Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters
Abstract
1. Introduction
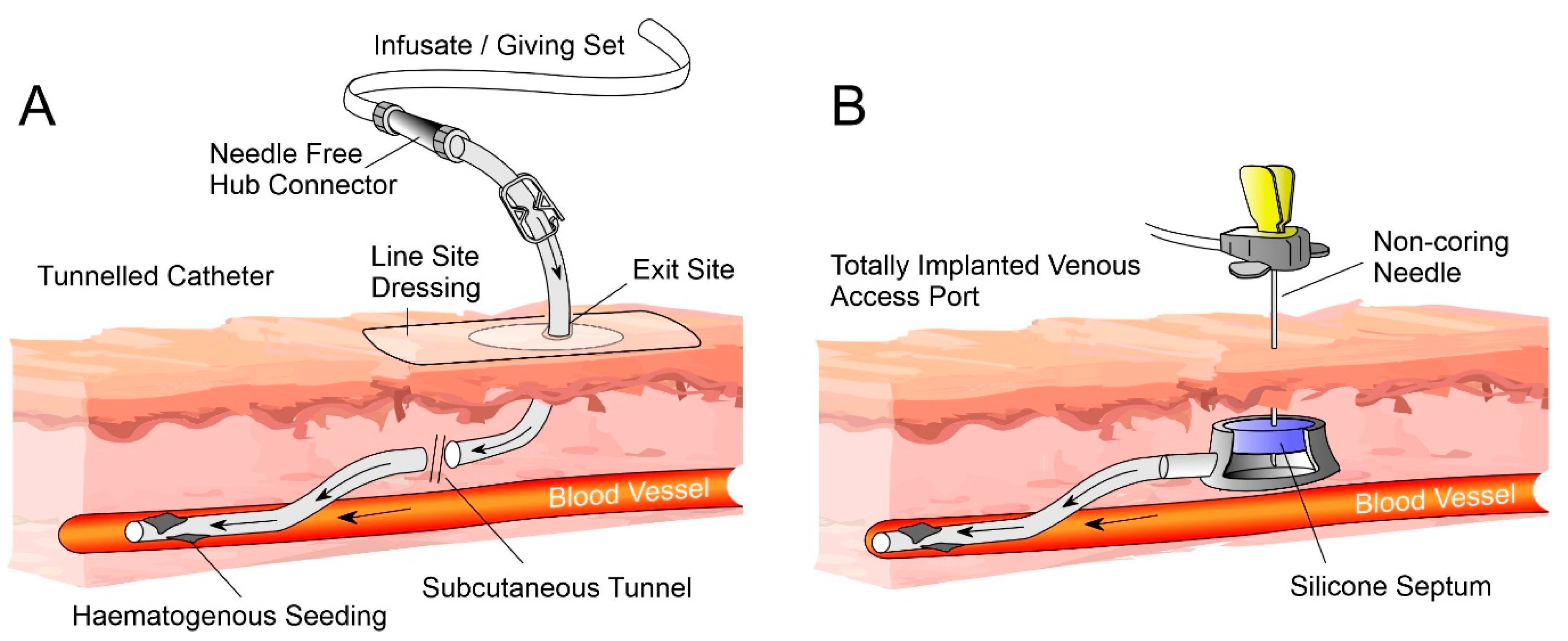
2. Catheter Components
3. Pathogens, Colonisation, Biofilms and Infection
4. Current Practice
4.1. Disinfection
4.2. Education and Aseptic Techniques
4.3. Catheter Locks
4.4. Barrier Caps
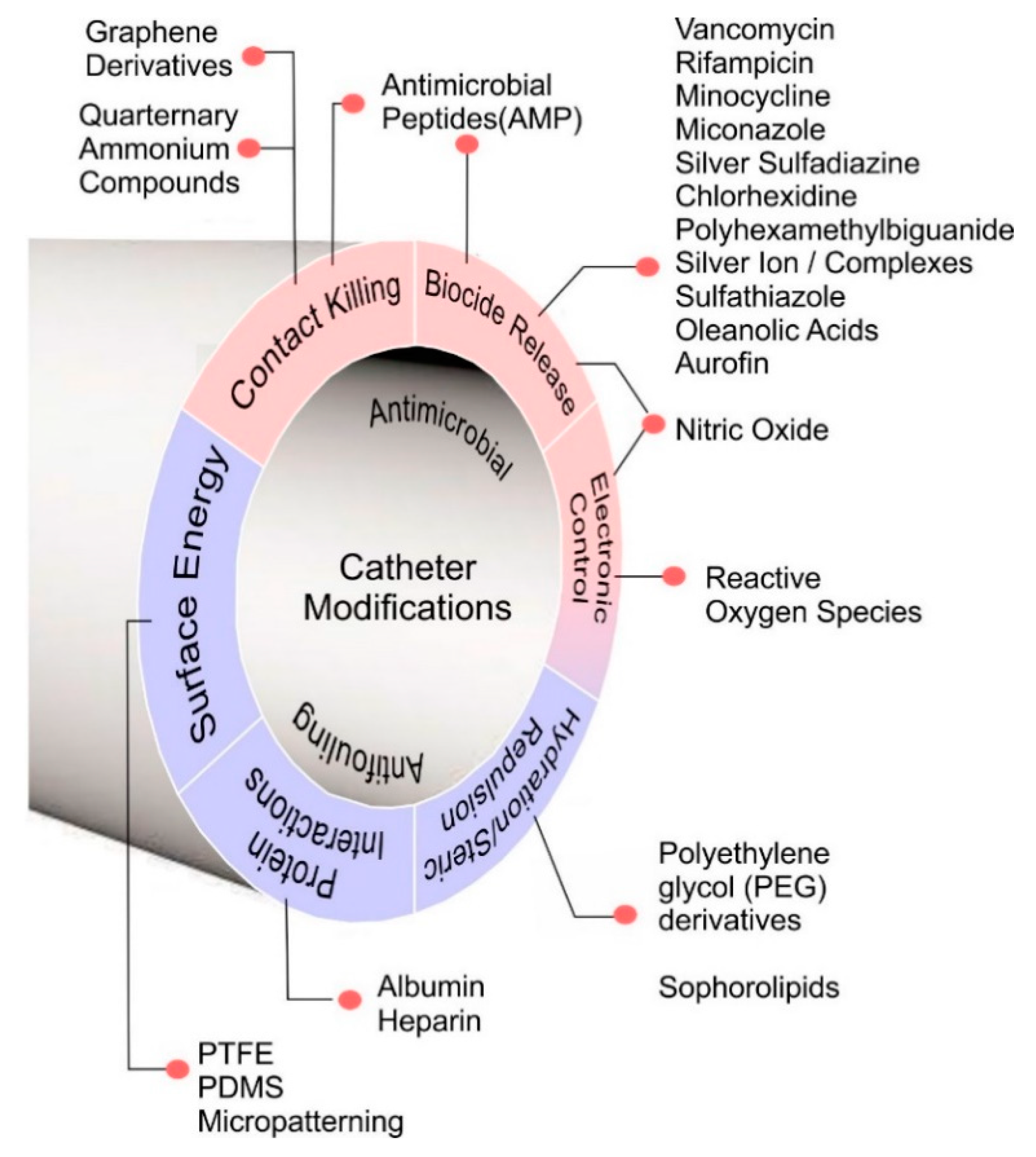
5. Catheter Designs and Antimicrobial Mechanisms
5.1. Biocide Release
5.2. Contact Kill Systems
5.3. Surface Hydration/Hydrophilicity
5.4. Protein Layer Interactions
5.5. Surface Energy
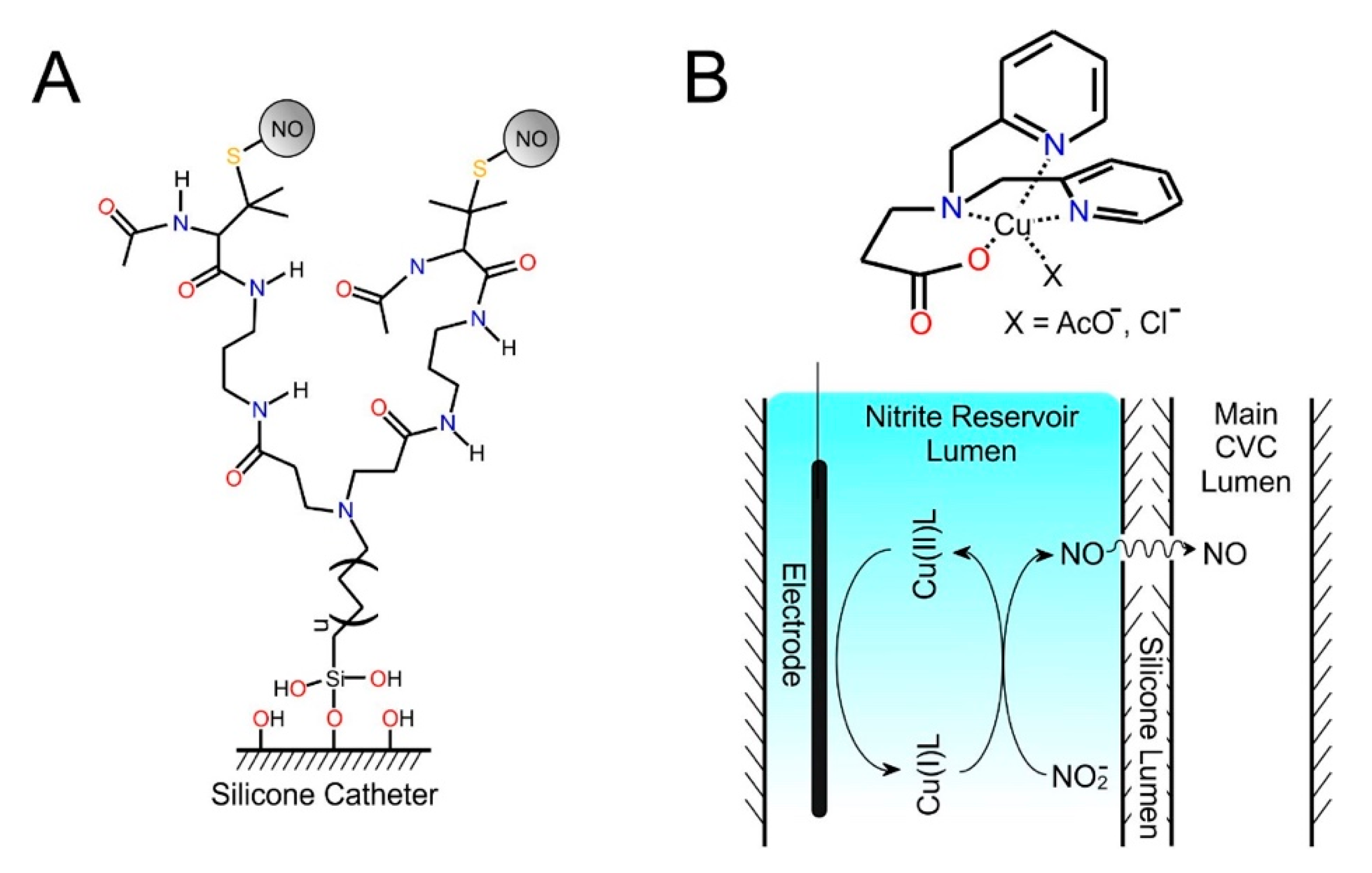
5.6. Smart/Electronic Materials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ripa, M.; Morata, L.; Rodriguez-Nunez, O.; Cardozo, C.; Puerta-Alcalde, P.; Hernandez-Meneses, M.; Ambrosioni, J.; Linares, L.; Bodro, M.; Valcárcel, A.; et al. Short-Term Peripheral Venous Catheter-Related Bloodstream Infections: Evidence for Increasing Prevalence of Gram-Negative Microorganisms from a 25-Year Prospective. Antimicrob. Agents Chemother. 2018, 62, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Gangoli, G.; Adams, E.; Hyde, R.; Broder, M.S.; Chang, E.; Reddy, S.R.; Tarbox, M.H.; Bentley, T.; Ovington, L.; et al. Increased Clinical and Economic Burden Associated with Peripheral Intravenous Catheter–Related Complications: Analysis of a US Hospital Discharge Database. Inqiry 2019, 56. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Nakamura, I.; Fujita, H.; Tsukimori, A.; Kobayashi, T.; Fukushima, S.; Fujii, T.; Matsumoto, T. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: A retrospective observational study. BMC Infect. Dis. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, S.; Marsh, N.; Ray-Barruel, G.; Flynn, J.; Larsen, E.; Rickard, C.M. Infection risks associated with peripheral vascular catheters. J. Infect. Prev. 2016, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Dahyot-Fizelier, C.; Mimoz, O. Prevention of central venous catheter-related infection in the intensive care unit. Crit. Care 2010, 14, 212. [Google Scholar] [CrossRef]
- Bouza, E. Intravascular catheter-related infections: A growth problem, the search for better solutions. Clin. Microbiol. Infect. 2002, 8, 255. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef]
- Mermel, L.A. Prevention of Intravascular Catheter–Related Infections. Ann. Intern. Med. 2000, 132, 391. [Google Scholar] [CrossRef]
- McGrath, E.; Du, W.; Rajpurkar, M. Preemptive Ethanol Lock Therapy in Pediatric Hematology/Oncology Patients with Catheter-Associated Bloodstream Infection: Impact on Length of Stay, Cost, and Catheter Salvage. Clin. Pediatr. (Phila) 2018, 57, 285–293. [Google Scholar] [CrossRef]
- Labriola, L. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin. Dial. 2019, 32, 402–405. [Google Scholar] [CrossRef]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Burillo, A.; Muñoz, P. Catheter-related infections: Diagnosis and intravascular treatment. Clin. Microbiol. Infect. 2002, 8, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Dimick, J.B.; Pelz, R.K.; Consunji, R.; Swoboda, S.M.; Hendrix, C.W.; Lipsett, P.A. Increased Resource Use Associated with Catheter-Related Bloodstream Infection in the Surgical Intensive Care Unit. Arch. Surg. 2001, 136, 229–234. [Google Scholar] [CrossRef]
- Saliba, P.; Hornero, A.; Cuervo, G.; Grau, I.; Jimenez, E.; García, D.; Tubau, F.; Martínez-Sánchez, J.M.; Carratalà, J.; Pujol, M. Mortality risk factors among non-ICU patients with nosocomial vascular catheter-related bloodstream infections: A prospective cohort study. J. Hosp. Infect. 2018, 99, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Curtis, N.; Worth, L.J.; Flynn, P.M. Central Line–associated Bloodstream Infection in Children. Pediatr. Infect. Dis. J. 2013, 32, 905–910. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Dellinger, E.P.; Gerberding, J.L.; Heard, S.O.; Maki, D.G.; Masur, H.; McCormick, R.D.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter–Related Infections. Clin. Infect. Dis. 2002, 35, 1281–1307. [Google Scholar] [CrossRef]
- Helder, O.K.; Kornelisse, R.F.; Reiss, I.K.M.; Ista, E. Disinfection practices in intravenous drug administration. Am. J. Infect. Control 2016, 44, 721–723. [Google Scholar] [CrossRef]
- Ista, E.; van der Hoven, B.; Kornelisse, R.F.; van der Starre, C.; Vos, M.C.; Boersma, E.; Helder, O.K. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 724–734. [Google Scholar] [CrossRef]
- Loveday, H.P.; Wilson, J.A.; Pratt, R.J.; Golsorkhi, M.; Tingle, A.; Bak, A.; Browne, J.; Prieto, J.; Wilcox, M. Epic3: National evidence-based guidelines for preventing healthcare-associated infections in nhs hospitals in england. J. Hosp. Infect. 2014, 86, S1–S70. [Google Scholar] [CrossRef]
- Smith, R.N.; Nolan, J.P. Central venous catheters. BMJ 2013, 347, f6570. [Google Scholar] [CrossRef]
- Wells, S. Venous access in oncology and haematology patients: Part two. Nurs. Stand. 2008, 23, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, F.; Cecero, E.; Degl’Innocenti, D.; Selmi, V.; Giua, R.; Villa, G.; Chelazzi, C.; Romagnoli, S.; Pittiruti, M. Infection of totally implantable venous access devices: A review of the literature. J. Vasc. Access 2018, 19, 230–242. [Google Scholar] [CrossRef]
- Moureau, N.L.; Flynn, J. Disinfection of Needleless Connector Hubs: Clinical Evidence Systematic Review. Nurs. Res. Pract. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. What is the predominant source of intravascular catheter infections? Clin. Infect. Dis. 2011, 52, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.J.; Moureau, N.L.; Sengupta, S. Quantitative assessment of reflux in commercially available needle-free IV connectors. J. Vasc. Access 2018, 19, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hadaway, L.; Richardson, D. Needleless connectors: A primer on terminology. J. Infus. Nurs. 2010, 33, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hadaway, L. Needleless connectors for IV catheters. Am. J. Nurs. 2012, 112, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.R.; Schilling, S.; Doellman, D.; Hutchinson, N.; Rickey, M.; Nelson, S. Central Venous Catheter Occlusion: A Prospective, Controlled Trial Examining the Impact of a Positive-Pressure Valve Device. J. Parenter. Enter. Nutr. 2004, 28, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Doellman, D.; Hutchinson NJacobs, B.R. The impact of needleless connector device design on central venous catheter occlusion in children: A prospective, controlled trial. J. Parenter. Enter. Nutr. 2006, 30, 85–90. [Google Scholar] [CrossRef]
- Hadaway, L. Needleless connectors: Improving practice, reducing risks. JAVA-J. Assoc. Vasc. Access 2011, 16, 20–24. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Murphy, C.; Hall, K.K.; Fogle, P.J.; Karchmer, T.B.; Harrington, G.; Salgado, C.; Giannetta, E.T.; Cameron, C.; Sherertz, R.J. Health Care–Associated Bloodstream Infections Associated with Negative- or Positive-Pressure or Displacement Mechanical Valve Needleless Connectors. Clin. Infect. Dis. 2009, 49, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, K.G.; Ellis, C.R.; Defaye, P.; Kennergren, C. Cardiac implantable electronic device infection in patients at risk. Arrhythmia Electrophysiol. Rev. 2016, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Tascini, C.; Tagliaferri, E.; Cori, A.D.; Soldati, E.; Leonildi, A.; Zucchelli, G.; Ciullo, I.; Menichetti, F. Microbiology of cardiac implantable electronic device infections. Europace 2012, 14, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Pronovost, P. Interventions to decrease catheter-related bloodstream infections in the ICU: The Keystone Intensive Care Unit Project. Am. J. Infect. Control 2008, 36, S171.e1–S171.e5. [Google Scholar] [CrossRef]
- Drews, F.A.; Bakdash, J.Z.; Gleed, J.R. Improving central line maintenance to reduce central line-associated bloodstream infections. Am. J. Infect. Control 2017, 45, 1224–1230. [Google Scholar] [CrossRef]
- Crnich, C.J.J.; Maki, D.G.G. The Promise of Novel Technology for the Prevention of Intravascular Device–Related Bloodstream Infection. II. Long-Term Devices. Clin. Infect. Dis. 2002, 34, 1362–1368. [Google Scholar] [CrossRef]
- Chernecky, C.; Waller, J. Comparative evaluation of five needleless intravenous connectors. J. Adv. Nurs. 2011, 67, 1601–1613. [Google Scholar] [CrossRef]
- Menyhay, S.; Maki, D. Disinfection of Needleless Catheter Connectors and Access Ports with Alcohol May Not Prevent Microbial Entry: The Promise of a Novel Antiseptic-Barrier Cap. Infect. Control Hosp. Epidemiol. 2006, 27, 23–27. [Google Scholar] [CrossRef]
- Rupp, M.E.; Sholtz, L.A.; Jourdan, D.R.; Marion, N.D.; Tyner, L.K.; Fey, P.D.; Iwen, P.C.; Anderson, J.R. Outbreak of Bloodstream Infection Temporally Associated with the Use of an Intravascular Needleless Valve. Clin. Infect. Dis. 2007, 44, 1408–1414. [Google Scholar] [CrossRef][Green Version]
- Macias, A.E.; Huertas, M.; Ponce de Leon, S.; Munoz, J.M.; Chavez, A.R.; Sifuentes-Osornio, J.; Romero, C.; Bobadilla, M. Contamination of intravenous fluids: A continuing cause of hospital bacteremia. Am. J. Infect. Control 2010, 38, 217–221. [Google Scholar] [CrossRef]
- Fowler, V.G.; Justice, A.; Moore, C.; Benjamin, D.K.; Woods, C.W.; Campbell, S.; Reller, L.B.; Corey, G.R.; Day, N.P.J.; Peacock, S.J. Risk Factors for Hematogenous Complications of Intravascular Catheter-Associated Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2005, 40, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.G.; Weiner, L.M.; Milstone, A.M.; Saiman, L.; Magill, S.S.; See, I. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011–2014. Infect. Control Hosp. Epidemiol. 2018, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alby-Laurent, F.; Lambe, C.; Ferroni, A.; Salvi, N.; Lebeaux, D.; Le Gouëz, M.; Castelle, M.; Moulin, F.; Nassif, X.; Lortholary, O.; et al. Salvage strategy for long-term central venous catheter-associated Staphylococcus aureus infections in children. Front. Pediatr. 2019, 6, 427. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Zanwar, S.; Jain, P.; Gokarn, A.; Devadas, S.K.; Punatar, S.; Khurana, S.; Bonda, A.; Pruthy, R.; Bhat, V.; Qureshi, S.; et al. Antibiotic lock therapy for salvage of tunneled central venous catheters with catheter colonization and catheter-related bloodstream infection. Transpl. Infect. Dis. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Pérez-Zárate, P.; Aragón-Piña, A.; Soria-Guerra, R.E.; González-Amaro, A.M.; Pérez-Urizar, J.; Pérez-González, L.F.; Martinez-Gutierrez, F. Risk factors and biofilm detection on central venous catheters of patients attended at tertiary hospital. Micron 2015, 78, 33–39. [Google Scholar] [CrossRef]
- Gominet, M.; Compain, F.; Beloin, C.; Lebeaux, D. Central venous catheters and biofilms: Where do we stand in 2017? Apmis 2017, 125, 365–375. [Google Scholar] [CrossRef]
- Murga, R.; Miller, J.M.; Donlan, R.M. Biofilm formation by gram-negative bacteria on central venous catheter connectors: Effect of conditioning films in a laboratory model. J. Clin. Microbiol. 2001, 39, 2294–2297. [Google Scholar] [CrossRef]
- Devries, M.; Mancos, P.S.; Valentine, M.J. Reducing bloodstream infection risk in central and peripheral intravenous lines: Initial data on passive intravenous connector disinfection. JAVA-J. Assoc. Vasc. Access 2014, 19, 87–93. [Google Scholar] [CrossRef]
- Menyhay, S.Z.; Maki, D.G. Preventing central venous catheter-associated bloodstream infections: Development of an antiseptic barrier cap for needleless connectors. Am. J. Infect. Control 2008, 36, S174.e1–S174.e5. [Google Scholar] [CrossRef]
- Soothill, J.S.; Bravery, K.; Ho, A.; Macqueen, S.; Collins, J.; Lock, P. A fall in bloodstream infections followed a change to 2% chlorhexidine in 70% isopropanol for catheter connection antisepsis: A pediatric single center before/after study on a hemopoietic stem cell transplant ward. Am. J. Infect. Control 2009, 37, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Macias, J.H.; Arreguin, V.; Munoz, J.M.; Alvarez, J.A.; Mosqueda, J.L.; Macias, A.E. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am. J. Infect. Control 2013, 41, 634–637. [Google Scholar] [CrossRef]
- Kaler, W.; Chinn, R. Successful Disinfection of Needleless Access Ports: A Matter of Time and Friction. J. Assoc. Vasc. Access 2007, 12, 140–142. [Google Scholar] [CrossRef]
- Mazher, M.A.; Kallen, A.; Edwards, J.R.; Donlan, R.M. An In Vitro evaluation of disinfection protocols used for needleless connectors of central venous catheters. Lett. Appl. Microbiol. 2013, 57, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Irwin, G.; Viney, M.; Watkins, L.; Morris, S.; Kirksey, K.; Brown, A. Optimal Disinfection Times for Needleless Intravenous Connectors. J. Assoc. Vasc. Access 2012, 17, 137–143. [Google Scholar] [CrossRef]
- Simmons, S.; Bryson, C.; Porter, S. “Scrub the hub”: Cleaning duration and reduction in bacterial load on central venous catheters. Crit. Care Nurs. Q. 2011, 34, 31–35. [Google Scholar] [CrossRef]
- Rupp, M.E.; Yu, S.; Huerta, T.; Cavalieri, R.J.; Alter, R.; Fey, P.D.; Van Schooneveld, T.; Anderson, J.R. Adequate Disinfection of a Split-Septum Needleless Intravascular Connector with a 5-Second Alcohol Scrub. Infect. Control Hosp. Epidemiol. 2012, 33, 661–665. [Google Scholar] [CrossRef]
- Caspari, L.; Epstein, E.; Blackman, A.; Jin, L.; Kaufman, D.A. Human factors related to time-dependent infection control measures: “Scrub the hub” for venous catheters and feeding tubes. Am. J. Infect. Control 2017, 45, 648–651. [Google Scholar] [CrossRef]
- Sannoh, S.; Clones, B.; Munoz, J.; Montecalvo, M.; Parvez, B. A multimodal approach to central venous catheter hub care can decrease catheter-related bloodstream infection. Am. J. Infect. Control 2010, 38, 424–429. [Google Scholar] [CrossRef]
- Young, E.M.; Commiskey, M.L.; Wilson, S.J. Translating evidence into practice to prevent central venous catheter- associated bloodstream infections: A systems-based intervention. Am. J. Infect. Control 2006, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.S.; Park, S.M.; Lee, J.M.; Song, J.Y.; Lee, S.J. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am. J. Infect. Control 2013, 41, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Hadaway, L. Intermittent Intravenous Administration Sets: Survey of Current Practices. J. Assoc. Vasc. Access. 2007, 12, 143–147. [Google Scholar] [CrossRef]
- Bond, A.; Teubner, A.; Taylor, M.; Cawley, C.; Abraham, A.; Dibb, M.; Chadwick, P.R.; Soop, M.; Carlson, G.; Lal, S. Assessing the impact of quality improvement measures on catheter related blood stream infections and catheter salvage: Experience from a national intestinal failure unit. Clin. Nutr. 2018, 37, 2097–2101. [Google Scholar] [CrossRef]
- Davis, J. Central-Line-Associated Bloodstream Infection: Comprehensive, Data-Driven Prevention. Pennsylvania Patient Saf Auth. Patient Saf. Advis. 2011, 8, 100–104. [Google Scholar]
- Goossens, G.A. Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs. Res. Pract. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Norris, L.A.B.; Kablaoui, F.; Brilhart, M.K.; Bookstaver, P.B. Systematic review of antimicrobial lock therapy for prevention of central-line-associated bloodstream infections in adult and pediatric cancer patients. Int. J. Antimicrob. Agents 2017, 50, 308–317. [Google Scholar] [CrossRef]
- Dang, F.P.; Li, H.J.; Wang, R.J.; Wu, Q.; Chen, H.; Ren, J.J.; Tian, J.H. Comparative efficacy of various antimicrobial lock solutions for preventing catheter-related bloodstream infections: A network meta-analysis of 9099 patients from 52 randomized controlled trials. Int. J. Infect. Dis. 2019, 87, 154–165. [Google Scholar] [CrossRef]
- Vassallo, M.; Dunais, B.; Roger, P.M. Antimicrobial lock therapy in central-line associated bloodstream infections: A systematic review. Infection 2015, 43, 389–398. [Google Scholar] [CrossRef]
- Krishnasami, Z.; Carlton, D.; Bimbo, L.; Taylor, M.E.; Balkovetz, D.F.; Barker, J.; Allon, M. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002, 61, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Basas, J.; Palau, M.; Ratia, C.; del Pozo, J.L.; Martín-Gomez, M.T.; Gomis, X.; Torrents, E.; Almirante, B.; Gavaldà, J. High-Dose Daptomycin is Effective as an Antibiotic Lock Therapy in a Rabbit Model of Staphylococcus epidermidis Catheter-Related Infection. Antimicrob. Agents Chemother. 2018, 62, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Carratalà, J. The antibiotic-lock technique for therapy of “highly needed” infected catheters. Clin. Microbiol. Infect. 2002, 8, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lei, J.H.; Su, X.J.; Wang, X.H. Ethanol locks for the prevention of catheter-related bloodstream infection: A meta-analysis of randomized control trials. BMC Anesthesiol. 2018, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arechabala, M.C.; Catoni, M.I.; Claro, J.C.; Rojas, N.P.; Rubio, M.E.; Calvo, M.A.; Letelier, L.M. Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Gudiol, C.; Nicolae, S.; Royo-Cebrecos, C.; Aguilar-Guisado, M.; Montero, I.; Martín-Gandul, C.; Perayre, M.; Berbel, D.; Encuentra, M.; Arnan, M.; et al. Administration of taurolidine-citrate lock solution for prevention of central venous catheter infection in adult neutropenic haematological patients: A randomised, double-blinded, placebo-controlled trial (TAURCAT). Trials 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Oto, J.; Imanaka, H.; Konno, M.; Nakataki, E.; Nishimura, M. A prospective clinical trial on prevention of catheter contamination using the hub protection cap for needleless injection device. Am. J. Infect. Control 2011, 39, 309–313. [Google Scholar] [CrossRef]
- Voor In ‘t Holt, A.F.; Helder, O.K.; Vos, M.C.; Schafthuizen, L.; Sülz, S.; van den Hoogen, A.; Ista, E. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2017, 69, 34–40. [Google Scholar] [CrossRef]
- Sweet, M.A.; Cumpston, A.; Briggs, F.; Craig, M.; Hamadani, M. Impact of alcohol-impregnated port protectors and needleless neutral pressure connectors on central line-associated bloodstream infections and contamination of blood cultures in an inpatient oncology unit. Am. J. Infect. Control 2012, 40, 931–934. [Google Scholar] [CrossRef]
- Hankins, R.; Majorant, O.D.; Rupp, M.E.; Cavalieri, R.J.; Fey, P.D.; Lyden, E.; Cawcutt, K.A. Microbial colonization of intravascular catheter connectors in hospitalized patients. Am. J. Infect. Control 2019, 47, 1489–1492. [Google Scholar] [CrossRef]
- Kamboj, M.; Blair, R.; Bell, N.; Son, C.; Huang, Y.T.; Dowling, M.; Lipitz-Snyderman, A.; Eagan, J.; Sepkowitz, K. Use of disinfection cap to reduce central-line—Associated bloodstream infection and blood culture contamination among hematology—Oncology patients. Infect. Control Hosp. Epidemiol. 2015, 36, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, S.M.; Van Wyck, D.B.; Njord, L.; Ziebol, R.J.; Lynch, L.E.; Killion, D.P. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J. Am. Soc. Nephrol. 2018, 29, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.G.; Casariego, G.J.N.; Romero, M.M.V.; García, J.G.; Diaz, R.R.; Perez, J.A.P. Reducing the degree of colonisation of venous access catheters by continuous passive disinfection. Eur. J. Hosp. Pharm. 2016, 23, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, L.; Mokrzycki, M.H. Prevention of hemodialysis catheter infections: Ointments, dressings, locks, and catheter hub devices. Hemodial. Int. 2018, 22, S75–S82. [Google Scholar] [CrossRef]
- Buchman, A.L.; Spapperi, J.; Leopold, P. A new central venous catheter cap: Decreased microbial growth and risk for catheter-related bloodstream infection. J. Vasc. Access 2009, 10, 11–21. [Google Scholar] [CrossRef]
- Mariyaselvam, M.; Hodges, E.; Richardson, J.; Steel, A.; Moondi, P.; Young, P. The coated antiseptic tip (CAT) syringe. J. Med. Eng. Technol. 2015, 39, 259–263. [Google Scholar] [CrossRef]
- Wildgruber, M.; Lueg, C.; Borgmeyer, S.; Karimov, I.; Braun, U.; Kiechle, M.; Meier, R.; Koehler, M.; Ettl, J.; Berger, H. Polyurethane versus silicone catheters for central venous port devices implanted at the forearm. Eur. J. Cancer 2016, 59, 113–124. [Google Scholar] [CrossRef]
- Yuh, J.; Yi, T.; Fen, M.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]
- Thomas, S.; Datta, J.; Haponiuk, J.; Reghunadhan, A. Polyurethane Polymers: Composites and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Akindoyo, J.; Beg, M.; Ghazali, S.; Islam, M.; Jeyaratnam, N.; Yuvaraj, A. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Ishihara, K.; Liu, Y.; Inoue, Y. Advances in Polyurethane Biomaterials; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Mathew, E.; Domínguez-Robles, J.; Larrañeta, E.; Lamprou, D.A. Fused deposition modelling as a potential tool for antimicrobial dialysis catheters manufacturing: New trends vs. conventional approaches. Coatings 2019, 9, 515. [Google Scholar] [CrossRef]
- Viola, G.M.; Rosenblatt, J.; Raad, I.I. Drug eluting antimicrobial vascular catheters: Progress and promise. Adv. Drug. Deliv. Rev. 2017, 112, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Villani, M.; Consonni, R.; Canetti, M.; Bertoglio, F.; Iervese, S.; Bruni, G.; Visai, L.; Iannace, S.; Bertini, F. Polyurethane-Based Composites: Effects of Antibacterial Fillers on the Physical-Mechanical Behavior of Thermoplastic Polyurethanes. Polymers 2020, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Barde, M.; Davis, M.; Rangari, S.; Mendis, H.C.; De La Fuente, L.; Auad, M.L. Development of antimicrobial-loaded polyurethane films for drug-eluting catheters. J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Zander, Z.K.; Chen, P.; Hsu, Y.H.; Dreger, N.Z.; Savariau, L.; McRoy, W.C.; Cerchiari, A.E.; Chambers, S.D.; Barton, H.A.; Becker, M.L. Post-fabrication QAC-functionalized thermoplastic polyurethane for contact-killing catheter applications. Biomaterials 2018, 178, 339–350. [Google Scholar] [CrossRef]
- May, R.M.; Magin, C.M.; Mann, E.E.; Drinker, M.C.; Fraser, J.C.; Siedlecki, C.A.; Brennan, A.B.; Reddy, S.T. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 2015, 4, 9–16. [Google Scholar] [CrossRef]
- Peng, C.; Vishwakarma, A.; Li, Z.; Miyoshi, T.; Barton, H.A.; Joy, A. Modification of a conventional polyurethane composition provides significant anti-biofilm activity against: Escherichia coli. Polym. Chem. 2018, 9, 3195–3198. [Google Scholar] [CrossRef]
- Francolini, I.; Silvestro, I.; Di Lisio, V.; Martinelli, A.; Piozzi, A. Synthesis, characterization, and bacterial fouling-resistance properties of polyethylene glycol-grafted polyurethane elastomers. Int. J. Mol. Sci. 2019, 20, 1001. [Google Scholar] [CrossRef]
- Yu, H.; Liu, L.; Li, X.; Zhou, R.; Yan, S.; Li, C.; Luan, S.; Yin, J.; Shi, H. Fabrication of polylysine based antibacterial coating for catheters by facile electrostatic interaction. Chem. Eng. J. 2019, 360, 1030–1041. [Google Scholar] [CrossRef]
- Lozeau, L.D.; Alexander, T.E.; Camesano, T.A. Surface-Tethered Antimicrobial Peptides: An Invention to Create Effective Antimicrobial Coatings. Technol. Innov. 2019, 20, 441–454. [Google Scholar] [CrossRef]
- McCoy, C.P.; Irwin, N.J.; Donnelly, L.; Jones, D.S.; Hardy, J.G.; Carson, L. Anti-Adherent Biomaterials for Prevention of Catheter Biofouling. Int. J. Pharm. 2018, 535, 420–427. [Google Scholar] [CrossRef]
- Machuca, J.; Lopez-Rojas, R.; Fernandez-Cuenca, F.; Pascual, A. Comparative activity of a polyhexanide–betaine solution against biofilms produced by multidrug-resistant bacteria belonging to high-risk clones. J. Hosp. Infect. 2019, 103, e92–e96. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, L.; Yang, H.; Zhou, R.; Che, C.; Li, X.; Li, C.; Luan, S.; Yin, J.; Shi, H. Water-Insoluble Polymeric Guanidine Derivative and Application in the Preparation of Antibacterial Coating of Catheter. ACS Appl. Mater. Interfaces 2018, 10, 39257–39267. [Google Scholar] [CrossRef] [PubMed]
- Pant, J.; Goudie, M.J.; Chaji, S.M.; Johnson, B.W.; Handa, H. Nitric oxide releasing vascular catheters for eradicating bacterial infection. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Goudie, M.J.; Singha, P.; Hopkins, S.P.; Brisbois, E.J.; Handa, H. Active Release of an Antimicrobial and Antiplatelet Agent from a Nonfouling Surface Modification. ACS Appl. Mater. Interfaces 2019, 11, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Sajeevan, S.E.; Chatterjee, M.; Paul, V.; Baranwal, G.; Kumar, V.A.; Bose, C.; Banerji, A.; Nair, B.G.; Prasanth, B.P.; Biswas, R. Impregnation of catheters with anacardic acid from cashew nut shell prevents Staphylococcus aureus biofilm development. J. Appl. Microbiol. 2018, 125, 1286–1295. [Google Scholar] [CrossRef]
- Lim, K.; Saravanan, R.; Chong, K.K.L.; Goh, S.H.M.; Chua, R.R.Y.; Tambyah, P.A.; Chang, M.W.; Kline, K.A.; Leong, S.S.J. Anhydrous polymer-based coating with sustainable controlled release functionality for facile, efficacious impregnation, and delivery of antimicrobial peptides. Biotechnol. Bioeng. 2018, 115, 2000–2012. [Google Scholar] [CrossRef]
- Bayston, R.; Ashraf, W.; Pelegrin, I.; Fowkes, K.; Bienemann, A.S.; Singleton, W.G.B.; Scott, I.S. An external ventricular drainage catheter impregnated with rifampicin, trimethoprim and triclosan, with extended activity against MDR Gram-negative bacteria: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019, 74, 2959–2964. [Google Scholar] [CrossRef]
- Liu, H.; Shukla, S.; Vera-González, N.; Tharmalingam, N.; Mylonakis, E.; Fuchs, B.B.; Shukla, A. Auranofin Releasing Antibacterial and Antibiofilm Polyurethane Intravascular Catheter Coatings. Front. Cell Infect. Microbiol. 2019, 9, 37. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Prevention Strategies; BSI Guidelines Library Infection Control CDC n.d.: Atlanta, GA, USA, 2011. [Google Scholar]
- Lai, N.M.; Chaiyakunapruk, N.; Lai, N.A.; O’Riordan, E.; Pau, W.S.C.; Saint, S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Narayana, J.L.; Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, G. Modulation of antimicrobial potency of human cathelicidin peptides against the ESKAPE pathogens and in vivo efficacy in a murine catheter-associated biofilm model. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1592–1602. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, Y.Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.J. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Schillaci, D.; Varvarà, P.; Cusimano, M.G.; Geraci, D.M.; Giuffrè, M.; Cavallaro, G.; Maida, C.M.; Giammona, G. Branched High Molecular Weight Glycopolypeptide with Broad-Spectrum Antimicrobial Activity for the Treatment of Biofilm Related Infections. ACS Appl. Mater. Interfaces 2018, 10, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Konopińska, K.K.; Schmidt, N.J.; Hunt, A.P.; Lehnert, N.; Wu, J.; Xi, C.; Meyerhoff, M.E. Comparison of Copper(II)-Ligand Complexes as Mediators for Preparing Electrochemically Modulated Nitric Oxide-Releasing Catheters. ACS Appl. Mater. Interfaces 2018, 10, 25047–25055. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.P.; Batka, A.E.; Hosseinzadeh, M.; Gregory, J.D.; Haque, H.K.; Ren, H.; Meyerhoff, M.R.; Lehnert, N. Nitric Oxide Generation on Demand for Biomedical Applications via Electrocatalytic Nitrite Reduction by Copper BMPA- And BEPA-Carboxylate Complexes. ACS Catal. 2019, 9, 7746–7758. [Google Scholar] [CrossRef]
- Maharubin, S.; Nayak, C.; Phatak, O.; Kurhade, A.; Singh, M.; Zhou, Y.; Tan, G. Polyvinylchloride coated with silver nanoparticles and zinc oxide nanowires for antimicrobial applications. Mater. Lett. 2019, 249, 108–111. [Google Scholar] [CrossRef]
- Stevens, K.N.J.; Croes, S.; Boersma, R.S.; Stobberingh, E.E.; van der Marel, C.; van der Veen, F.H.; Knetsch, M.L.W.; Koole, L.H. Hydrophilic surface coatings with embedded biocidal silver nanoparticles and sodium heparin for central venous catheters. Biomaterials 2011, 32, 1264–1269. [Google Scholar] [CrossRef]
- Lotlikar, S.R.; Gallaway, E.; Grant, T.; Popis, S.; Whited, M.; Guragain, M.; Rogers, R.; Hamilton, S.; Gerasimchuk, N.G.; Patrauchan, M.A. Polymeric composites with silver (I) cyanoximates inhibit biofilm formation of gram-positive and gram-negative bacteria. Polymers 2019, 11, 1018. [Google Scholar] [CrossRef]
- Redfern, J.; Geerts, L.; Seo, J.W.; Verran, J.; Tosheva, L.; Wee, L.H. Toxicity and Antimicrobial Properties of ZnO@ZIF-8 Embedded Silicone against Planktonic and Biofilm Catheter-Associated Pathogens. ACS Appl. Nano Mater. 2018, 1, 1657–1665. [Google Scholar] [CrossRef]
- Balne, P.K.; Harini, S.; Dhand, C.; Dwivedi, N.; Chalasani, M.L.S.; Verma, N.K.; Barathi, V.A.; Beuerman, R.; Agrawal, R.; Lakshminarayanan, R. Surface characteristics and antimicrobial properties of modified catheter surfaces by polypyrogallol and metal ions. Mater. Sci. Eng. C 2018, 90, 673–684. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, J.; Yadav, K.; Mitra, M.; Pal, S.; Ray, A.; Gupta, S.; Medatwal, N.; Gupta, R.; Mishra, D.; et al. Cholic Acid-Derived Amphiphile which Combats Gram-Positive Bacteria-Mediated Infections via Disintegration of Lipid Clusters. ACS Biomater. Sci. Eng. 2019, 5, 4764–4775. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; Álvarez De Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acid Derivatives as a Promising Treatment against Bacterial Biofilm in Nosocomial Infections: An in Vitro and in Vivo Study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhães, F.D.; Pinto, A.M.; Gonçalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon N. Y. 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Yu, K.; Mei, Y.; Kizhakkedathu, J.N. Polymer brush-based approaches for the development of infection-resistant surfaces. J. Mater. Chem. B 2014, 2, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Valotteau, C.; Baccile, N.; Humblot, V.; Roelants, S.; Soetaert, W.; Stevens, C.V.; Dufrêne, Y.F. Nanoscale antiadhesion properties of sophorolipid-coated surfaces against pathogenic bacteria. Nanoscale Horiz. 2019, 4, 975–982. [Google Scholar] [CrossRef]
- Baumann Kreuziger, L.; Jaffray, J.; Carrier, M. Epidemiology, diagnosis, prevention and treatment of catheter-related thrombosis in children and adults. Thromb. Res. 2017, 157, 64–71. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.H.; Reiss, U.; Wilimas, J.A.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef]
- Geerts, W. Central venous catheter-related thrombosis. Hematology 2014, 2014, 306–311. [Google Scholar] [CrossRef]
- Roth, Y.; Lewitus, D.Y. The grafting of multifunctional antithrombogenic chemical networks on polyurethane intravascular catheters. Polymers 2020, 12, 1131. [Google Scholar] [CrossRef]
- Smith, R.S.; Zhang, Z.; Bouchard, M.; Li, J.; Lapp, H.S.; Brotske, G.R.; Lucchnio, D.L.; Weaver, D.; Roth, L.A.; Coury, A.; et al. Vascular Catheters with a Nonleaching Poly-Sulfobetaine Surface Modification Reduce Thrombus Formation and Microbial Attachment. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl. Mater. Interfaces 2014, 6, 11385–11393. [Google Scholar] [CrossRef]
- Alomari, A.I.; Falk, A. The Natural History of Tunneled Hemodialysis Catheters Removed or Exchanged: A Single-Institution Experience. J. Vasc. Interv. Radiol. 2007, 18, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Van Rooden, C.J.; Schippers, E.F.; Barge, R.M.Y.; Rosendaal, F.R.; Guiot, H.F.L.; Van Der Meer, F.J.M.; Meinders, E.; Huisman, M.V. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: A prospective study. J. Clin. Oncol. 2005, 23, 2655–2660. [Google Scholar] [CrossRef]
- Mehall, J.R.; Saltzman, D.A.; Jackson, R.J.; Smith, S.D. Fibrin sheath enhances central venous catheter infection. Crit. Care Med. 2002, 30, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Albadawi, H.; Patel, N.; Khademhosseini, A.; Zhang, Y.S.; Naidu, S.; Knuttinen, G.; Oklu, R. Anti-fouling strategies for central venous catheters. Cardiovasc. Diagn. Ther. 2017, 7, S246–S257. [Google Scholar] [CrossRef]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef]
- Ps, S.; Shah, N. Heparin-bonded catheters for prolonging the patency of central venous catheters in children (Review) summary of findings for the main comparison. Cochrane Database Syst. Rev. 2014, 3–5. [Google Scholar] [CrossRef]
- Falk, A. The Role of Surface Coatings on Central Venous and Hemodialysis Catheters. Buyers Guid 2009, 2009, 51–53. [Google Scholar]
- Arepally, G.M. Clinical platelet disorders heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef]
- Chong, B. Heparin-induced thrombocytopenia. J. Thromb. Haemost. 2003, 1, 1471–1478. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling coatings of poly(dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.F.; Berger, R.; Butt, H.J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic polymeric superhydrophobic surfaces and nanostructures: From fabrication to applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Li, M.; Phair, J.; Cardosi, M.F.M.F.; Davis, J. Nanostructuring carbon fibre probes for use in central venous catheters. Anal. Chim. Acta 2014, 812, 1–5. [Google Scholar] [CrossRef]
- Davis, J.; Molina, M.T.; Leach, C.P.; Cardosi, M.F. Plasma-polyplumbagin-modified microfiber probes: A functional material approach to monitoring vascular access line contamination. ACS Appl. Mater. Interfaces 2013, 5, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Casimero, C.; McConville, A.; Fearon, J.J.; Lawrence, C.L.; Taylor, C.M.; Smith, R.B.; Davis, J. Sensor systems for bacterial reactors: A new flavin-phenol composite film for the in situ voltammetric measurement of pH. Anal. Chim. Acta 2018, 1027, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Alonso-Arce, M.; Schmidt, C.; Valderas, D.; Sedano, B.; Legarda, J.; Arizti, F.; Gómez, E.; Aguinaga, A.; Del Pozo, J.L.; et al. Smart central venous port for early detection of bacterial biofilm related infections. Biomed. Microdevices 2014, 16, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Mihu, M.M.R.M.; Cabral, V.; Pattabhi, R.; Tar, M.M.T.M.; Davies, K.K.P.K.; Friedman, A.J.A.; Martinez, L.R.; Nosanchuk, J.D. Sustained nitric oxide-releasing nanoparticles interfere with methicillinresistant staphylococcus aureus adhesion and biofilm formation in a rat central venous catheter model. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]



| Action | Negative | Neutral | Positive | Anti-Reflux |
|---|---|---|---|---|
| Disconnection | Blood refluxes into catheter | Blood refluxes into catheter | Fluid moves towards patient | Fluid restricted by diaphragm |
| Connection | Fluid moves toward patient | Fluid moves toward patient | Blood refluxes into catheter | Fluid restricted by diaphragm |
| Clamping sequence | Clamp before disconnection | No specified clamping | Clamp after disconnection | No specified clamping |
| Commercial Examples | BD Smartsite | ICU Medical Microclave Clear | Braun Ultrasite | ICU Medical Neutron |
| BD Q-Syte | Baxter One-Link | BD MaxPlus | Nexus TKO-5 | |
| Baxter Interlink | RyMed Invision | Braun Caresite | Nexus TKO-6P | |
| ICU Medical Clave* | Nexus NIS-6P |
| Pathogen | Prevalence |
|---|---|
| Coagulase-negative staphylococci (i.e., S. epidermidis) | 20.9% |
| Staphylococcus aureus | 18.1% |
| Escherichia coli | 7.4% |
| Klebsiella pneumoniae/Klebsiella oxytoca | 9.4% |
| Enterococcus faecalis | 9.1% |
| Manufacturer | Product | Antimicrobial Agent | Mode of Action |
|---|---|---|---|
| Kimal | Altius® ProActiv+ | Polyhexamethylene biguanide (PHMB) | Contact |
| Teleflex | ARROWg+ard® | Chlorhexidine and silver sulfadiazine | Eluting |
| Chlorag+ard® | Chlorhexidine only | ||
| Cook Medical | Spectrum® | Minocycline & Rifampin | Eluting |
| B. Braun | Certofix® | Polyhexamethylene biguanide (PHMB) | Contact |
| Edward Lifesciences | Vantex CVC Oligon | Silver ions | |
| AMC Thromboshield | Benzalkonium chloride with heparin coating | Contact |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casimero, C.; Ruddock, T.; Hegarty, C.; Barber, R.; Devine, A.; Davis, J. Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters. Medicines 2020, 7, 49. https://doi.org/10.3390/medicines7090049
Casimero C, Ruddock T, Hegarty C, Barber R, Devine A, Davis J. Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters. Medicines. 2020; 7(9):49. https://doi.org/10.3390/medicines7090049
Chicago/Turabian StyleCasimero, Charnete, Todd Ruddock, Catherine Hegarty, Robert Barber, Amy Devine, and James Davis. 2020. "Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters" Medicines 7, no. 9: 49. https://doi.org/10.3390/medicines7090049
APA StyleCasimero, C., Ruddock, T., Hegarty, C., Barber, R., Devine, A., & Davis, J. (2020). Minimising Blood Stream Infection: Developing New Materials for Intravascular Catheters. Medicines, 7(9), 49. https://doi.org/10.3390/medicines7090049






