Phytochemical Modulation of MiRNAs in Colorectal Cancer
Abstract
:1. Colorectal Cancer and Its Preventability
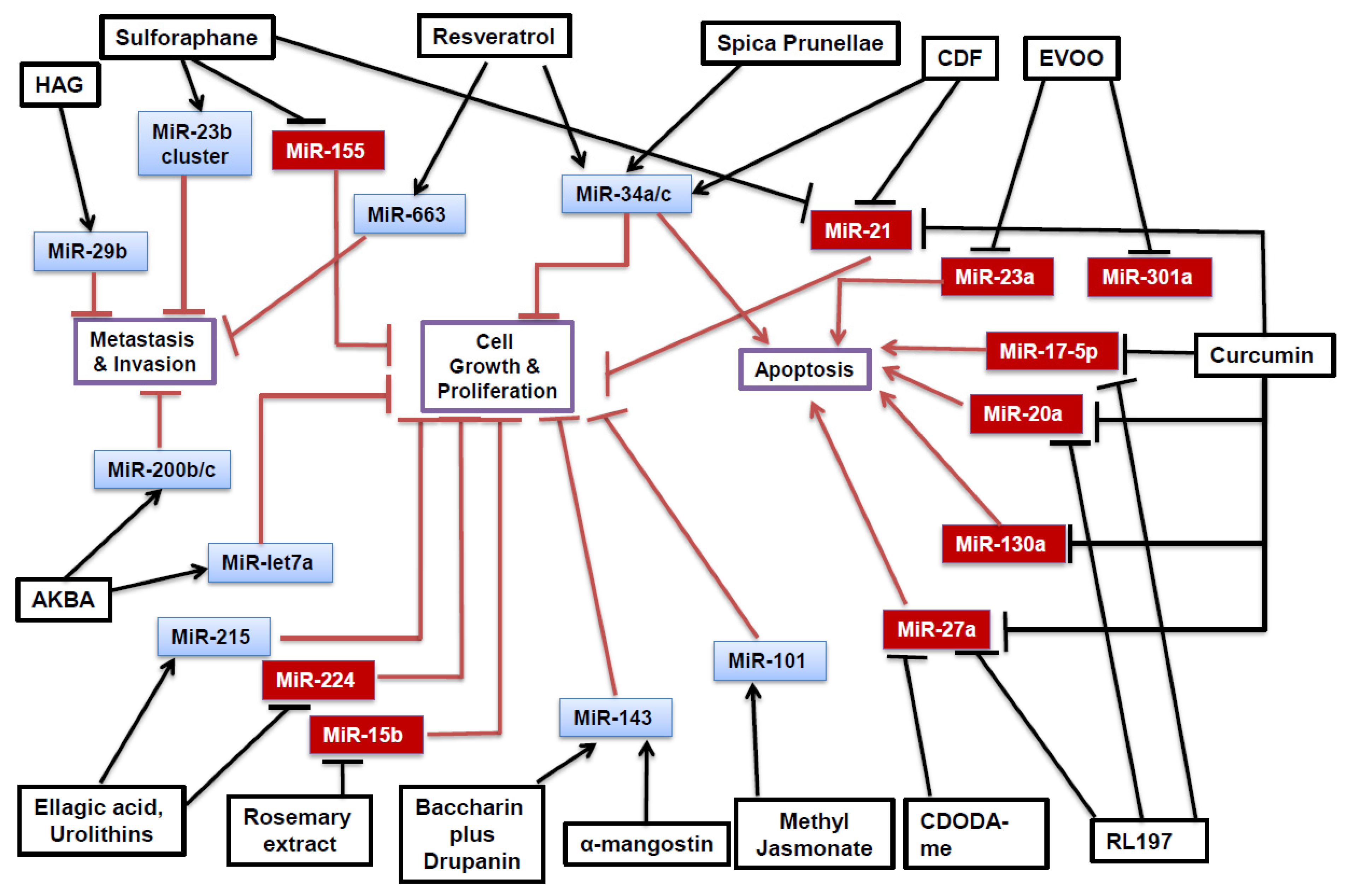
2. Phytochemicals and MiRNAs
2.1. MiRNA Processing
2.2. Curcumin
2.3. Difluorinated Curcumin
2.4. RL197
2.5. Resveratrol
2.6. Grape Seed Extract
2.7. Baccharin and Drupanin
2.8. Methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate
2.9. Sulforaphane
2.10. Walnuts
2.11. Extra Virgin Olive Oil
2.12. α-Mangostin
2.13. Boswellic Acid (AKBA)
2.14. Plum Polyphenols
2.15. Spica Prunellae
2.16. Ellagitannins
2.17. Rosemary Extract
2.18. Methyl Jasmonate
2.19. American Ginseng
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef]
- Baena, R.; Salinas, P. Diet and colorectal cancer. Maturitas 2015, 80, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D. Related disease—Related cause? Lancet 1969, 294, 1229–1231. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Giovannucci, E.L.; Colditz, G.A.; Hunter, D.J.; Stampfer, M.J.; Rosner, B.; Speizer, F.E.; Willett, W.C. Dietary fiber and the risk of colorectal cancer and adenoma in women. N. Engl. J. Med. 1999, 340, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N. Engl. J. Med. 1990, 323, 1664–1672. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Ward, E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1688–1694. [Google Scholar] [CrossRef]
- Pathy, S.; Lambert, R.; Sauvaget, C.; Sankaranarayanan, R. The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis. Colon Rectum 2012, 55, 900–906. [Google Scholar] [PubMed]
- Sinha, R.; Anderson, D.; McDonald, S.; Greenwald, P. Cancer risk and diet in India. J. Postgrad. Med. 2003, 49, 222–228. [Google Scholar] [PubMed]
- Rastogi, T.; Devesa, S.; Mangtani, P.; Mathew, A.; Cooper, N.; Kao, R.; Sinha, R. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int. J. Epidemiol. 2007, 37, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Barnes, I.; Kan, S.W.; Beral, V. Cancer incidence in British Indians and British whites in Leicester, 2001–2006. Br. J. Cancer 2010, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Tony Kong, A.-N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.; Tattersall, M. On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef]
- Goel, A.; Boland, C.R. Epigenetics of colorectal cancer. Gastroenterology 2012, 143, 1442–1460.e1. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharm. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef]
- Denzler, R.; McGeary, S.E.; Title, A.C.; Agarwal, V.; Bartel, D.P.; Stoffel, M. Impact of microRNA levels, target-site complementarity, and cooperativity on competing endogenous RNA-regulated gene expression. Mol. Cell 2016, 64, 565–579. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Arora, S.; Averett, C.; Singh, S.; Singh, A.P. Modulation of microRNAs by phytochemicals in cancer: Underlying mechanisms and translational significance. Biomed. Res. Int. 2015, 2015, 848710. [Google Scholar] [CrossRef]
- Hwang, H.W.; Mendell, J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2006, 94, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.E.S.; Slack, F.J. Cancer issue: The role of microRNAs in cancer. Yale J. Biol. Med. 2006, 79, 131–140. [Google Scholar]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Palamarchuk, A.; Efanov, A.; Maximov, V.; Aqeilan, R.I.; Croce, C.M.; Pekarsky, Y. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005, 65, 11282–11286. [Google Scholar] [CrossRef]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin suppresses the colon cancer proliferation by inhibiting Wnt/β-Catenin pathways via miR-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Segditsas, S.; Tomlinson, I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 2006, 25, 7531–7537. [Google Scholar] [CrossRef]
- Padhye, S.; Yang, H.; Jamadar, A.; Cui, Q.C.; Chavan, D.; Dominiak, K.; McKinney, J.; Banerjee, S.; Dou, Q.P.; Sarkar, F.H. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009, 26, 1874–1880. [Google Scholar] [CrossRef]
- Roy, S.; Levi, E.; Majumdar, A.P.; Sarkar, F.H. Expression of miR-34 is lost in colon cancer which can be re-expressed by a novel agent CDF. J. Hematol. Oncol. 2012, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Ji, Z.Z.; Zheng, P.S. Notch1 regulates the growth of human colon cancers. Cancer 2010, 116, 5207–5218. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yu, Y.; Padhye, S.B.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS ONE 2013, 8, e68543. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Downes, C.P. PTEN function: How normal cells control it and tumour cells lose it. Biochem. J. 2004, 382, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ahmad, A.; Banerjee, S.; Padhye, S.; Dominiak, K.; Schaffert, J.M.; Wang, Z.; Philip, P.A.; Sarkar, F.H. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010, 70, 3606–3617. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007, 12, 4839–4854. [Google Scholar] [CrossRef]
- Jančík, S.; Drábek, J.; Radzioch, D.; Hajdúch, M. Clinical relevance of KRAS in human cancers. BioMed Res. Int. 2010, 2010, 150960. [Google Scholar] [CrossRef]
- Saud, S.M.; Li, W.; Morris, N.L.; Matter, M.S.; Colburn, N.H.; Kim, Y.S.; Young, M.R. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 2014, 35, 2778–2786. [Google Scholar] [CrossRef]
- Altamemi, I.; Murphy, E.A.; Catroppo, J.F.; Zumbrun, E.E.; Zhang, J.; McClellan, J.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of microRNAs in resveratrol-mediated mitigation of colitis-associated tumorigenesis in Apc(Min/+) mice. J. Pharm. Exp. 2014, 350, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and withaferin A inhibited the signaling in colorectal cancer cells. Mediat. Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharm. 2010, 80, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Schiemann, W.P. The TGF-β paradox in human cancer: An update. Future Oncol. 2009, 5, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef]
- Derry, M.M.; Somasagara, R.R.; Raina, K.; Kumar, S.; Gomez, J.; Patel, M.; Agarwal, R.; Agarwal, C. Target identification of grape seed extract in colorectal cancer using drug affinity responsive target stability (DARTS) technique: Role of endoplasmic reticulum stress response proteins. Curr. Cancer Drug Targets 2014, 14, 323–336. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Athar, M. Grape seeds: Ripe for cancer chemoprevention. Cancer Prev. Res. 2013, 6, 617–621. [Google Scholar] [CrossRef]
- Derry, M.M.; Raina, K.; Balaiya, V.; Jain, A.K.; Shrotriya, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R.; Agarwal, C. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: Interlinking miRNA with cytokine signaling and inflammation. Cancer Prev. Res. 2013, 6, 625–633. [Google Scholar] [CrossRef]
- D’ignazio, L.; Bandarra, D.; Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016, 283, 413–424. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.-X.; Han, L.; You, Y.-P.; Jiang, T.; Pu, P.-Y.; Kang, C.-S. MicroRNA roles in beta-catenin pathway. Mol. Cancer 2010, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Sampson, V.B.; Rong, N.H.; Han, J.; Yang, Q.; Aris, V.; Soteropoulos, P.; Petrelli, N.J.; Dunn, S.P.; Krueger, L.J. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007, 67, 9762–9770. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Das, S.K.; Hashimoto, T.; Sowa, Y.; Yoshida, T.; Sakai, T.; Matsuura, Y.; Kanazawa, K. Artepillin C in Brazilian propolis induces G0/G1 arrest via stimulation of Cip1/p21 expression in human colon cancer cells. Mol. Carcinog. 2005, 44, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Yamada, N.; Ohta, S.; Ichihara, K.; Akao, Y. Propolis cinnamic acid derivatives induce apoptosis through both extrinsic and intrinsic apoptosis signaling pathways and modulate of miRNA expression. Phytomedicine 2014, 21, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Chintharlapalli, S.; Papineni, S.; Abdelrahim, M.; Abudayyeh, A.; Jutooru, I.; Chadalapaka, G.; Wu, F.; Mertens-Talcott, S.; Vanderlaag, K.; Cho, S.D.; et al. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer cells. Int. J. Cancer 2009, 125, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Passer, B.J.; Nancy-Portebois, V.; Amzallag, N.; Prieur, S.; Cans, C.; de Climens, A.R.; Fiucci, G.; Bouvard, V.; Tuynder, M.; Susini, L. The p53-inducible TSAP6 gene product regulates apoptosis and the cell cycle and interacts with Nix and the Myt1 kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Kala, R.; Tollefsbol, T.O. Mechanisms for the Inhibition of Colon Cancer Cells by Sulforaphane through Epigenetic Modulation of MicroRNA-21 and Human Telomerase Reverse Transcriptase (hTERT) Down-regulation. Curr. Cancer Drug Targets 2018, 18, 97–106. [Google Scholar] [CrossRef]
- Slaby, O.; Sachlova, M.; Brezkova, V.; Hezova, R.; Kovarikova, A.; Bischofová, S.; Sevcikova, S.; Bienertova-Vasku, J.; Vasku, A.; Svoboda, M. Identification of microRNAs regulated by isothiocyanates and association of polymorphisms inside their target sites with risk of sporadic colorectal cancer. Nutr. Cancer 2013, 65, 247–254. [Google Scholar] [CrossRef] [PubMed]
- San, K.; Horita, M.; Ganapathy, A.; Chinnadurai, G.; Ezekiel, U.R. Deregulated expression of microRNA-200b/c and SUZ12, a Polycomb repressive complex 2 subunit, in chemoresistant colorectal cancer cells. Genes Cancer 2017, 8, 673–681. [Google Scholar] [PubMed]
- Castilla, M.Á.; Moreno-Bueno, G.; Romero-Pérez, L.; Van De Vijver, K.; Biscuola, M.; López-García, M.Á.; Prat, J.; Matías-Guiu, X.; Cano, A.; Oliva, E. Micro-RNA signature of the epithelial–mesenchymal transition in endometrial carcinosarcoma. J. Pathol. 2011, 223, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, Y.; Yang, J.; Zhou, Y.; Li, J.; Yin, S.; Sun, C.; Ma, M.; Huang, Y.; Xi, J.J. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat. Commun. 2011, 2, 554. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Croce, C.M.; Michaille, J.-J. miR-155: On the crosstalk between inflammation and cancer. Int. Rev. Immunol. 2009, 28, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, K.; Hatziapostolou, M.; Karagiannides, I.; Polytarchou, C.; Jaeger, S.; Iliopoulos, D.; Pothoulakis, C. Neurotensin signaling activates microRNAs-21 and-155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology 2011, 141, 1749–1761.e1. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Naudin, C.; Letourneur, M.; Polrot, M.; Renoir, J.-M.; Lazar, V.; Dessen, P.; Roche, S.; Bertoglio, J.; Pierre, J. Suppressor of cytokine signaling 1 modulates invasion and metastatic potential of colorectal cancer cells. Mol. Oncol. 2014, 8, 942–955. [Google Scholar] [CrossRef]
- Bolling, B.W.; McKay, D.L.; Blumberg, J.B. The phytochemical composition and antioxidant actions of tree nuts. Asia Pac. J. Clin. Nutr. 2010, 19, 117–123. [Google Scholar] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Biological properties of olive oil phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Di Bonaventura, M.V.M.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Iinuma, M.; Naoe, T.; Nozawa, Y.; Akao, Y. Characterized mechanism of alpha-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 2007, 15, 5620–5628. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Naoe, T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol. Rep. 2006, 16, 845–850. [Google Scholar] [CrossRef]
- Moussaieff, A.; Mechoulam, R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2009, 61, 1281–1293. [Google Scholar] [CrossRef]
- Takahashi, M.; Sung, B.; Shen, Y.; Hur, K.; Link, A.; Boland, C.R.; Aggarwal, B.B.; Goel, A. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 2012, 33, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E. Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle 2009, 8, 843–852. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Datta, P. Regulation of EMT in colorectal cancer: A culprit in metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Kim, H.; Talcott, S.T.; Turner, N.D.; Byrne, D.H.; Mertens-Talcott, S.U. Plum polyphenols inhibit colorectal aberrant crypt foci formation in rats: Potential role of the miR-143/protein kinase B/mammalian target of rapamycin axis. Nutr. Res. 2016, 36, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, L.; Feng, J.; Lin, W.; Cai, Q.; Peng, J. Spica Prunellae extract suppresses the growth of human colon carcinoma cells by targeting multiple oncogenes via activating miR-34a. Oncol. Rep. 2017, 38, 1895–1901. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, L.; Zhuang, Q.; Zhao, J.; Cao, Z.; Zeng, J.; Lin, S.; Xu, W.; Peng, J. Spica prunellae promotes cancer cell apoptosis, inhibits cell proliferation and tumor angiogenesis in a mouse model of colorectal cancer via suppression of stat3 pathway. BMC Complement. Altern. Med. 2013, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy–6H–dibenzopyran–6–one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.V.; Yamanaka, S.; Vazquez, C.; Mori, Y.; Cheng, Y.; Abraham, J.M.; Bayless, T.M.; Harpaz, N.; Selaru, F.M.; Meltzer, S.J. MicroRNA-224 negatively regulates p21 expression during late neoplastic progression in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Karaayvaz, M.; Pal, T.; Song, B.; Zhang, C.; Georgakopoulos, P.; Mehmood, S.; Burke, S.; Shroyer, K.; Ju, J. Prognostic significance of miR-215 in colon cancer. Clin. Colorectal Cancer 2011, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key-associated molecular hallmarks: MicroRNA cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res. 2016, 60, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; He, K.; Roller, M.; Lai, C.-S.; Shao, X.; Pan, M.-H.; Ho, C.-T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martín-Hernández, R.; García-Risco, M.R.; Fornari, T.; Reglero, G.; De Molina, A.R. Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS ONE 2014, 9, e98556. [Google Scholar] [CrossRef]
- Fernández, L.P.; Sánchez-Martínez, R.; Vargas, T.; Herranz, J.; Martín-Hernández, R.; Mendiola, M.; Hardisson, D.; Reglero, G.; Feliu, J.; Redondo, A. The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance. Sci. Rep. 2018, 8, 8485. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Akimoto, M.; Kiyota, H.; Ishii, Y.; Ishikura, H.; Honma, Y. Gene expression profiles in differentiating leukemia cells induced by methyl jasmonate are similar to those of cytokinins and methyl jasmonate analogs induce the differentiation of human leukemia cells in primary culture. Leukemia 2009, 23, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Roberts, C.W. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, Y. Methyl jasmonate induces the apoptosis of human colorectal cancer cells via downregulation of EZH2 expression by microRNA‑101. Mol. Med. Rep. 2017, 15, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.; Le, P.M.; Davis, T.; Hofseth, A.B.; Chumanevich, A.; Chumanevich, A.A.; Wargovich, M.J.; Nagarkatti, M.; Nagarkatti, P.S.; Windust, A. A hexane fraction of American ginseng suppresses mouse colitis and associated colon cancer: Anti-inflammatory and proapoptotic mechanisms. Cancer Prev. Res. 2012, 5, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.; Cui, X.; Le, P.M.; Hofseth, A.B.; Windust, A.; Nagarkatti, M.; Nagarkatti, P.S.; Schetter, A.J.; Harris, C.C.; Hofseth, L.J. A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS ONE 2013, 8, e75034. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Shen, L.; Liu, C.-C.; An, C.-Y.; Ji, H.-F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016, 6, 20872. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Liu, L.; Wang, W.; Fung, T.T.; Wu, K.; Smith-Warner, S.A.; Cao, Y.; Hu, F.B.; Ogino, S.; Fuchs, C.S. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018, 4, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Gillen, C.; Walmsley, R.; Prior, P.; Andrews, H.; Allan, R. Ulcerative colitis and Crohn’s disease: A comparison of the colorectal cancer risk in extensive colitis. Gut 1994, 35, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting inflammation in cancer prevention and therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Toiyama, Y.; Okugawa, Y.; Tanaka, K.; Araki, T.; Uchida, K.; Hishida, A.; Uchino, M.; Ikeuchi, H.; Hirota, S.; Kusunoki, M. A panel of methylated microRNA biomarkers for identifying high-risk patients with ulcerative colitis-associated colorectal cancer. Gastroenterology 2017, 153, 1634–1646.e8. [Google Scholar] [CrossRef]
| Phytochemical | Cell Line | MiRNA Affected | Target(s) and Effect | Reference |
|---|---|---|---|---|
| Curcumin | RKO, SW480 | miR-17-5p↓ miR-20a↓ miR-27a↓ | ZBTB10↑, ZBTB4↑ Sp1↓, Sp3↓, Sp4↓ | [25] |
| RKO, HCT116 | miR-21↓ | PDCD4↑ | [26] | |
| SW480 | miR-130a↓ | Wnt↓, B-catenin↓, Nkd2↑ | [28] | |
| CDF | HCT-116, SW620, HT-29 | miR-21↓ | PTEN↑ | [33] |
| HCT-116, SW620 | miR-34a↑ miR-34c↑ | Notch-1↓ | [31] | |
| RL197 | RKO, SW480 | miR-17-5p↓ miR-20a↓ miR-27a↓ | ZBTB10↑, ZBTB4↑ Sp1↓, Sp3↓, Sp4↓ | [25] |
| Resveratrol | DLD-1, SW480 | miR-34a↑ | E2F3↓, Sirt1↓ | [45] |
| SW480 | miR-663↑ | TGFβ1↓ | [43] | |
| Baccharin + Drupanin | DLD-1 | miR-143↑ | MAPK/Erk5↓ C-myc↓ | [55] |
| CDODA-Me | RKO, SW480 | miR-27a↓ | ZBTB10↑, Myt1↑ Sp1↓, Sp3↓, Sp4↓ | [57] |
| Sulforaphane | RKO | miR-21↓ | hTERT↓, HDAC1↓ | [59] |
| NCM460, NCM356 | miR-23b↑ miR-27b↑ | FZD7↓ MAP3K1↓ | [60] [63] | |
| NCM460, NCM356 | miR-155↓ | SOCS1↑, AKT↓ | [60,66] | |
| EVOO | Caco-2 | miR-23a↓ miR-301a↓ | CB1↑ | [71] |
| A-mangostin | DLD-1 | miR-143↑ | MAPK/Erk5 ↓ | [73] |
| AKBA | SW620, HT29, HCT116 | miR-200b↑ miR-200c↑ miR-let7a↑ | Vimentin↓ CDK6↓ E-cadherin↑ | [76] |
| Spica Prunellae | HCT-8 | miR-34a↑ | Notch-1↓ Notch-2↓ Bcl-2↓ | [82] |
| Ellagic Acid and Urolithins | HT-29, Caco-2 | miR-215↑ miR-224↓ | CDKN1A↑ | [87] |
| Rosemary Extract | SW480 | miR-15b↓ | GCNT3↑ | [90] |
| Methyl Jasmonate | SW620 | miR-101↑ | EZH2↓ | [92] |
| HAG | HCT116, DLD-1, LOVO | miR-29b↑ | MMP-2↓ | [96] |
| Phytochemical | Cell Line Tested | miRNA Affected | Target(s) and Effect | Reference |
|---|---|---|---|---|
| Resveratrol | APCCKO/Krasmut mice | miR-96↑ | Kras↓ | [40] |
| ApcMin/+ mice | miR-101b↑ miR-455↑ | IL-6↓, TNF-α↓ | [41] | |
| Grape Seed Extract | Azoxymethane (AOM)-induced colon tumors in A/J Mice | miR-19a↑ miR-20a↑ miR-103↓ miR-135b↓ miR-148a↓ miR-196a↓ miR-205↓ miR-let7a↑ | NF-κB↓ β-catenin↓ pERK1/2↓ HIF-1α↓ Kras↓ VEGF↓ C-myc↓ | [48] |
| Walnuts | HT-29 injected into mice | miR-297a↑ miR-467c↓ miR-1903↓ miR-3068↓ | Cyclooxygenase enzymes↓ FAT4↑ FGFR2↑ NCOA3↑ LMO4↑ PIGR↑ SNARP↑ RBM25↑ | [69] |
| Plum Polyphenols | AOM-induced colon tumors in Sprague-Dawley rats | miR-143↑ | Akt↓, mTOR↓ | [81] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathy, A.; Ezekiel, U. Phytochemical Modulation of MiRNAs in Colorectal Cancer. Medicines 2019, 6, 48. https://doi.org/10.3390/medicines6020048
Ganapathy A, Ezekiel U. Phytochemical Modulation of MiRNAs in Colorectal Cancer. Medicines. 2019; 6(2):48. https://doi.org/10.3390/medicines6020048
Chicago/Turabian StyleGanapathy, Aravinda, and Uthayashanker Ezekiel. 2019. "Phytochemical Modulation of MiRNAs in Colorectal Cancer" Medicines 6, no. 2: 48. https://doi.org/10.3390/medicines6020048
APA StyleGanapathy, A., & Ezekiel, U. (2019). Phytochemical Modulation of MiRNAs in Colorectal Cancer. Medicines, 6(2), 48. https://doi.org/10.3390/medicines6020048




