm ultrasound is a noninvasive radiological exam that can be performed at the bedside. To assess the diaphragm, the patient is positioned in the semi-recumbent position, ideally on spontaneous breathing. Two approaches are classically used to assess diaphragm function.
4.1. The Subcostal Approach
Diaphragm motion is measured at the mid-clavicular line or at the antero-axillary line in the patient. For right hemi-diaphragm analysis, the liver is used as an acoustic window, whereas the spleen is used for left hemidiaphragm analysis. The operator visualizes the right diaphragm as a bright line covering the liver. The normal diaphragm moves toward the probe (supplementary information), and we record an upward motion from M mode tracing (Figure 1). Normal values were published, with high intra- and inter-reproducibility [19]. During quiet breathing, the normal diaphragm motion was reported to at 13.4 ± 1.8 mm [19]. In the study by Boussuges et al. [19], normal values of diaphragm motion during a deep inspiration were 70 ± 6 mm for males and at 57 ± 1 mm for females. During tidal breathing, diaphragm weakness is defined by a diaphragm inspiratory motion less than 10–15 mm [20,21,22]. While the analysis of right hemidiaphragm motion is easily performed in routine practice, left hemidiaphragm motion is harder to assess, since during the inspiration phase, the descending lung and the bowel interposition decrease the visibility of diaphragm mobility. Finally, tissue Doppler imaging coupled to a sniff maneuver can be used to assess diaphragm function (Figure 2), and normal values have been published [23].
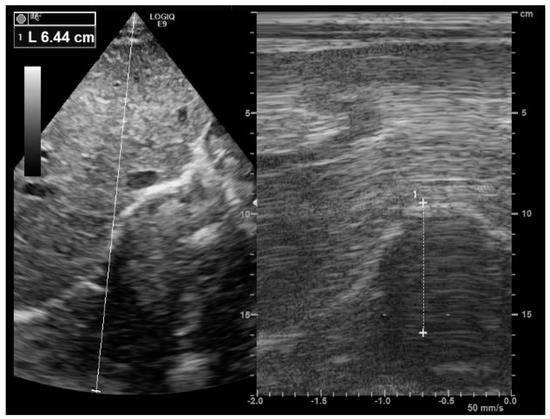
Figure 1.
Right diaphragm ultrasound. Note the normal inspiratory motion (64 mm) of the hemidiaphragm from the subcostal view. After the visualization of the right hemidiaphragm (bright line) using a B-mode (image on the left), an M mode was applied (image on the right) to record diaphragm motion.
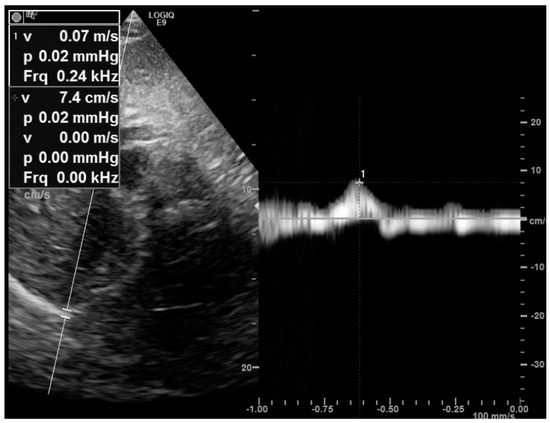
Figure 2.
Measurement of the right peak sniff tissue Doppler imaging velocity from the subcostal view. The diaphragm velocity was recorded during a sniff maneuver. Here is a reduced peak sniff velocity (7 cm/s) in a patient with muscular dystrophy.
4.2. The Apposition Zone Approach
The apposition zone is the area of the chest within the abdomen that reaches the lower ribcage. To assess the diaphragm from the apposition zone, it is essential to use a linear high-frequency probe. For the echographic procedure, the probe has to be positioned on the mid-axillary line or on the antero-axillary line, perpendicular to the zone of apposition, in the 8th to 11th intercostal space. The diaphragm can be visualized as a hypo-echogenic central layer surrounded by two hyper-echogenic layers, namely, the pleural line and the peritoneum. Figure 3 shows diaphragm thickness measurement from the apposition zone using ultrasound at end-expiration and at end-inspiration, providing diaphragm thickening.
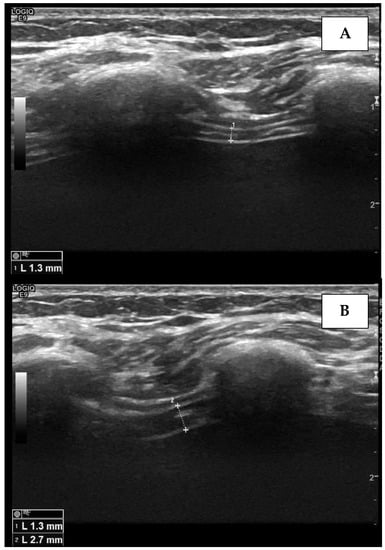
Figure 3.
Measurement of the right diaphragm thickness (dotted line) in the end-expiratory phase (A) and end-inspiratory phase (B), using ultrasound. The diaphragm is visualized as a hypo-echogenic central layer surrounded by two hyper-echogenic lines, namely, the pleural line and the peritoneum.
Normal values have been published [20]. The normal diaphragm end-expiratory thickness is more than 1.7 mm [24]. Diaphragm thickness may vary with gender and body composition. In a study that included 150 healthy subjects with a mean body mass index (BMI) of 27.9 ± 4.7 kg/m², the normal right expiratory diaphragm thickness was 3.8 ± 1.5 mm for males and 2.7 ± 1 mm for females [20]. In the study by Ueki et al. [25], the normal diaphragm thickness was 1.7 ± 0.2 mm at rest, reaching 4.5 ± 0.9 mm at total lung capacity (TLC). In another study that included 109 healthy participants with a mean BMI of 24.1 ± 3.6 kg/m², Carrillo-Esper et al. [26] reported a mean diaphragm thickness of 1.9 ± 0.4 mm for men and 1.4 ± 0.03 mm for women. From the end-expiratory diaphragm thickness and end-inspiratory diaphragm thickness, it is possible to calculate the diaphragm thickening fraction (TF) = (end-inspiratory thickness-end-expiratory thickness)/end-expiratory thickness). Diaphragm TF is reproducible [27], and a diaphragm TF value below 20% is a marker of diaphragm paresis [17] and diaphragm paralysis [21]. Diaphragm dysfunction can be defined by a diaphragm TF < 20–36% [21]. Table 1 summarizes the normal values of diaphragm ultrasound parameters

Table 1.
Normal values of diaphragm parameters determined by ultrasound.
5. Diaphragm Dysfunction in ICU
Diaphragm dysfunction is common in ICU and affects prognosis, respiratory outcomes, length of hospital stay, and weaning process [4]. In ICU, the prevalence of diaphragm dysfunction at admission was reported to be up to 64% by Demoule et al. [35], while in patients on weaning trials, the prevalence reached 23% to 80% [22,24,36]. The mechanisms of diaphragm dysfunction are multiple and include mechanical ventilation-induced diaphragm disuse, sepsis, use of drugs (propofol, steroid, neuromuscular blockers), and excessive loading [37].
The consequences of diaphragm dysfunction are severe, resulting in an increase of mechanical ventilation duration [22,36], risk of re-intubation, and mortality [35]. Hence, monitoring diaphragm function with ultrasound may be useful. In fact, diaphragm ultrasound parameters cut-off to predict weaning trial success or failure from mechanical ventilation have been published [38]. Thus, Pirompanich et al. [39] reported a right diaphragmatic TF cut-off ≥ 26% to predict successful weaning. Ferrari et al. [40] reported a cut-off value of diaphragm TF > 36%, for predicting success weaning in ICU patients on spontaneous breathing. In the study by DiNino et al. [24], a diaphragm TF ≥ 30% could predict extubation success.
Other authors focused on diaphragm excursion. In fact, the magnitude of diaphragm excursion during inspiration can predict the success of a weaning trial [41,42]. Diaphragm excursion is considered abnormal if it is lower than 10–14 mm [19]. In the study by Kim et al. [22], a diaphragm weakness defined by diaphragm inspiratory motion <10 mm was associated with weaning features. The diaphragm inspiratory motion cut-off to predict successful extubation was 11 mm, using a T tube trial, in the study by Jiang et al. [43]. A right inspiratory motion cut-off > 10 mm was reported by Jung-Wan Yoo et al. [44] to predict successful extubation. Finally, a combined index has been proposed to predict weaning trial success, namely, the diaphragm excursion time index, which is the product of diaphragm excursion and inspiratory time [45]. In the study by Palkar et al. [45], during a spontaneous breathing trial, the diaphragmatic excursion time index was 2.42 ± 1.55 cm.s in the group with successful weaning trial versus 1.64 ± 1.19 cm.s (p < 0.03) in the group with failure weaning trial. Spadaro et al. [46] proposed another index, that is, the diaphragmatic RSBI (respiratory rate/diaphragmatic motion), to predict weaning failure. A diaphragmatic RSBI cut-off > 1.3 can predict weaning failure, whereas a cut-off diaphragm motion ≤ 14 mm can predict weaning failure [46]. Table 2 summarizes the cut-off values of diaphragmatic ultrasound parameters used to predict weaning outcome in ICU patients.

Table 2.
Diaphragm ultrasound parameters cut-off values for predicting success from mechanical ventilation weaning in ICU patients.
6. Diaphragm Dysfunction in Cardiac Surgery
Diaphragm dysfunction is frequent after cardiac surgery, particularly after coronary artery bypass graft (CABG) [1] and in patients with diabetes mellitus [47]. In addition, obesity and arterial hypertension may be risk factors for post-operative diaphragmatic dysfunction [4]. Preoperative inspiratory muscle weakness may predict the postoperative duration of mechanical ventilation in patients undergoing cardiac surgery [5]. Clinically, patients with bilateral diaphragm paralysis disclose orthopnea, rapid shallow breathing, and thoraco-abdominal paradoxus in the supine position [48]. The abdominal paradoxus, an inward motion of the abdomen during inspiration, is related to a flaccid diaphragm, drawn up by the contraction of the intercostal muscles during inspiration. Physical examination may find an increase of the respiratory rate, the utilization of accessory inspiratory muscles, and a contraction of the abdominal expiratory muscles. On physical exam, the analysis of ribcage expansion as well as of abdomen displacement may indirectly provide information regarding diaphragm features.
In practice, we distinguish two situations, in the context of phrenic features after cardiac surgery:
- The management of patients in cardiac ICU after surgery, in the context of a weaning trial
- The management of patients with moderate symptoms who may reveal diaphragm features in cardiac rehabilitation unit.
In cardiac ICU, DD after cardiac surgery is frequent. In the study by Moury et al. [10], DD, defined by a diaphragm TF < 20% during a spontaneous breathing trial, was present with an incidence reaching 75%. Length of surgery, cross-clamp time, and cardio-pulmonary bypass time negatively affect diaphragm thickening [10]. In addition, propofol and remifentanil were associated with a decrease of diaphragm TF [10]. In the study by Diehl et al. [3], that included 13 patients with DD, after cardiac surgery, the authors reported high morbidity and mortality, with the occurrence of nosocomial pneumonia, cardiorespiratory arrest after early extubation, prolonged mechanical ventilation, and death. In a recent French study that included 3577 patients, the presence of DD after cardiac surgery was associated with the risk of post-operative pneumonia, non-invasive ventilation, and invasive ventilation and an increase of ICU hospital length of stay [4]. Table 3 summarizes studies that assessed diaphragm function and clinical outcomes of adult patients after cardiac surgery.

Table 3.
Studies that analyzed diaphragm dysfunction and outcomes after cardiac surgery.
It is essential to provide risk stratification for patients undergoing cardiac surgery. Preoperative diaphragm function may be used as a prognostic tool. Indeed, preoperative diaphragmatic function has been reported to be associated with pulmonary outcomes after surgery [2]. A recent study reported an increase of post-operative pulmonary complications that include atelectasis, pneumonia, and prolonged mechanical ventilation in patients with a pre-operative diaphragm TF below 38% [2]. Hence, diaphragm ultrasound may help assess operative risk, in addition with the EuroSCORE II (European System for Cardiac Operative Risk Evaluation), in patients undergoing cardiac surgery [2].
7. Management of Diaphragmatic Dysfunction after Cardiac Surgery
The diagnosis of diaphragmatic failure after a cardiac surgery relies on clinic, radiology, and blood gas exchange analyses. Blood gas exchange analysis may reveal acute respiratory acidosis. PFT cannot be performed immediately after sternotomy. Diaphragm elevation on chest X ray (Figure 4) may warrant specific exploration; however, it is not very specific (44%), as patients with pneumonia, atelectasis, and diaphragmatic eventration may also present this radiological sign [54]. Fluoroscopy can show classical diaphragm paradoxical motion during the sniff maneuver. The measurement of trans-diaphragmatic pressure provides clues for diaphragm paralysis, but this invasive technique is not routinely performed. A phrenic nerve electrophysiological study can be performed; however, this technique is not routinely performed in cardiac ICU. Furthermore, after cardiac surgery, patients are frequently equipped with temporary external pacemakers connected to epicardial electrodes, which confound electromyograms [17].
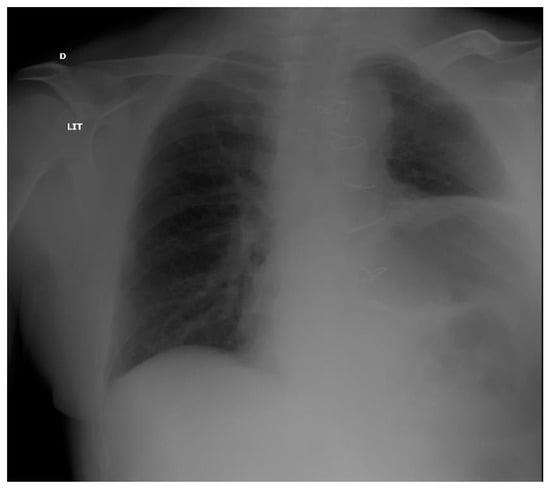
Figure 4.
Chest X ray in a post-operative patient with diaphragm paralysis. Note ascension of the left diaphragm.
Conversely, diaphragm ultrasound can be done routinely in cardiac ICU after surgery. In patients with diaphragm dysfunction documented with ultrasound, spontaneous breathing trial (SBT) must be cautious, and CPAP (continuous positive airway pressure) can be proposed to support the respiratory status after extubation. The use of diaphragm ultrasound may reduce the time to extubation, according to a recent randomized trial [55]. In fact, using a cut-off of diaphragm TF ≥ 30% in ICU patients undergoing SBT, Mc Cool et al. [55] reported a significant reduction of the time from ultrasound to extubation in an interventional group in comparison with a group receiving usual care. Further studies in patients undergoing cardiac surgery are warranted in the post-operative period to determine the role of ultrasound in clinical practice.
8. Conclusions
Diaphragm is the main inspiratory muscle.
The assessment of diaphragm function relies on invasive and non-invasive tests.
Ultrasound can be used to assess diaphragm function. Diaphragm dysfunction is relatively frequent in patients after cardiac surgery. This feature affects patients’ prognosis. Performing diaphragm ultrasound may help to stratify and manage patients in the context of cardiac surgery.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicines9010005/s1. Video S1: The video shows the normal diaphragmatic motion from the right subcostal view using a cardiac probe.
Author Contributions
Conceptualization: A.F., L.S.N., N.M., F.L. and D.A.; Writing original draft: A.F., L.S.N., F.L. and D.A.; Scientific intellectual contributions: A.F., N.M., L.S.N., D.O., H.P., J.B., D.A. and F.L.; Writing and reviewing: all authors; Validation: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bruni, A.; Garofalo, E.; Pasin, L.; Serraino, G.F.; Cammarota, G.; Longhini, F.; Landoni, G.; Lembo, R.; Mastroroberto, P.; Navalesi, P.; et al. Diaphragmatic Dysfunction after Elective Cardiac Surgery: A Prospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3336–3344. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Eljaiek, R.; Rodrigue, É.; Lamarche, Y.; Girard, M.; Wang, H.T.; Levesque, S.; Denault, A.Y. Preoperative Diaphragm Function is Associated with Postoperative Pulmonary Complications after Cardiac Surgery. Crit. Care Med. 2019, 47, e966–e974. [Google Scholar] [CrossRef]
- Diehl, J.L.; Lofaso, F.; Deleuze, P.; Similowski, T.; Lemaire, F.; Brochard, L. Clinically relevant diaphragmatic dysfunction after cardiac operations. J. Thorac. Cardiovasc. Surg. 1994, 107, 487–498. [Google Scholar] [CrossRef]
- Laghlam, D.; Lê, M.P.; Srour, A.; Monsonego, R.; Estagnasié, P.; Brusset, A.; Squara, P. Diaphragm Dysfunction After Cardiac Surgery: Reappraisal. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, E.R.; Steffens, É.; Windmöller, P.; Fontela, P.C.; da Cruz, D.T.; Battisti, I.D.E. Preoperative expiratory and inspiratory muscle weakness to predict postoperative outcomes in patients undergoing elective cardiac surgery. J. Card. Surg. 2020, 35, 128–134. [Google Scholar] [CrossRef]
- He, S.; Chen, B.; Li, W.; Yan, J.; Chen, L.; Wang, X.; Xiao, Y. Ventilator-associated pneumonia after cardiac surgery: A meta-analysis and systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3148–3155.e5. [Google Scholar] [CrossRef] [PubMed]
- Moury, P.-H.; Cuisinier, A.; Durand, M.; Bosson, J.-L.; Chavanon, O.; Payen, J.-F.; Jaber, S.; Albaladejo, P. Diaphragm thickening in cardiac surgery: A perioperative prospective ultrasound study. Ann. Intensive Care 2019, 9, 50. [Google Scholar] [CrossRef]
- Aguirre, V.J.; Sinha, P.; Zimmet, A.; Lee, G.A.; Kwa, L.; Rosenfeldt, F. Phrenic nerve injury during cardiac surgery: Mechanisms, management and prevention. Heart Lung Circ. 2013, 22, 895–902. [Google Scholar] [CrossRef]
- Dimopoulou, I.; Daganou, M.; Dafni, U.; Karakatsani, A.; Khoury, M.; Geroulanos, S.; Jordanoglou, J. Phrenic nerve dysfunction after cardiac operations: Electrophysiologic evaluation of risk factors. Chest 1998, 113, 8–14. [Google Scholar] [CrossRef]
- Berrizbeitia, L.D.; Tessler, S.; Jacobowitz, I.J.; Kaplan, P.; Cunningham, J.N. Effect of sternotomy and coronary bypass surgery on postoperative pulmonary mechanics: Comparison of internal mammary and saphenous vein bypass grafts. Chest 1989, 96, 873–876. [Google Scholar] [CrossRef]
- Rochester, D.F. The diaphragm: Contractile properties and fatigue. J. Clin. Investig. 1985, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Benditt, J.O. Esophageal and gastric pressure measurements. Respir. Care 2005, 50, 68–77. [Google Scholar] [PubMed]
- Wilcox, P.G.; Pardy, R.L. Diaphragmatic weakness and paralysis. Lung 1989, 167, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Troyer, A.D.; Wilson, T.A. Action of the diaphragm on the rib cage. J. Appl. Physiol. 2016, 121, 391–400. [Google Scholar] [CrossRef]
- Gibson, G.J. Diaphragmatic paresis: Pathophysiology, clinical features, and investigation. Thorax 1989, 44, 960–970. [Google Scholar] [CrossRef]
- Steier, J.; Kaul, S.; Seymour, J.; Jolley, C.; Rafferty, G.; Man, W.; Luo, Y.M.; Roughton, M.; Polkey, M.I.; Moxham, J. The value of multiple tests of respiratory muscle strength. Thorax 2007, 62, 975–980. [Google Scholar] [CrossRef]
- Laveneziana, P.; Albuquerque, A.; Aliverti, A.; Babb, T.; Barreiro, E.; Dres, M.; Dubé, B.-P.; Fauroux, B.; Gea, J.; Guenette, J.A.; et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019, 53, 1801214. [Google Scholar] [CrossRef]
- Santos, D.B.; Desmarais, G.; Falaize, L.; Ogna, A.; Cognet, S.; Louis, B.; Orlikowski, D.; Prigent, H.; Lofaso, F. Twitch mouth pressure for detecting respiratory muscle weakness in suspicion of neuromuscular disorder. Neuromuscul. Disord. 2017, 27, 518–525. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Boon, A.J.; Ba, C.J.H.; Ghahfarokhi, L.S.; Strommen, J.A.; Watson, J.C.; Sorenson, E.J. Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle Nerve 2013, 47, 884–889. [Google Scholar] [CrossRef]
- Gottesman, E.; McCool, F.D. Ultrasound evaluation of the paralyzed diaphragm. Am. J. Respir. Crit. Care Med. 1997, 155, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Suh, H.J.; Hong, S.-B.; Koh, Y.; Lim, C.-M. Diaphragm dysfunction assessed by ultrasonography: Influence on weaning from mechanical ventilation. Crit. Care Med. 2011, 39, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil, A.; Nguyen, L.S.; Ogna, A.; Stojkovic, T.; Meng, P.; Mompoint, D.; Carlier, R.; Prigent, H.; Clair, B.; Behin, A.; et al. Diaphragm sniff ultrasound: Normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS ONE 2019, 14, e0214288. [Google Scholar] [CrossRef] [PubMed]
- DiNino, E.; Gartman, E.J.; Sethi, J.M.; McCool, F.D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014, 69, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Ueki, J.; De Bruin, P.F.; Pride, N.B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 1995, 50, 1157–1161. [Google Scholar] [CrossRef]
- Carrillo-Esper, R.; Pérez-Calatayud, Á.A.; Arch-Tirado, E.; Díaz-Carrillo, M.A.; Garrido-Aguirre, E.; Tapia-Velazco, R.; Peña-Pérez, C.A.; Espinoza-de Los Monteros, I.; Meza-Márquez, J.M.; Flores-Rivera, O.I.; et al. Standardization of sonographic diaphragmthickness evaluations in healthy volunteers. Respir. Care 2016, 61, 920–924. [Google Scholar] [CrossRef]
- Goligher, E.C.; Laghi, F.; Detsky, M.E.; Farias, P.; Murray, A.; Brace, D.; Brochard, L.J.; Bolz, S.S.; Rubenfeld, G.D.; Kavanagh, B.P.; et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: Feasibility, reproducibility and validity. Intensive Care Med. 2015, 41, 642–649. [Google Scholar] [CrossRef]
- Wait, J.L.; Nahormek, P.A.; Yost, W.T.; Rochester, D.P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. 1989, 67, 1560–1568. [Google Scholar] [CrossRef]
- Cohen, E.; Mier, A.; Heywood, P.; Murphy, K.; Boultbee, J.; Guz, A. Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax 1994, 49, 885–889. [Google Scholar] [CrossRef]
- Kantarci, F.; Mihmanli, I.; Demirel, M.K.; Harmanci, K.; Akman, C.; Aydogan, F.; Mihmanli, A.; Uysal, O. Normal diaphragmatic motion and the effects of body composition: Determination with M-mode sonography. J. Ultrasound Med. 2004, 23, 255–260. [Google Scholar] [CrossRef]
- Testa, A.; Soldati, G.; Giannuzzi, R.; Berardi, S.; Portale, G.; Silveri, N.G. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 2011, 37, 44–52. [Google Scholar] [CrossRef]
- Orde, S.R.; Boon, A.J.; Firth, D.G.; Villarraga, H.R.; Sekiguchi, H. Diaphragm assessment by two dimensional speckle tracking imaging in normal subjects. BMC Anesthesiol. 2016, 16, 43. [Google Scholar] [CrossRef]
- Scarlata, S.; Mancini, D.; Laudisio, A.; Benigni, A.; Incalzi, R.A. Reproducibility and Clinical Correlates of Supine Diaphragmatic Motion Measured by M-Mode Ultrasonography in Healthy Volunteers. Respiration 2018, 96, 259–266. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Herkenrath, S.; Henke, C.; Langenbruch, L.; Schneppe, M.; Randerath, W.; Young, P.; Brix, T.; Boentert, M. Evaluation of Respiratory Muscle Strength and Diaphragm Ultrasound: Normative Values, Theoretical Considerations, and Practical Recommendations. Respiration 2020, 99, 369–381. [Google Scholar] [CrossRef]
- Demoule, A.; Jung, B.; Prodanovic, H.; Molinari, N.; Chanques, G.; Coirault, C.; Matecki, S.; Duguet, A.; Similowski, T.; Jaber, S. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am. J. Respir. Crit. Care Med. 2013, 188, 213–219. [Google Scholar] [CrossRef]
- Jung, B.; Moury, P.H.; Mahul, M.; De Jong, A.; Galia, F.; Prades, A.; Albaladejo, P.; Chanques, G.; Molinari, N.; Jaber, S. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016, 42, 853–861. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Dres, M.; Dubé, B.-P.; Mayaux, J.; Delemazure, J.; Reuter, D.; Brochard, L.; Similowski, T.; Demoule, A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am. J. Respir. Crit. Care Med. 2017, 195, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pirompanich, P.; Romsaiyut, S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J. Intensive Care 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; De Filippi, G.; Elia, F.; Panero, F.; Volpicelli, G.; Aprà, F. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit. Ultrasound J. 2014, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Dres, M.; Goligher, E.C.; Dubé, B.-P.; Morawiec, E.; Dangers, L.; Reuter, D.; Mayaux, J.; Similowski, T.; Demoule, A. Diaphragm function and weaning from mechanical ventilation: An ultrasound and phrenic nerve stimulation clinical study. Ann. Intensive Care 2018, 8, 53. [Google Scholar] [CrossRef]
- Dres, M.; Demoule, A. Diaphragm dysfunction during weaning from mechanical ventilation: An underestimated phenomenon with clinical implications. Crit. Care 2018, 22, 73. [Google Scholar] [CrossRef]
- Jiang, J.R.; Tsai, T.H.; Jerng, J.S.; Yu, C.J.; Wu, H.D.; Yang, P.C. Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest 2004, 126, 179–185. [Google Scholar] [CrossRef]
- Yoo, J.W.; Lee, S.J.; Lee, J.D.; Kim, H.C. Comparison of clinical utility between diaphragm excursion and thickening change using ultrasonography to predict extubation success. Korean J. Intern. Med. 2018, 33, 331–339. [Google Scholar] [CrossRef]
- Palkar, A.; Narasimhan, M.; Greenberg, H.; Singh, K.; Koenig, S.; Mayo, P.; Gottesman, E. Diaphragm Excursion-Time Index: A New Parameter Using Ultrasonography to Predict Extubation Outcome. Chest 2018, 153, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Grasso, S.; Mauri, T.; Corte, F.D.; Alvisi, V.; Ragazzi, R.; Cricca, V.; Biondi, G.; Di Mussi, R.; Marangoni, E.; et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit. Care 2016, 20, 305. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kato, H.; Tsujimoto, S.; Kitamura, R. Diabetes mellitus, internal thoracic artery grafting, and risk of an elevated hemidiaphragm after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 1994, 8, 437–440. [Google Scholar] [CrossRef]
- Chan, C.K.; Loke, J.; Virgulto, J.A.; Mohsenin, V.; Ferranti, R.; Lammertse, T. Bilateral diaphragmatic paralysis: Clinical spectrum, prognosis, and diagnostic approach. Arch. Phys. Med. Rehabil. 1988, 69, 976–979. [Google Scholar] [PubMed]
- Markand, O.N.; Moorthy, S.; Mahomed, Y.; King, R.D.; Brown, J.W. Postoperative phrenic nerve palsy in patients with open-heart surgery. Ann. Thorac. Surg. 1985, 39, 68–73. [Google Scholar] [CrossRef]
- Canbaz, S.; Turgut, N.; Halici, U.; Balci, K.; Ege, T.; Duran, E. Electrophysiological evaluation of phrenic nerve injury during cardiac surgery--a prospective, controlled, clinical study. BMC Surg. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- DeVita, M.A.; Robinson, L.R.; Rehder, J.; Hattler, B.; Cohen, C. Incidence and natural history of phrenic neuropathy occurring during open heart surgery. Chest 1993, 103, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Merino-Ramirez, M.A.; Juan, G.; Ramón, M.; Cortijo, J.; Rubio, E.; Montero, A.; Morcillo, E. Electrophysiologic evaluation of phrenic nerve and diaphragm function after coronary bypass surgery: Prospective study of diabetes and other risk factors. J. Thorac. Cardiovasc. Surg. 2006, 132, 530–536.e2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tralhão, A.; Cavaleiro, P.; Arrigo, M.; Lopes, J.-P.; Lebrun, M.; Rivas-Lasarte, M.; Le Pimpec-Barthes, F.; Latrémouille, C.; Achouh, P.; Pirracchio, R.; et al. Early changes in diaphragmatic function evaluated using ultrasound in cardiac surgery patients: A cohort study. J. Clin. Monit. 2019, 34, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Doorduin, J.; van Hees, H.W.; van der Hoeven, J.G.; Heunks, L.M. Monitoring of the respiratory muscles in the critically ill. Am. J. Respir. Crit. Care Med. 2013, 187, 20–27. [Google Scholar] [CrossRef]
- McCool, F.D.; Oyieng’o, D.O.; Koo, P. The Utility of Diaphragm Ultrasound in Reducing Time to Extubation. Lung 2020, 198, 499–505. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).