In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum rotundifolium
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Preparation of the Extract
2.3. Major Compounds, Phenolic Content, and Antioxidant Activity
2.4. Cell Culture and Differentiation
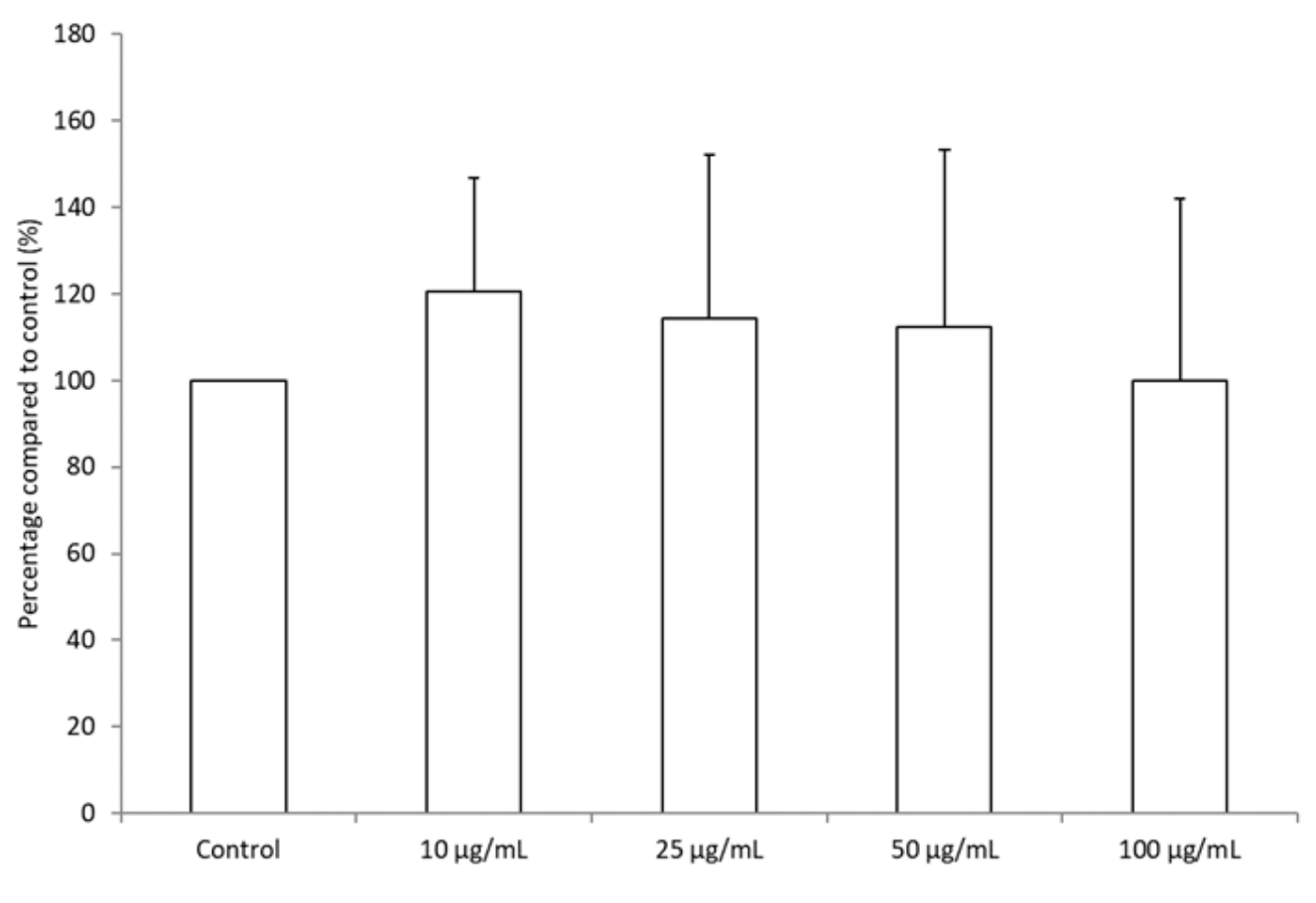
2.5. Leukocyte Viability
2.6. Kinetics of ROS Production by Leukocytes
2.7. PBMC Preparation from Human Blood
2.8. Chemotaxis of PMNs Incubated with BRE
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Determination of Cytokine Concentrations
2.11. Western Blot Analysis for NF-κB
2.12. Statistical Analysis
3. Results
3.1. BRE Content and Antioxidant Properties
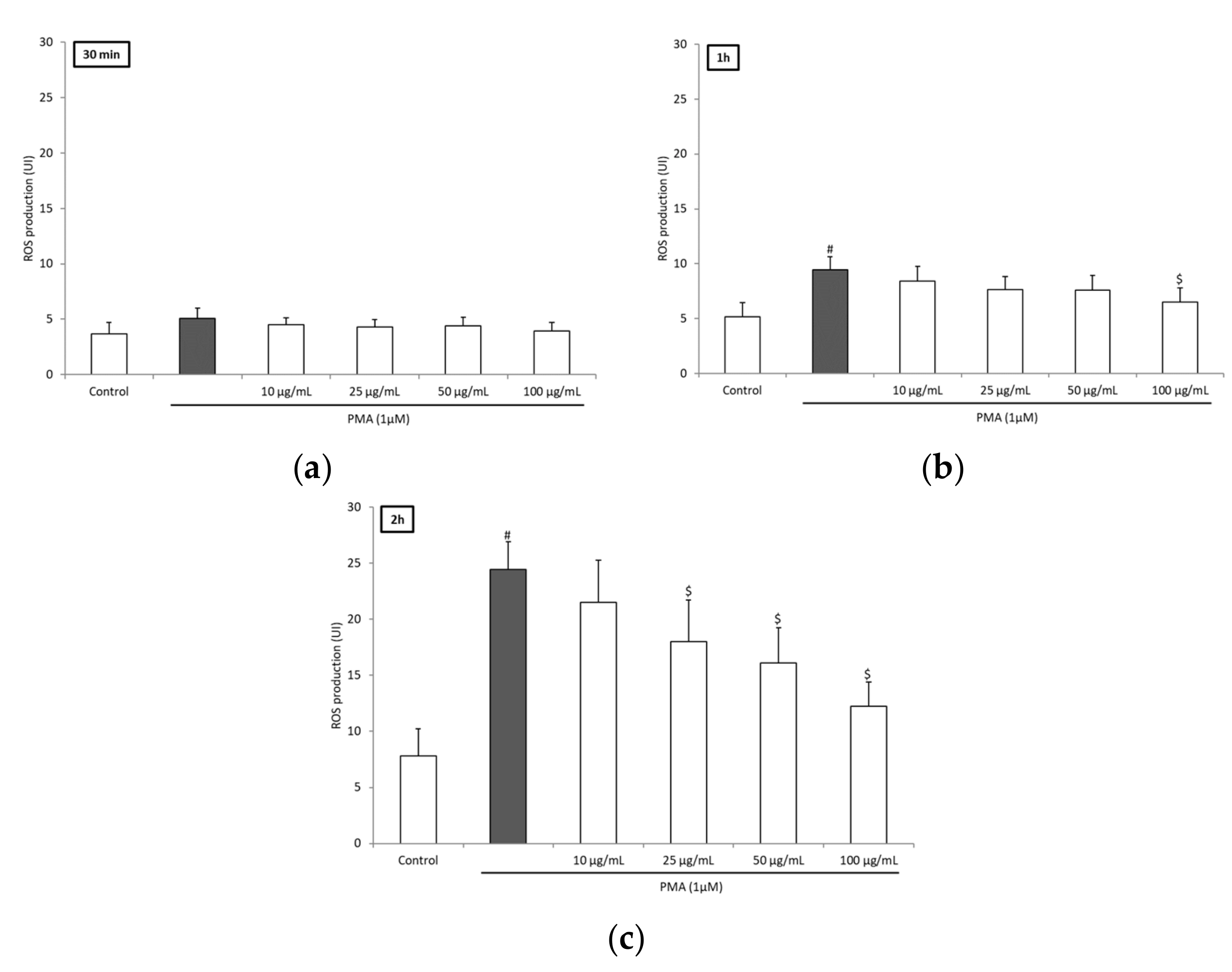
3.2. BRE Inhibited ROS Production of Blood Leukocytes
3.3. BRE Enhanced PMNs Chemotactic Index
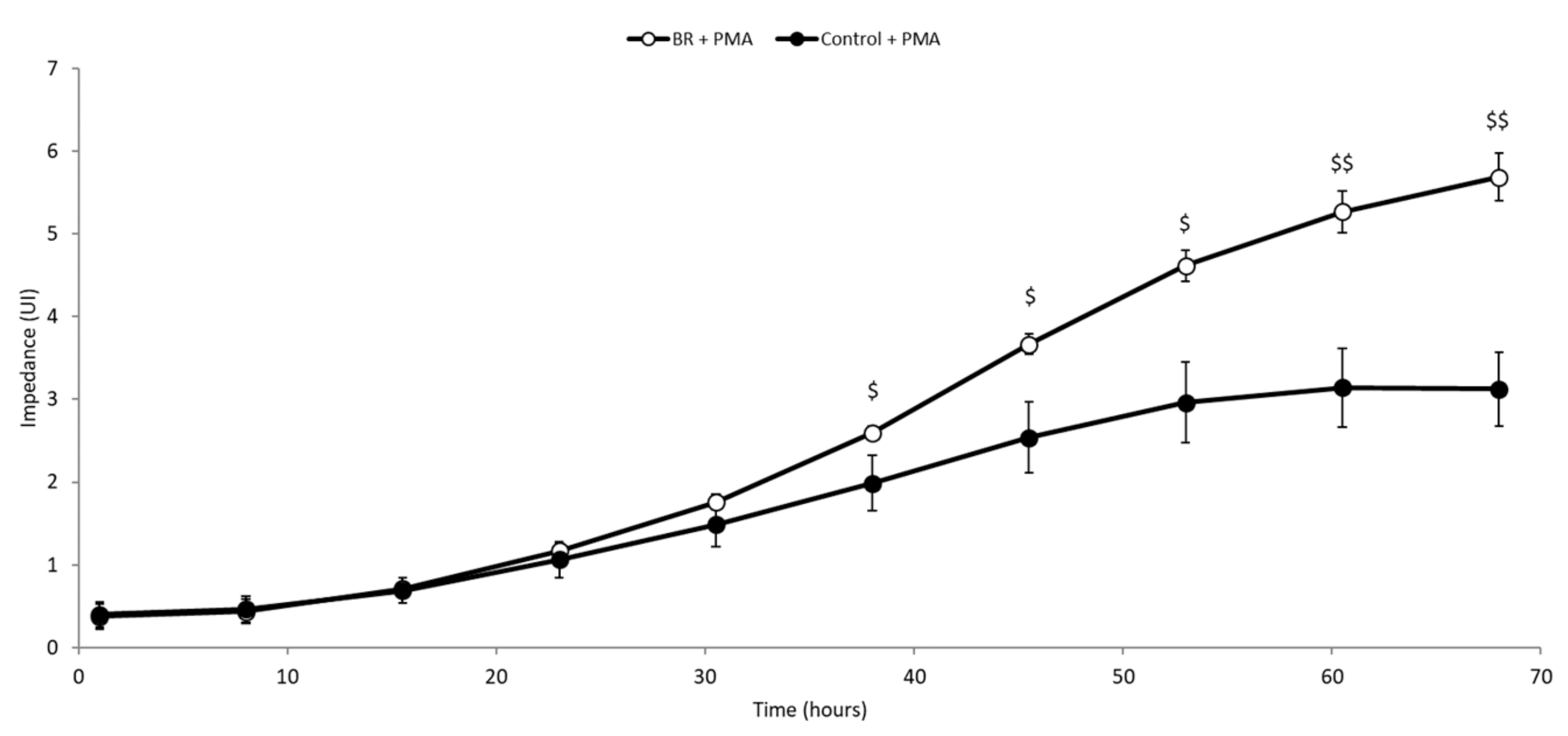
3.4. BRE Promoted the Differentiation of THP-1 Cells
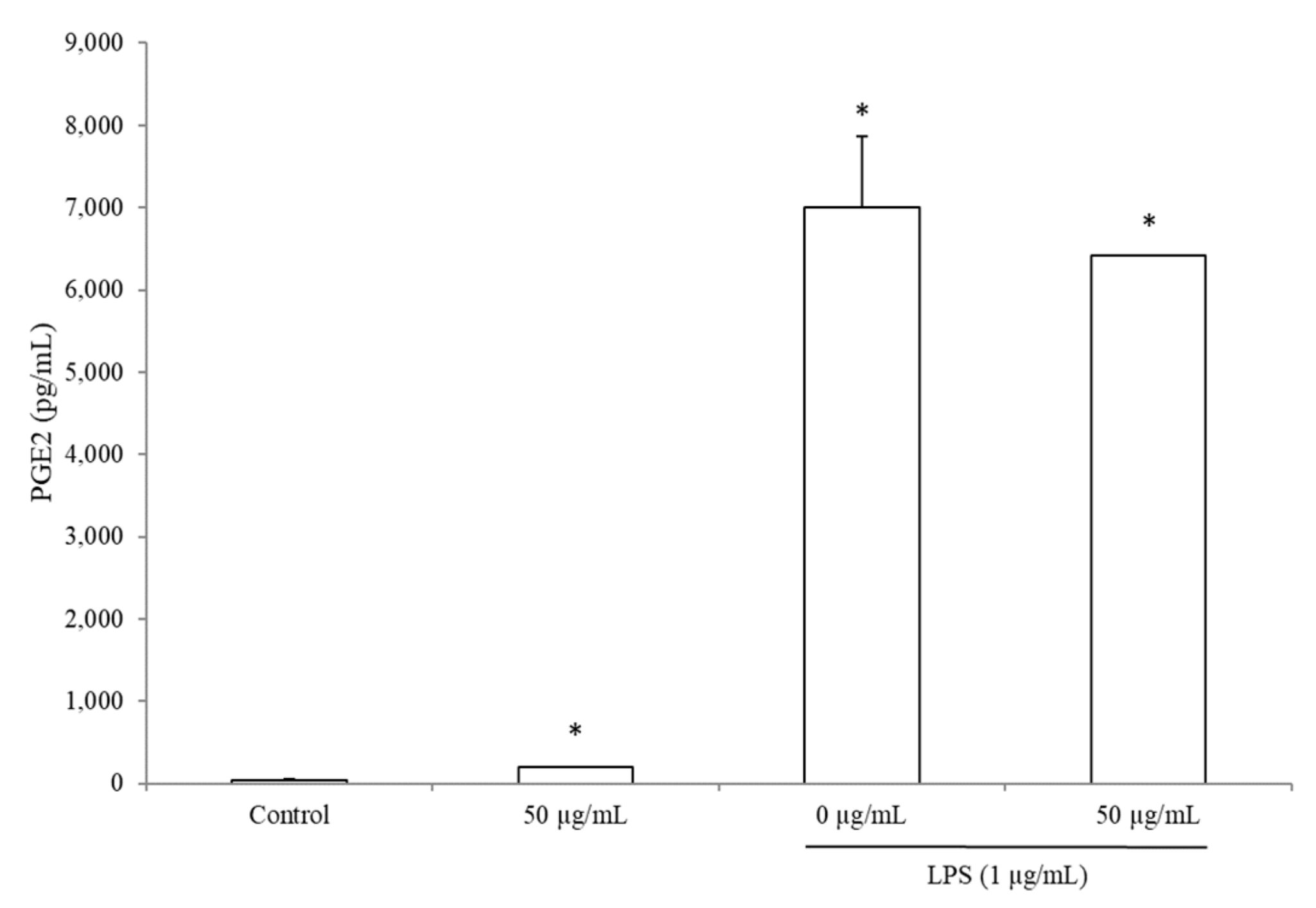
3.5. BRE Increased PGE2 Concentration without LPS
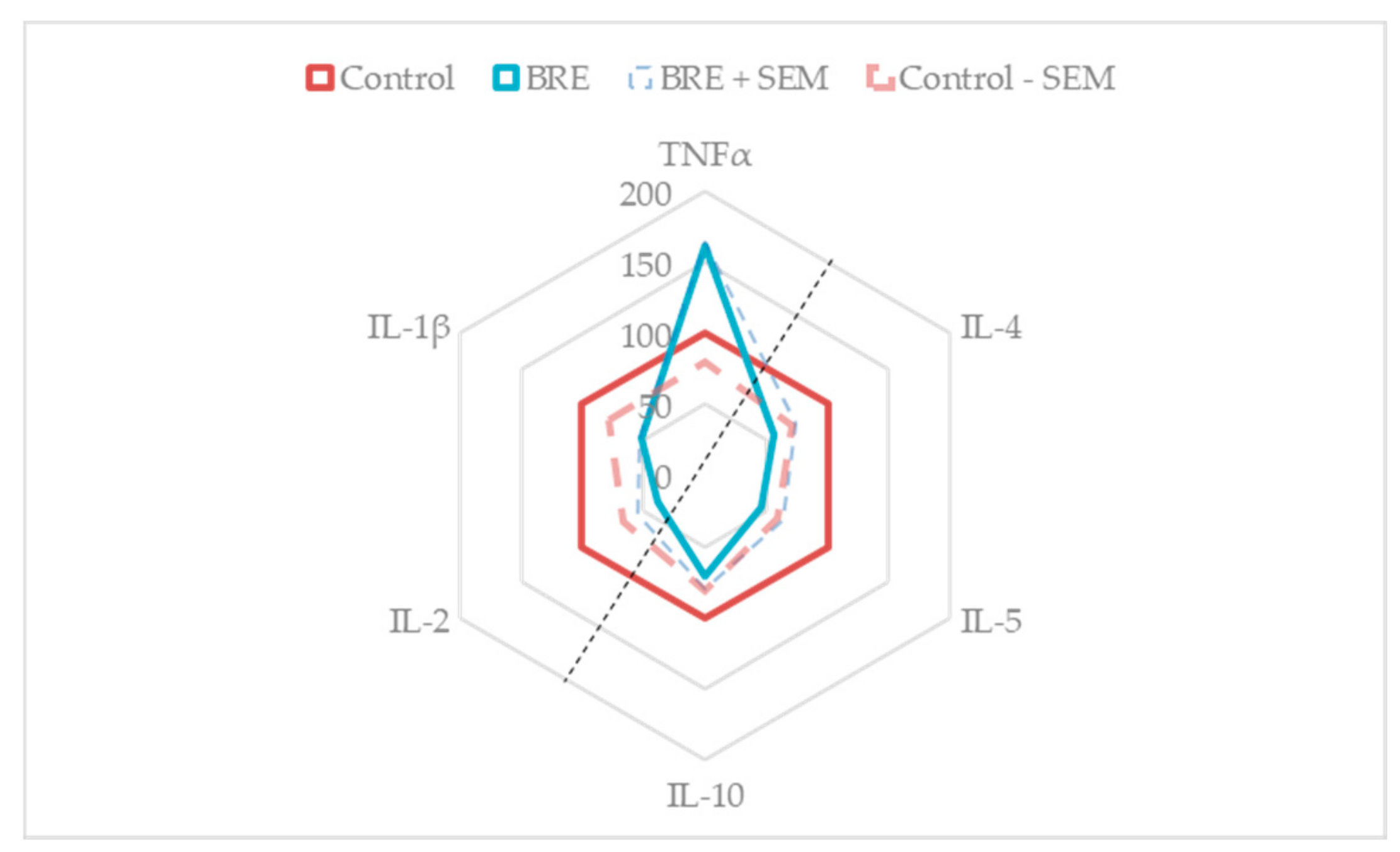
3.6. BRE Impacted PBMCs Cytokines Secretion
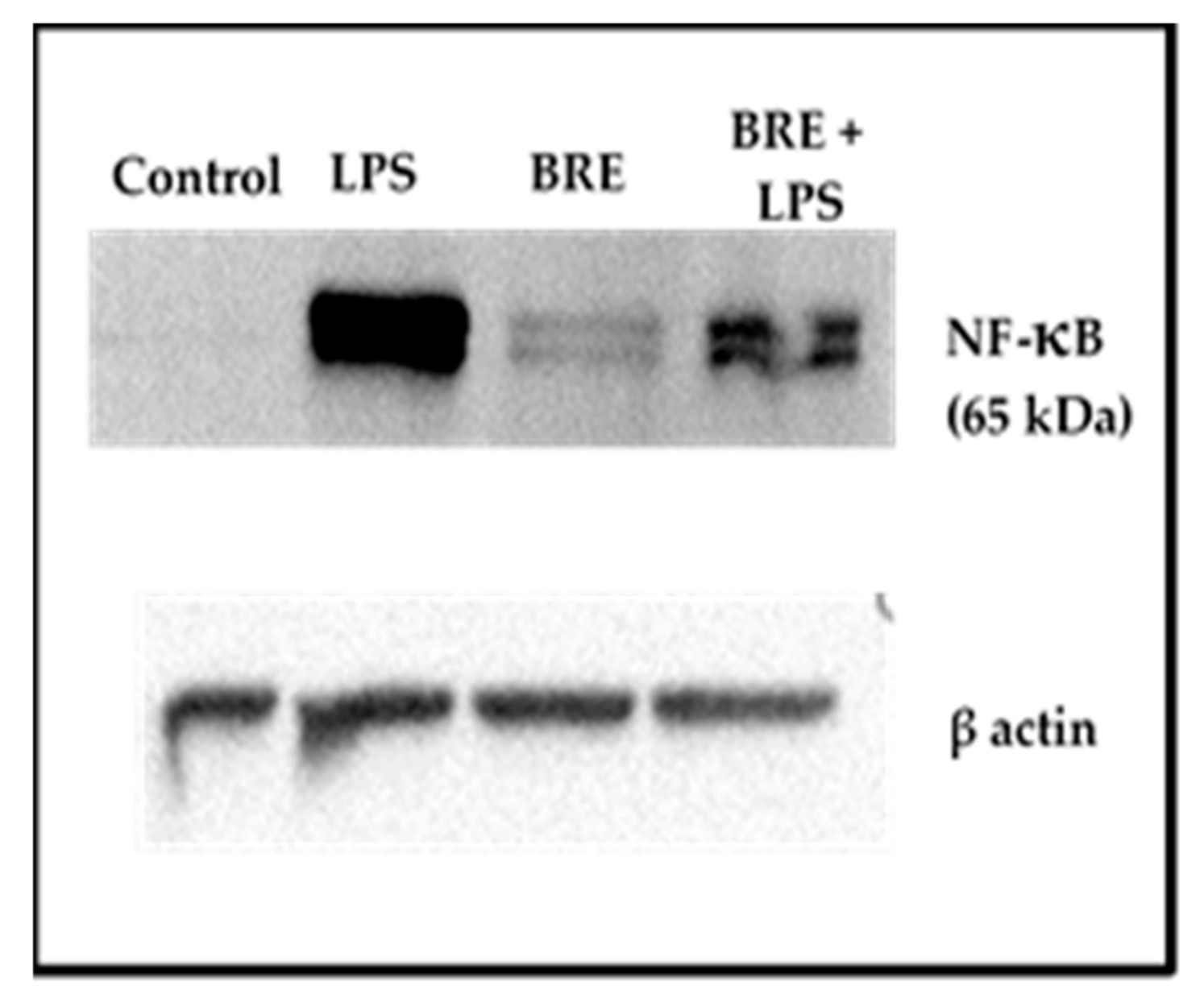
3.7. BRE Showed a Dual Effect on NF-κB Activation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ashour, M.L.; Wink, M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action: Bupleurum: Phytochemistry and pharmacology. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017, 55, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Giner, R.M.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Ríos, J.-L. In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium. Life Sci. 2001, 68, 1199–1206. [Google Scholar] [CrossRef]
- Fujioka, T.; Yoshida, K.; Fujii, H.; Nagao, T.; Okabe, H.; Mihashi, K. Antiproliferative constituents from Umbelliferae plants VI. New ursane-type saikosaponin analogs from the fruits of Bupleurum rotundifolium. Chem. Pharm. Bull. (Tokyo) 2003, 51, 365–372. [Google Scholar] [CrossRef]
- Nakahara, Y.; Okawa, M.; Kinjo, J.; Nohara, T. Oleanene Glycosides of the Aerial Parts and Seeds of Bupleurum falcatum and the Aerial Parts of Bupleurum rotundifolium, and Their Evaluation as Anti-hepatitis Agents. Chem. Pharm. Bull. (Tokyo) 2011, 59, 1329–1339. [Google Scholar] [CrossRef]
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. BioMed Res. Int. 2017, 2017, 1–22. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Dubost, N.; Ou, B.; Beelman, R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Ordonez, A.; Gomez, J.; Vattuone, M.; Isla, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Meda, N.R.; Fraisse, D.; Gnoula, C.; Vivier, M.; Felgines, C.; Senejoux, F. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Eur. Food Res. Technol. 2017, 243, 1659–1666. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.-P.; Guillot, J. Potential anti-inflammatory effects ofMelaleuca alternifolia essential oil on human peripheral blood leukocytes. Phytother. Res. 2006, 20, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Takano-Ishikawa, Y.; Watanabe, J.; Goto, M.; Rao, L.J.M.; Ramalakshmi, K. Antioxidant potential of green and black teas of selected South India cultivars. Jpn. Agric. Res. Q. JARQ 2012, 46, 81–87. [Google Scholar] [CrossRef][Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Gevrenova, R.; Kondeva-Burdina, M.; Denkov, N.; Zheleva-Dimitrova, D. Flavonoid profiles of three Bupleurum species and in vitrohepatoprotective activity of Bupleurum flavum Forsk. Pharmacogn. Mag. 2015, 11, 14. [Google Scholar] [CrossRef]
- Cai, Z.; Li, W.; Wang, H.; Yan, W.; Zhou, Y.; Wang, G.; Cui, J.; Wang, F. Antitumor effects of a purified polysaccharide from Rhodiola rosea and its action mechanism. Carbohydr. Polym. 2012, 90, 296–300. [Google Scholar] [CrossRef]
- Song, F.-L.; Gan, R.-Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef]
- Jariyapamornkoon, N.; Yibchok-anun, S.; Adisakwattana, S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Z.; Guo, X.-T.; Chen, J.-W.; Zhao, Y.; Cong, X.; Jiang, Z.-L.; Cao, R.-F.; Cui, K.; Gao, S.-S.; Tian, W.-R. Saikosaponin-D Attenuates Heat Stress-Induced Oxidative Damage in LLC-PK 1 Cells by Increasing the Expression of Anti-Oxidant Enzymes and HSP72. Am. J. Chin. Med. 2014, 42, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, S.-C.; Chung, I.-K.; Cheon, W.-H.; Ku, S.-K. Antioxidant and Protective Effects of Bupleurum falcatum on the L-Thyroxine-Induced Hyperthyroidism in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar]
- Kim, Y.-H.; Kim, Y.-W.; Oh, Y.-J.; Back, N.-I.; Chung, S.-A.; Chung, H.-G.; Jeong, T.-S.; Choi, M.-S.; Lee, K.-T. Protective Effect of the Ethanol Extract of the Roots of Brassica rapa on Cisplatin-Induced Nephrotoxicity in LLC-PK1 Cells and Rats. Biol. Pharm. Bull. 2006, 29, 2436–2441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014, 5, 352. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Sethi, G. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef]
- Bremner, P.; Tang, S.; Birkmayer, H.; Fiebich, B.; Munoz, E.; Marquez, N.; Rivera, D.; Heinrich, M. Phenylpropanoid NF-κB inhibitors from Bupleurum fruticosum. Planta Med. 2004, 70, 914–918. [Google Scholar] [CrossRef]
- Ushio, Y.; Abe, H. The Effects of Saikosaponin on Macrophage Functions and Lymphocyte Proliferation. Planta Med. 1991, 57, 511–514. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, A. Cxcl8 (IL-8) Mediates Neutrophil Recruitment and Behavior in the Zebrafish Inflammatory Response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 1993, 177, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Espinoza, M.; Manogue, K.R.; Cerami, A. Induction of the chemokine beta peptides, MIP-1 alpha and MIP-1 beta, by lipopolysaccharide is differentially regulated by immunomodulatory cytokines gamma-IFN, IL-10, IL-4, and TGF-beta. Mol. Med. 1998, 4, 648–657. [Google Scholar] [CrossRef]
- Mühl, H.; Dinarello, C.A. Macrophage inflammatory protein-1 alpha production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor N(G)-monomethyl-L-arginine. J. Immunol. 1997, 159, 5063–5069. [Google Scholar] [PubMed]
- Mbonye, U.R.; Wada, M.; Rieke, C.J.; Tang, H.-Y.; DeWitt, D.L.; Smith, W.L. The 19-amino Acid Cassette of Cyclooxygenase-2 Mediates Entry of the Protein into the Endoplasmic Reticulum-associated Degradation System. J. Biol. Chem. 2006, 281, 35770–35778. [Google Scholar] [CrossRef]
- Kalinski, P. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 2001, 97, 3466–3469. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Bryn, T.; Yaqub, S.; Mahic, M.; Henjum, K.; Aandahl, E.M.; Tasken, K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int. Immunol. 2008, 20, 235–245. [Google Scholar] [CrossRef]
- Neurath, M.F.; Finotto, S.; Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 2002, 8, 567–573. [Google Scholar] [CrossRef]
- Sokol, C.L.; Barton, G.M.; Farr, A.G.; Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008, 9, 310–318. [Google Scholar] [CrossRef] [PubMed]
| RT 1 (min) | [M − H]− (m/z) | Compound |
|---|---|---|
| 3.84 | 191.0549 | Quinic acid 2 |
| 4.1 | 341.1085 | Saccharose 2 |
| 6.83 | 191.0186 | Citric acid 2 |
| 12.92 | 353.0875 | Chlorogenic acid 2 |
| 14.31 | 353.0875 | Cryptochlorogenic acid 2 |
| 15.88 | 367.1029 | 3-O-feruloylquinic acid 3 |
| 17.52 | 367.1030 | Feruloylquinic acid Isomer 3 |
| 18.46 | 463.0880 | Isoquercitrin 2 |
| 20.72 | 623.1614 | Narcissin 3 |
| 22.95 | 477.1035 | Isorhamnetin-3-O-glucoside 3 |
| ORAC (µM TE 2) | DPPH (µM TE 2) | Iron (II) Chelation (µM EDTAE 3) | Flavonoid Content (mg RE 4) | Total Phenolic Content (mg GAE 5) |
|---|---|---|---|---|
| 1277 ± 852 | 198 ± 8.5 | 47.3 ± 3.23 | 13.9 ± 0.2 | 47 ± 0.3 |
| Migration | BRE Concentration (µg/mL) | |
|---|---|---|
| 0 | 50 | |
| SM 2 | 75 ± 22 | 31.3 ± 9 * |
| DM 3 | 90 ± 24 | 67.5 ± 7 |
| DM/SM | 1.3 ± 0.4 | 2.3 ± 0.6 * |
| Without PHA | PHA (5 µg/mL) | |||||
|---|---|---|---|---|---|---|
| BRE Concentration (µg/mL) | BRE Concentration (µg/mL) | |||||
| 0 | 50 | Variation 1 | 0 | 50 | Variation 1 | |
| IL-2 | 15.4 ± 11 | 4.5 ± 0.7 | NS | 327.9 ± 188 | 125.6 ± 101.3 | NS |
| IL-4 | 1.4 ± 1 | 3.5 ± 0.9 | (++) | 198.7 ± 96.6 | 112.2 ± 28.8 | NS |
| IL-10 | 14.4 ± 7.3 | 10.6 ± 6.1 | NS | 10,354.5 ± 3830 | 10,284.7 ± 1350 | NS |
| IL-5 | ND | ND | 33.9 ± 23.1 | 15.37 ± 11.9 | NS | |
| IL12p70 | 0.73 ± 0.6 | 0.24 ± 0.3 | NS | 40.73 ± 11.9 | 17.1 ± 10.5 | (−−) |
| IL-1β | 9.2 ± 9.1 | 952 ± 890 | (+) | 2548.5 ± 402.2 | 1334.7 ± 33.5 | (−−) |
| TNFα | 15.9 ± 9.2 | 715 ± 216 | (++) | 4637.2 ± 1518 | 7538.7 ± 185.5 | (++) |
| IL-8 | 535.7 ± 196 | 816 ± 5.8 | (+) | 748.9 ± 65 | 1197 ± 19 | (++) |
| MIP1α | 4.2 ± 2.7 | 204 ± 163 | (+) | 1757.8 ± 578 | 1026.1 ± 20.7 | (−) |
| MIP1β | 137 ± 107 | 3300 ± 2890 | (++) | 6400.1 ± 603.5 | 6275.6 ± 359.2 | NS |
| IL-12p70/ IL-10 | 0.05 ± 0.03 | 0.03 ± 0.05 | NS | 0.001 ± 0.0009 | 0.003 ± 0.003 | (−) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Senejoux, F.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D.; et al. In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum rotundifolium. Medicines 2019, 6, 101. https://doi.org/10.3390/medicines6040101
Cholet J, Decombat C, Vareille-Delarbre M, Gainche M, Berry A, Senejoux F, Ripoche I, Delort L, Vermerie M, Fraisse D, et al. In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum rotundifolium. Medicines. 2019; 6(4):101. https://doi.org/10.3390/medicines6040101
Chicago/Turabian StyleCholet, Juliette, Caroline Decombat, Marjolaine Vareille-Delarbre, Maël Gainche, Alexandre Berry, François Senejoux, Isabelle Ripoche, Laetitia Delort, Marion Vermerie, Didier Fraisse, and et al. 2019. "In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum rotundifolium" Medicines 6, no. 4: 101. https://doi.org/10.3390/medicines6040101
APA StyleCholet, J., Decombat, C., Vareille-Delarbre, M., Gainche, M., Berry, A., Senejoux, F., Ripoche, I., Delort, L., Vermerie, M., Fraisse, D., Felgines, C., Ranouille, E., Berthon, J.-Y., Priam, J., Saunier, E., Tourrette, A., Troin, Y., Thebaud, G., Chalard, P., & Caldefie-Chezet, F. (2019). In Vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum rotundifolium. Medicines, 6(4), 101. https://doi.org/10.3390/medicines6040101





