Modulation of Th1/Th2 Cytokine Balance by Quercetin In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of CD4+ T Cells
2.3. Cell Culture
2.4. Assay for Cytokines
2.5. Assay for Transcription Factor Activities
2.6. Assay for mRNA Expression
2.7. Statistical Analysis
3. Results
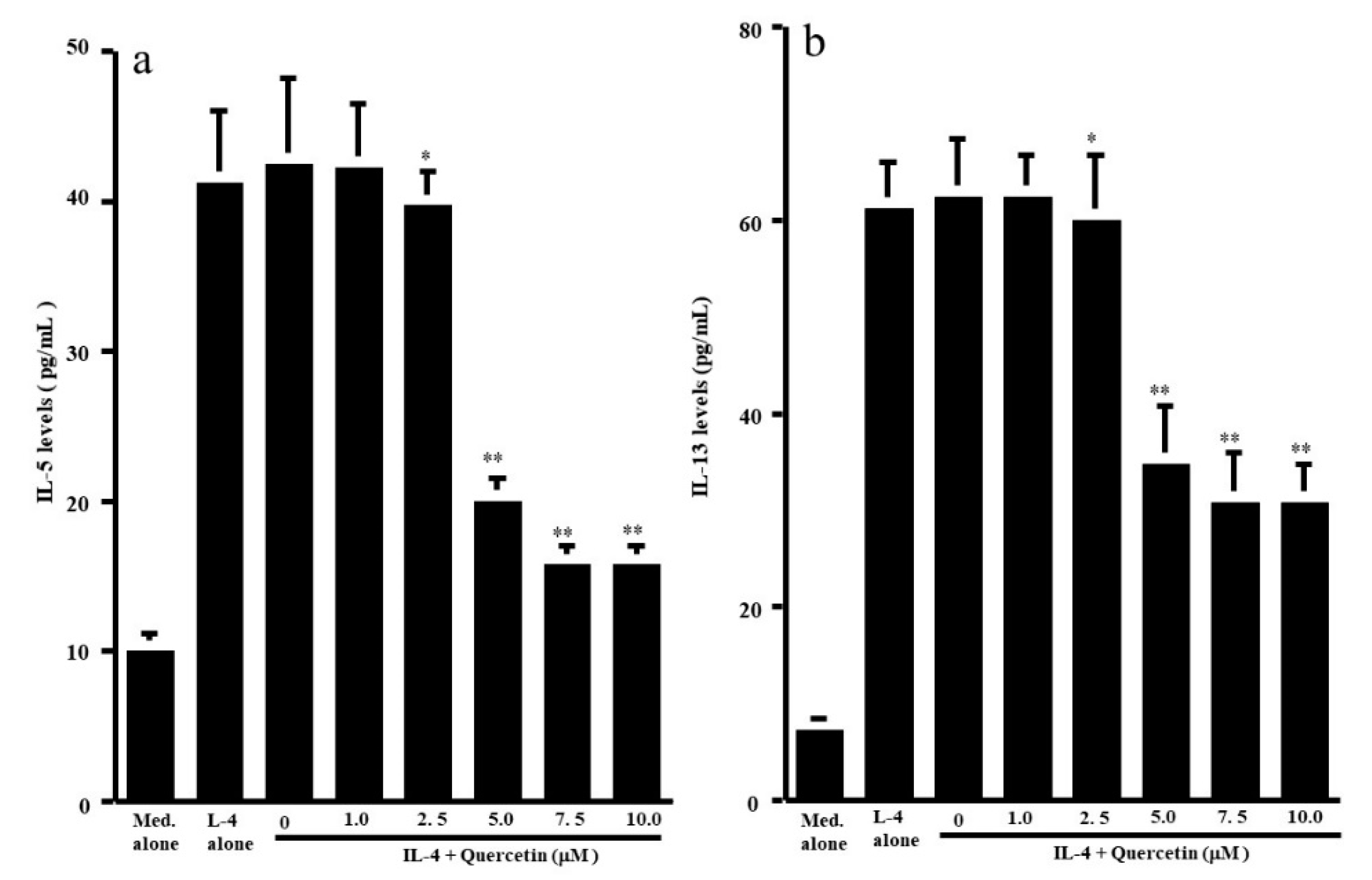
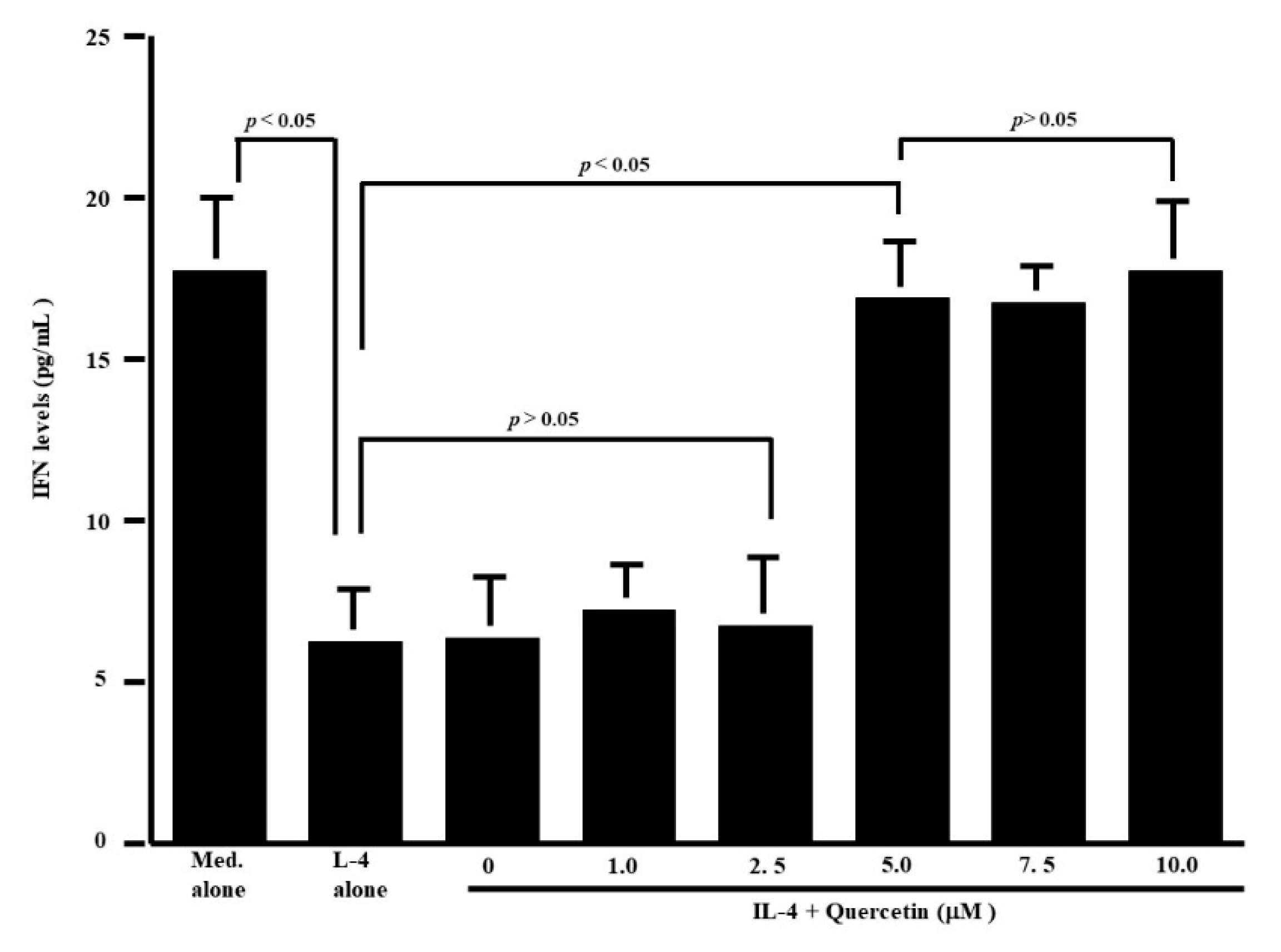
3.1. Influence of Quercetin on the Production of T-Cell Cytokines
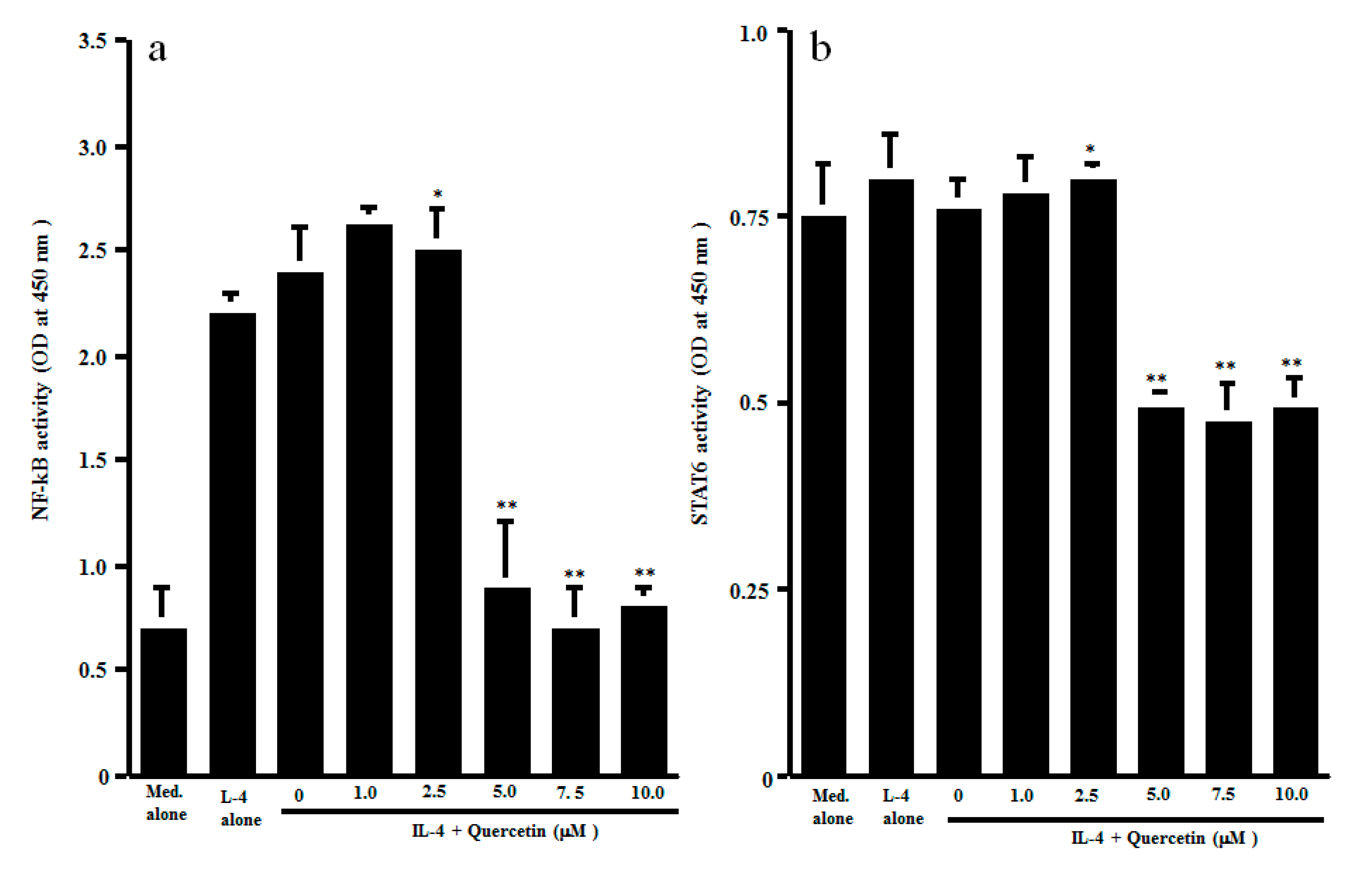
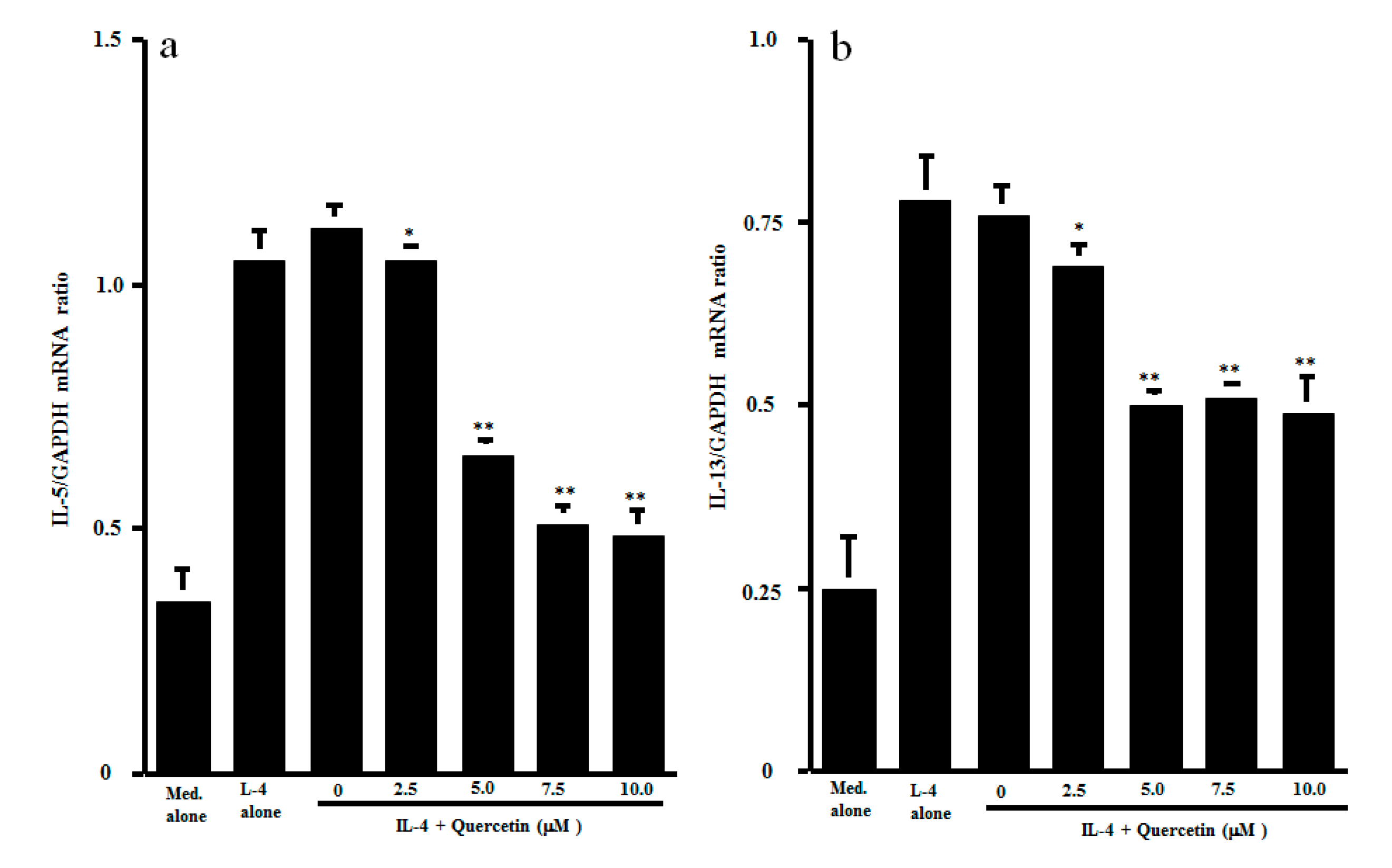
3.2. Influence of Quercetin on Transcription Factor Activation and Cytokine mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pawankar, R.; Mori, S.; Ozu, C.; Kimura, S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac. Allergy 2011, 1, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Jimenez, F.; Pavon-Romero, G.; Juarez-Martinez, L.L.; Teran, L.M. Allergic rhinitis. J. Allergy Ther. 2012, 5, 2–7. [Google Scholar]
- Kumazawa, T.; Takimoto, H.; Matsumoto, T.; Kawaguchi, S. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr. Pharm. Des. 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Lee, S.; Hong, S. Effects of acupuncture and moxibustion in a mouse model of allergic rhinitis. Otolaryngol. Head Neck Surg. 2011, 146, 19–25. [Google Scholar] [CrossRef]
- Jeong, K.T.; Kim, S.G.; Lee, J.; Park, Y.N.; Park, H.H.; Park, N.Y.; Kim, K.J.; Lee, H.; Lee, Y.J.; Lee, E. Anti-allergic effect of a Korean traditional medicine, Biyeom-Tang on mast cells and allergic rhinitis. BMC Comp. Alternt. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Ebihara, N.; Asano, K.; Sunagawa, M. Suppressive activity of quercetin on nitric oxide production from nasal epithelial cells in vitro. Evid. Based Complement. Alternat. Med. 2018. [Google Scholar] [CrossRef]
- Edo, Y.; Otaki, A.; Asano, K. Enhancement of thioredoxin production from nasal epithelial cells by quercetin in vitro and in vivo. Medicines 2018, 5, 124. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Moussa, S.A.A.; Qaid, H.A.Y.; Ai-Ayed, M.S. Potential effects of different natural antioxidants on inflammatory damage and oxidative-mediated hepatotoxity induced by gold nanoparticle. Int. J. Nanomed. 2018, 13, 7931–7938. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Staurengo-Ferrari, L.; Zarpelon, A.; Pinho-Ribeiro, F.A.; Ruiz-Miyazaki, K.W.; Vicentini, F.T.M.C.; Vignoli, J.A.; Camilios-Neto, D.; Georgetti, S.R.; Baracat, M.M.; et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomed. Pharmacother. 2018, 102, 175–184. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.J.; Choi, J.; Frei, B. Qiercetin affects glutathione levels and redox ratio in human aortic endothelial cells not through oxidation but formation and cellular export of quercetin-glutathione conjugates and upregulation of glutamate-cysteine ligase. Redox Biol. 2016, 9, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kashiwabara, M.; Abe, S.; Asano, K. Suppressive activity of quercetin on the production of eosinophil chemoattaractants from eosinophils in vitro. In Vivo 2014, 28, 515–522. [Google Scholar]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Advances Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kashiwabara, M.; Asano, K. Inhibitory action of quercetin on eosinophil activation in vitro. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, C.M.; Jung, I.D.; Lee, J.S.; Jeong, Y.I.; Chang, J.H.; Chun, S.H.; Kim, M.J.; Choi, I.W.; Ahn, S.C.; et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmac. 2009, 9, 261–267. [Google Scholar] [CrossRef]
- Jafarinia, M.; Hosseini, M.S.; Kasiri, N.; Fazel, N.; Fathi, F.; Hakemi, M.G.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 1–11. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Nair, M.P.N.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Kumar, N.; Nair, R.E.; Schwartz, S.A. The flavonoid, quercetin, differentially regulated Th-1(IFNγ) and Th-2 (IL-4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochem. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef]
- Kanai, K.; Asano, K.; Watanabe, S.; Kyo, Y.; Suzaki, H. Epinastine hydrochloride antagonism against interleukin-4-mediated T cell cytokine imbarance in vitro. Int. Arch. Allergy Immunol. 2006, 140, 43–52. [Google Scholar] [CrossRef]
- Kashiwabara, M.; Kazuhito Asano, K.; Mizuyoshi, T.; Kobayashi, H. Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Complement. Alternat. Med. 2016, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Rad. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced release of nitric oxide. Chem. Biol. Interact. 2001, 137, 43–58. [Google Scholar] [CrossRef]
- Kimura, M.; Okafuji, I.; Yoshida, T. Theophyllin suppresses IL-5 and IL-13 production, and lymphocyte proliferation upon stimulation with house dust mite in asthmatic children. Int. Arch. Allergy Immunol. 2003, 131, 189–194. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.E. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998, 102, 165–169. [Google Scholar] [CrossRef]
- Bochner, B.S.; Klunk, D.A.; Sterbinsky, S.A.; Coffman, R.L.; Schleimer, R.P. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J. Immunol. 1995, 154, 799–803. [Google Scholar]
- Teixeira, L.K.; Fonseca, B.P.F.; Barboza, B.A.; Viola, J.P.B. The role of interferon-γ on immune and allergic responses. Mem. Inst. Oswaldo Cruz 2005, 100, 137–144. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Sastre, J.; Davila, I. Dupilumab: A new paradigm for treatment of allergic diseases. J. Investig. Allergol. Clin. Immunol. 2018, 28, 139–150. [Google Scholar] [CrossRef]
- Duran, A.; Rodriguez, A.; Martin, P.; Serrano, M.; Flores, J.M.; Leitges, M.; Diaz-Meco, M.T.; Moscat, J. Crosstalk between PKCzeta and IL-4/STAT6 pathway during T-cell-mediated hepatitis. EMBO J. 2004, 23, 4595–4605. [Google Scholar] [CrossRef]
- Thieu, V.T.; Nguyen, E.T.; McCarthy, B.P.; Bruns, H.A.; Kapur, R.; Chang, C.H.; Kaplan, M.H. IL-4-stimulated NF-κB activity is required for STAT6 DNA binding. J. Leukoc. Biol. 2007, 82, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Todoroki, M.; Nishida, Y.; Tanabe, M.; Misawa, M. A novel STAT6 inhibitor AS1517499 ameliorates antigen-induced bronchial hypercontractility in mice. Am. J. Respir. Cell Mol. Biol. 2009, 41, 516–524. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, C.H.; Kim, S.Y.; Eun, J.S.; Shin, T.Y. Antiallergic effect of Artemisia iwayomogi on mast cell-mediated allergy model. Exp. Biol. Med. 2005, 230, 82–88. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Raifer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, F.M.; Chao, P.D.L.; Chen, C.P.; Jeng, K.C.; Hsu, H.B.; Lee, S.T.; Wu, W.T.; Lin, W.W. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on hemooxygenase-1 induction in mouse BV-2 microglia. Eur. J. Pharmacol. 2005, 521, 9–20. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Furuta, A.; Asano, K.; Kobayashi, H. Modulation of Th1/Th2 Cytokine Balance by Quercetin In Vitro. Medicines 2020, 7, 46. https://doi.org/10.3390/medicines7080046
Tanaka Y, Furuta A, Asano K, Kobayashi H. Modulation of Th1/Th2 Cytokine Balance by Quercetin In Vitro. Medicines. 2020; 7(8):46. https://doi.org/10.3390/medicines7080046
Chicago/Turabian StyleTanaka, Yoshihito, Atsuko Furuta, Kazuhito Asano, and Hitome Kobayashi. 2020. "Modulation of Th1/Th2 Cytokine Balance by Quercetin In Vitro" Medicines 7, no. 8: 46. https://doi.org/10.3390/medicines7080046
APA StyleTanaka, Y., Furuta, A., Asano, K., & Kobayashi, H. (2020). Modulation of Th1/Th2 Cytokine Balance by Quercetin In Vitro. Medicines, 7(8), 46. https://doi.org/10.3390/medicines7080046





