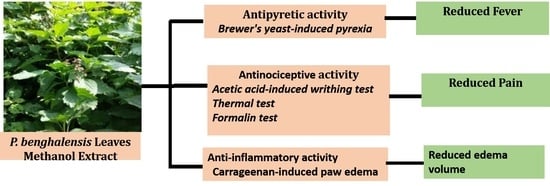
Antipyretic, Antinociceptive, and Anti-Inflammatory Activities from Pogostemon benghalensis Leaf Extract in Experimental Wister Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Plant Material
2.3. Preparation of Plant Extract
2.4. Animals and Ethical Approval
2.5. Acute Toxicity Studies
2.6. Acetic Acid-Induced Writhing Test
2.7. Thermal Test
2.8. Formalin Test
2.9. Antipyretic Activity
2.10. Anti-Inflammatory Activity
2.11. Data Analysis
3. Results
3.1. Acute Toxicity Study
3.2. Acetic Acid-Induced Writhing Reflex
3.3. Thermal Test
3.4. Formalin Test
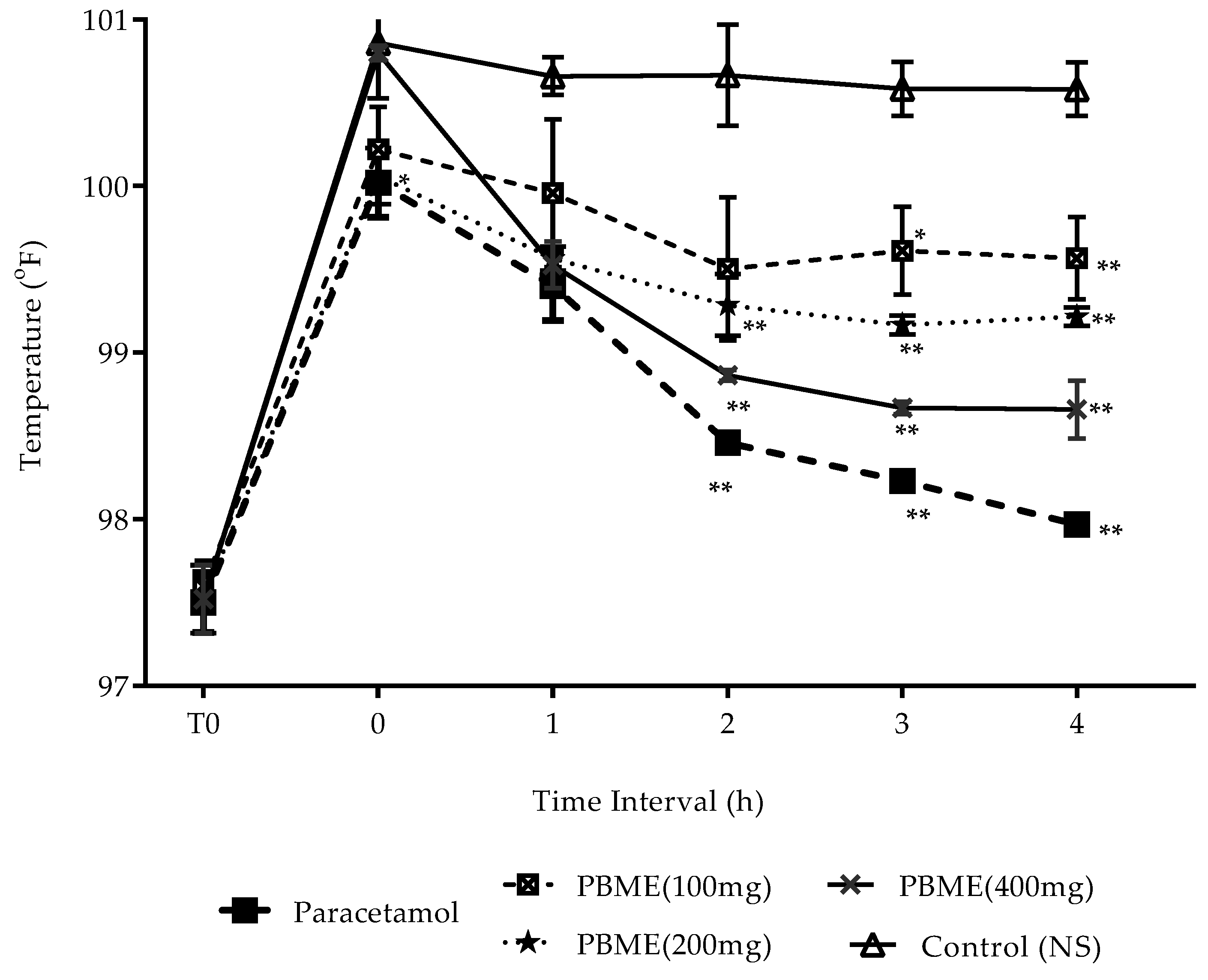
3.5. Antipyretic Effect
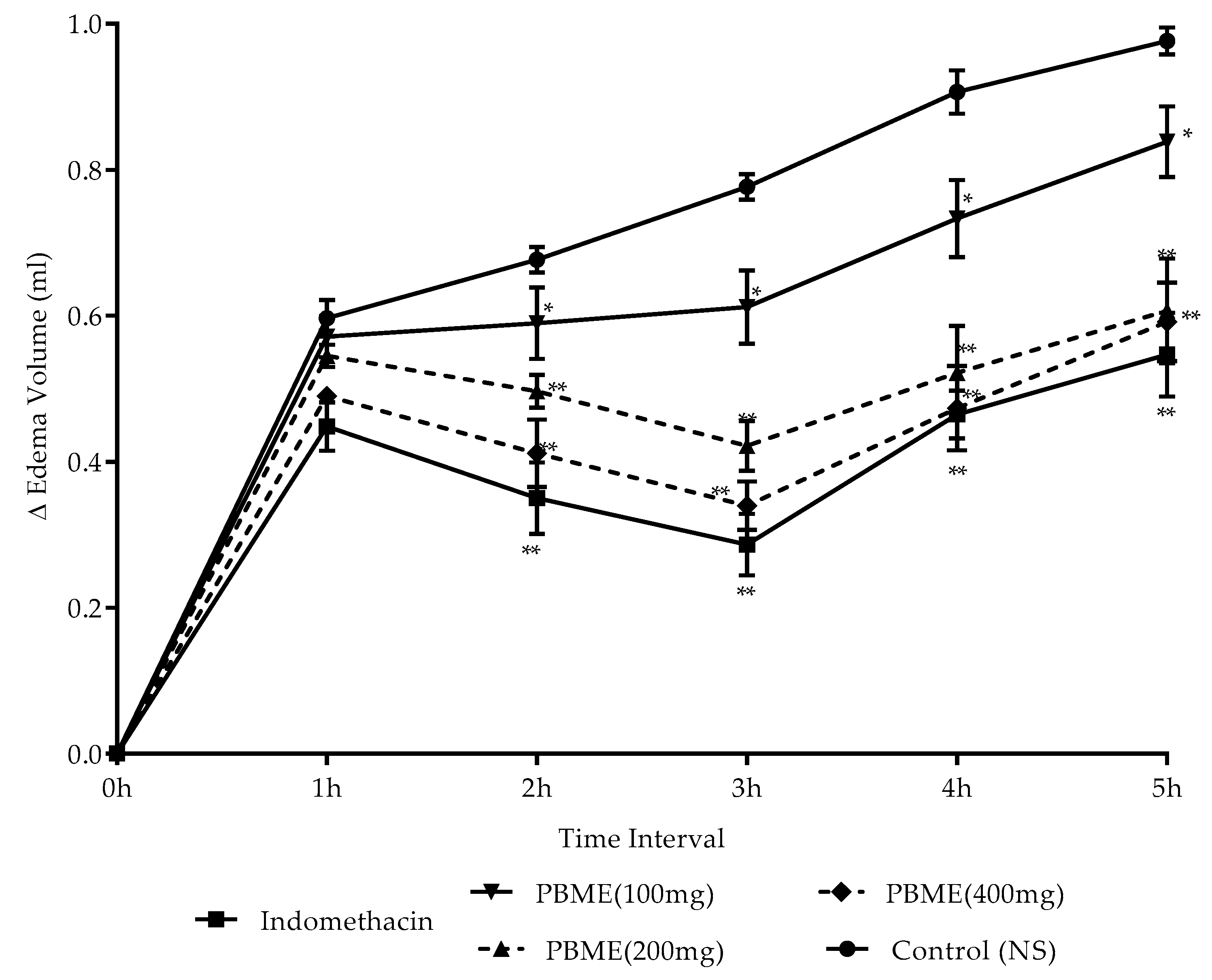
3.6. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Merskey, H.; Bogduk, N. Classification of Chronic Pain, 2nd ed.; IASP Press, International Association for the Study of Pain: Seattle, WA, USA, 1994; pp. 210–214. [Google Scholar]
- Tirumalasetty, J.; Ubedulla, S.; Chandrasekhar, N.; Kishan, P.; Rasamal, K. Evaluation of antipyretic activity of alcoholic extract of Vitex nigundo leaves in PGE1 induced pyrexia model in Albino rats. J. Chem. Pharm. Res. 2012, 4, 3015–3019. [Google Scholar]
- Punchard, N.A.; Whelan, C.J.; Adcock, I. The Journal of Inflammation. J. Inflamm. 2004, 1, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Labianca, R.; Sarzi-Puttini, P.; Zuccaro, S.M.; Cherubino, P.; Vellucci, R.; Fornasari, D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin. Drug. Investig. 2012, 32, 53–63. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Gurung, S.; Škalko-Basnet, N. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J. Ethnopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef]
- Bodeker, G.; Ong, C.-K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; World Health Organization: Kobe, Japan, 2005; Volume 1. [Google Scholar]
- Braun, L.A.; Tiralongo, E.; Wilkinson, J.M.; Spitzer, O.; Bailey, M.; Poole, S.; Dooley, M. Perceptions, use and attitudes of pharmacy customers on complementary medicines and pharmacy practice. BMC Complement. Altern. Med. 2010, 10, 38. [Google Scholar] [CrossRef]
- Liem, A. “I’ve Only Just Heard About It”: Complementary and Alternative Medicine Knowledge and Educational Needs of Clinical Psychologists in Indonesia. Medicina 2019, 55, 333. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.; Danakhu, K.; Kuwar, P.; Gurung, R.; Koirala, N. Total Phenolic, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Uprety, Y.; Poudel, R.C.; Gurung, J.; Chettri, N.; Chaudhary, R.P. Traditional use and management of NTFPs in Kangchenjunga Landscape: Implications for conservation and livelihoods. J. Ethnobiol. Ethnomed. 2016, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Dangol, D.R. Economic uses of forest plant resources in western Chitwan, Nepal. Banko Janakari 2002, 12, 56–64. [Google Scholar] [PubMed]
- Manandhar, N.P. Useful Wild Plants of Nepal; Franz Steiner Verlag Wiesbaden GMBH: Kathmandu, Nepal, 1989. [Google Scholar]
- Das, S.; Dash, S.K.; Padhy, S.N. Ethno-medicinal Informations from Orissa State, India, A Review. J. Hum. Ecol. 2003, 14, 165–227. [Google Scholar] [CrossRef]
- Ashwini, S.; Khade, A.; Basawaraj, H.; Shrishail, G. A comprehensive review on Pogostemon benghalensis (Burm. f.) O. Kuntze. Res. Rev. J. Pharmacogn. Phytochem. 2013, 1, 10–15. [Google Scholar]
- Ghimire, K.; Bastakoti, R.R. Ethnomedicinal knowledge and healthcare practices among the Tharus of Nawalparasi district in central Nepal. For. Ecol. Manag. 2009, 257, 2066–2072. [Google Scholar] [CrossRef]
- Patel, M.; Antala, B.; Dowerah, E.; Senthilkumar, R.; Lahkar, M. Antitumor activity of Pogostemon benghalensis Linn. on ehrlich ascites carcinoma tumor bearing mice. J. Cancer. Res. Ther. 2014, 10, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, J.; Tajo, A.; Minija, J.; Deena, M.; Sreeranjini, K.; Leeja, L.; Sivadasan, M.; Alfarhan, A. Antimicrobial activity of the essential oils of three species of Pogostemon. J. Environ. Biol. 2014, 35, 795. [Google Scholar] [PubMed]
- Taylor, R.S.L.; Manandhar, N.P.; Hudson, J.B.; Towers, G.H.N. Antiviral activities of Nepalese medicinal plants. J. Ethnopharmacol. 1996, 52, 157–163. [Google Scholar] [CrossRef]
- Pahari, S.K.; Singh, S.P.; Banmali, M.P.; Thaler, F.J.L.; Rathour, M.S.S. Ethical Guidelines for the Care and Use of Animals in Health Research in Nepal; Nepal Health Research Council: Kathmandu, Nepal, 2005. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD: Paris, France, 2008. [Google Scholar]
- Pingsusaen, P.; Kunanusorn, P.; Khonsung, P.; Chiranthanut, N.; Panthong, A.; Rujjanawate, C. Investigation of anti-inflammatory, antinociceptive and antipyretic activities of Stahlianthus involucratus rhizome ethanol extract. J. Ethnopharmacol. 2015, 162, 199–206. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Zakaria, Z.A.; Bujarimin, A.S.; Somchit, M.N.; Israf, D.A.; Moin, S. Evaluation of Moringa oleifera Aqueous Extract for Antinociceptive and Anti-Inflammatory Activities in Animal Models. Pharm. Biol. 2008, 46, 838–845. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanisms of fever production and lysis: Lessons from experimental LPS fever. Compr. Physiol. 2011, 4, 1563–1604. [Google Scholar]
- Malvar, D.d.C.; Soares, D.M.; Fabrício, A.S.C.; Kanashiro, A.; Machado, R.R.; Figueiredo, M.J.; Rae, G.A.; de Souza, G.E.P. The antipyretic effect of dipyrone is unrelated to inhibition of PGE(2) synthesis in the hypothalamus. Br. J. Pharmacol. 2011, 162, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Do Malvar, D.C.; Aguiar, F.A.; de Vaz, A.L.L.; Assis, D.C.; de Melo, M.C.; Jabor, V.A.; Kalapothakis, E.; Ferreira, S.H.; Clososki, G.C.; de Souza, G.E. Dipyrone metabolite 4-MAA induces hypothermia and inhibits PGE2-dependent and -independent fever while 4-AA only blocks PGE2-dependent fever. Br. J. Pharmacol. 2014, 171, 3666–3679. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, N.; Delporte, C.; Givernau, M.; Cassels, B.; Valenzuela, A.; Speisky, H. Anti-inflammatory and antipyretic effects of boldine. Agents Actions 1994, 42, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Huang, W.-T.; Cheng, B.-C.; Hsu, C.-C.; Lin, M.-T. The flavonoid baicalin protects against cerebrovascular dysfunction and brain inflammation in experimental heatstroke. Neuropharmacology 2007, 52, 1024–1033. [Google Scholar] [CrossRef]
- Dickenson, A. Mechanisms of central hypersensitivity: Excitatory amino acid mechanisms and their control. In The Pharmacology of Pain; Springer: Geneva, Switzerland, 1997; pp. 167–210. [Google Scholar]
- Umukoro, S.; Ashorobi, R.B. Further studies on the antinociceptive action of aqueous seed extract of Aframomum melegueta. J. Ethnopharmacol. 2007, 109, 501–504. [Google Scholar] [CrossRef]
- Deraedt, R.; Jouquey, S.; Delevallée, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Adzu, B.; Amos, S.; Kapu, S.D.; Gamaniel, K.S. Anti-inflammatory and anti-nociceptive effects of Sphaeranthus senegalensis. J. Ethnopharmacol. 2003, 84, 169–173. [Google Scholar] [CrossRef]
- Oh, Y.-C.; Jeong, Y.H.; Cho, W.-K.; Ha, J.-H.; Gu, M.J.; Ma, J.Y. Anti-Inflammatory and Analgesic Effects of Pyeongwisan on LPS-Stimulated Murine Macrophages and Mouse Models of Acetic Acid-Induced Writhing Response and Xylene-Induced Ear Edema. Int. J. Mol. Sci. 2015, 16, 1232–1251. [Google Scholar] [CrossRef]
- Jia, Q.; Su, W.; Peng, W.; Li, P.; Wang, Y. Anti-diarrhoea and analgesic activities of the methanol extract and its fractions of Jasminum amplexicaule Buch.-Ham.(Oleaceae). J. Ethnopharmacol. 2008, 119, 299–304. [Google Scholar] [CrossRef]
- Pini, L.A.; Vitale, G.; Ottani, A.; Sandrini, M. Naloxone-reversible antinociception by paracetamol in the rat. J. Pharmacol. Exp. Ther. 1997, 280, 934–940. [Google Scholar] [PubMed]
- Patel, R.; Montagut-Bordas, C.; Dickenson, A.H. Calcium channel modulation as a target in chronic pain control. Br. J. Pharmacol. 2018, 175, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Neto, J.F.; Do Nascimento, L.G.; Felipe, C.F.B.; De Sousa, D.P. Analgesic Potential of Essential Oils. Molecules 2016, 21, 20. [Google Scholar] [CrossRef]
- Maione, F.; Minosi, P.; Di Giannuario, A.; Raucci, F.; Chini, M.G.; De Vita, S.; Bifulco, G.; Mascolo, N.; Pieretti, S. Long-Lasting Anti-Inflammatory and Antinociceptive Effects of Acute Ammonium Glycyrrhizinate Administration: Pharmacological, Biochemical, and Docking Studies. Molecules 2019, 24, 2453. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sahbaie, P.; Zheng, M.; Ritchie, J.; Peltz, G.; Mogil, J.S.; Clark, J.D. Expression genetics identifies spinal mechanisms supporting formalin late phase behaviors. Mol. Pain 2010, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Gutstein, H.B. Opioid analgesics. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 11th ed.; Laurence, L., Brunton, J.S., Lazo, K., Parker, L., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2005; pp. 569–619. [Google Scholar]
- Khan, I.; Nisar, M.; Ebad, F.; Nadeem, S.; Saeed, M.; Khan, H.; Samiullah; Khuda, F.; Karim, N.; Ahmad, Z. Anti-inflammatory activities of Sieboldogenin from Smilax china Linn.: Experimental and computational studies. J. Ethnopharmacol. 2009, 121, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzyme Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Pandey, R.P.; Parajuli, P.; Jung, H.J.; Sohng, J.K. Methylation and subsequent glycosylation of 7,8-dihydroxyflavone. J. Biotechnol. 2014, 184, 128–137. [Google Scholar] [CrossRef] [PubMed]
| Treatment Groups | Dose (mg/kg, p.o.) | No. Writhings | Inhibition % |
|---|---|---|---|
| Control | 37.33 ± 1.03 | ||
| PBME | 100 | 25.00 ± 1.41 * | 33.03 |
| 200 | 18.50 ± 1.05 * | 50.44 | |
| 400 | 11.33 ± 0.81 * | 69.65 | |
| PBME (400 mg) + naloxone (5 mg/kg) | 13.11 ± 0.89 | 64.88 | |
| Indomethacin (10 mg/kg) | 10 | 11.67± 1.21 * | 68.74 |
| Sample | Dose (mg/kg, p.o) | Latency Time (min) | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 240 | ||
| Control | 14.8 ± 0.7 | 15.4 ± 1.0 | 15.2 ± 0.8 | 14.8 ± 1.0 | 15.2 ± 0.7 | |
| PBME | 100 | 15.0 ± 1.1 | 16.1 ± 0.7 | 18.1 ± 1.1 * | 18.4 ± 1.2 *,#,¤ | 17.3 ±1.2 *,#,¤ |
| 200 | 15.2 ± 0.8 | 16.0 ± 1.41 | 17.6 ± 1.5 * | 20.4 ± 1.2 *,#,¤ | 19.2 ± 1.5 *#,¤ | |
| 400 | 15.0 ± 0.5 * | 21.0 ± 0.7 * | 24.0 ± 0.7 *,# | 26.0 ± 1.0 *,# | 25.0 ± 0.9 *,# | |
| Morphine | 5 | 15.0 ± 1.2 * | 22.0 ± 1.2 *,# | 25.0 ± 1.6 *,#,¤ | 27.0 ± 0.8 *,#,¤ | 26.0 ± 1.1 *,# |
| PBME (400 mg) + naloxone (5mg/kg) | 14.8 ± 0.7 | 20.4 ± 1.4 *,# | 23.2 ± 0.7 *,# | 24.9 ± 0.5 *,# | 24.0 ± 0.5 *,# | |
| Morphine (5 mg/kg) + naloxone (5 mg/kg) | 15.0 ± 0.2 *,¤ | 15.3 ± 0.6 *,¤ | 15.2 ± 0.8 *,¤ | 15.1 ± 0.3 *,¤ | 15.1 ± 0.7 *,¤ | |
| Sample | Dose (mg/kg, p.o.) | Duration of Licking (s) (Inhibition %) | |
|---|---|---|---|
| Initial Phase (05–10 min) | Late Phase (20–30 min) | ||
| Control | 66.0 ± 1.58 | 63.6 ± 3.28 | |
| PBME | 100 | 34.0 ± 1.58 * (48.48) | 27.8 ± 0.83 * (56.29) |
| 200 | 26.2± 0.83 * (60.303) | 20.4 ± 1.51 * (67.925) | |
| 400 | 17.4 ± 0.55 * (73.64) | 9.40 ± 1.14 * (85.22) | |
| Aspirin | 100 | 63.0 ± 1.00 (04.54) | 7.4 ± 1.14 * (88.36) |
| Morphine | 5 | 15.8 ± 0.84 * (76.06) | 9.8 ± 1.30 * (84.59) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryal, S.; Adhikari, B.; Panthi, K.; Aryal, P.; Mallik, S.K.; Bhusal, R.P.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J.; Koirala, N. Antipyretic, Antinociceptive, and Anti-Inflammatory Activities from Pogostemon benghalensis Leaf Extract in Experimental Wister Rats. Medicines 2019, 6, 96. https://doi.org/10.3390/medicines6040096
Aryal S, Adhikari B, Panthi K, Aryal P, Mallik SK, Bhusal RP, Salehi B, Setzer WN, Sharifi-Rad J, Koirala N. Antipyretic, Antinociceptive, and Anti-Inflammatory Activities from Pogostemon benghalensis Leaf Extract in Experimental Wister Rats. Medicines. 2019; 6(4):96. https://doi.org/10.3390/medicines6040096
Chicago/Turabian StyleAryal, Sushant, Balkrishna Adhikari, Kasmira Panthi, Pramod Aryal, Shyam Kumar Mallik, Ram Prasad Bhusal, Bahare Salehi, William N. Setzer, Javad Sharifi-Rad, and Niranjan Koirala. 2019. "Antipyretic, Antinociceptive, and Anti-Inflammatory Activities from Pogostemon benghalensis Leaf Extract in Experimental Wister Rats" Medicines 6, no. 4: 96. https://doi.org/10.3390/medicines6040096
APA StyleAryal, S., Adhikari, B., Panthi, K., Aryal, P., Mallik, S. K., Bhusal, R. P., Salehi, B., Setzer, W. N., Sharifi-Rad, J., & Koirala, N. (2019). Antipyretic, Antinociceptive, and Anti-Inflammatory Activities from Pogostemon benghalensis Leaf Extract in Experimental Wister Rats. Medicines, 6(4), 96. https://doi.org/10.3390/medicines6040096