Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations
Abstract
:1. Introduction
2. P407-Based Hydrogels
3. Biocompatible Nanocarrier-Loaded Poloxamer 407 Hydrogels
3.1. Vesicular Delivery Systems Embedded in P407 Hydrogels
3.1.1. Liposomes
3.1.2. Niosomes
3.1.3. Ethosomes
3.2. Solid Lipid Nanoparticles (SLNs)
3.3. Polymeric Micro- and Nanoparticles Embedded in P407 Hydrogels
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosco, D.; Celia, C.; Cilurzo, F.; Trapasso, E.; Paolino, D. Colloidal carriers for the enhanced delivery through the skin. Expert Opin. Drug Deliv. 2008, 5, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(r)—The first fda-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Pitorre, M.; Gondé, H.; Haury, C.; Messous, M.; Poilane, J.; Boudaud, D.; Kanber, E.; Rossemond Ndombina, G.A.; Benoit, J.P.; Bastiat, G. Recent advances in nanocarrier-loaded gels: Which drug delivery technologies against which diseases? J. Control. Release 2017, 266, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R. Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels … A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Maolin, Z.; Jun, L.; Min, Y.; Hongfei, H. The swelling behaviour of radiation prepared semi-interpenetrating polymer networks composed of polyNIPAAm and hydrophilic polymers. Radiat. Phys. Chem. 2000, 58, 397–400. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; van Goor, H.; van Hest, J.C.M.; Hoogenboomb, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogels from soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, E159. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2016, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Hoarea, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Maria Giannitelli, S.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Schmolka, I.R. Physical basis for poloxamer interactions. Ann. N. Y. Acad. Sci. 1994, 720, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takats, Z.; Vekey, K.; Hegedus, L. Qualitative and quantitative determination of poloxamer surfactants by mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K. Recent progress in biomedical applications of pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Veyries, M.L.; Couarraze, G.; Geiger, S.; Agnely, F.; Massias, L.; Kunzli, B.; Faurisson, F.; Rouveix, B. Controlled release of vancomycin from poloxamer 407 gels. Int. J. Pharm. 1999, 192, 183–193. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic f-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Wen, Y.; Ban, J.; Mo, Z.; Zhang, Y.; An, P.; Liu, L.; Xie, Q.; Du, Y.; Xie, B.; Zhan, X.; et al. A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone. Nanotechnology 2018, 29, 425101. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.J.; Bentley, M.V.; Farah, M.; Bretas, R.E.; Marchetti, J.M. Rheological characterization of Poloxamer 407 lidocaine hydrochloride gels. Eur. J. Pharm. Sci. 2002, 17, 161–167. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Álvarez, R.; López Cortés, L.E.; Molina, J.; Cisneros, J.M.; Pachón, J. Optimizing the Clinical Use of Vancomycin. Antimicrob. Agents Chemother. 2016, 60, 2601–2609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevher, E.; Orhan, Z.; Mülazimoǧlu, L.; Şensoy, D.; Alper, M.; Yildiz, A.; Özsoy, Y. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int. J. Pharm. 2006, 317, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.P.; Jayasuriya, A.C. Effect of dual delivery of antibiotics (vancomycin and cefazolin) and BMP-7 from chitosan microparticles on Staphylococcus epidermidis and pre-osteoblasts in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, A.C.; Akkari, A.C.; Ferreira, I.R.; Maruyama, C.R.; Pascoli, M.; Guilherme, V.A.; de Paula, E.; Fraceto, L.F.; de Lima, R.; Melo Pda, S.; et al. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: Sol–gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int. J. Nanomed. 2015, 10, 2391–2401. [Google Scholar] [CrossRef]
- Park, Y.J.; Yong, C.S.; Kim, H.M.; Rhee, J.D.; Oh, Y.K.; Kim, C.K.; Choi, H.G. Effect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gel. Int. J. Pharm. 2003, 263, 105–111. [Google Scholar] [CrossRef]
- Lee, J.W.; Lim, T.H.; Park, J.B. Intradiscal drug delivery system for the treatment of low back pain. J. Biomed. Mater. Res. A 2010, 92, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Reilly, M.J.; Bhatia, S.K.; Sakhitab, N.; Archambault, J.D.; Bhatia, S.R. Effect of pharmaceuticals on thermoreversible gelation of PEO-PPO-PEO copolymers. Colloids Surf. B Biointerfaces 2008, 63, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kizilbash, A.; Ngô-Minh, C.T. Review of extended-release formulations of Tramadol for the management of chronic non-cancer pain: Focus on marketed formulations. J. Pain Res. 2014, 7, 149–161. [Google Scholar] [CrossRef]
- Desmeules, J.A.; Piguet, V.; Collart, L.; Dayer, P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br. J. Clin. Pharmacol. 1996, 41, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Dong, Z.Q.; Ma, F.; Bauer, W.R.; Wang, X.; Wu, G.C. Involvement of serotonin 2A receptors in the analgesic effect of tramadol in mono-arthritic rats. Brain Res. 2008, 1210, 76–83. [Google Scholar] [CrossRef]
- Bravo, L.; Mico, J.A.; Berrocoso, E. Discovery and development of tramadol for the treatment of pain. Expert Opin. Drug Discov. 2017, 12, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.K.D.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; de Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesia: Physico-chemical characterization and pharmacological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 299–307. [Google Scholar] [CrossRef]
- Kuthiala, G.; Chaudhary, G. Ropivacaine: A review of its pharmacology and clinical use Indian. J. Anaesth. 2011, 55, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.; Stacey, B. Multimodal treatment of chronic pain. Med. Clin. N. Am. 2016, 100, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ur-Rehman, T.; Tavelin, S.; Grobner, G. Chitosan in situ gelation for improved drug loading and retention in poloxamer 407 gels. Int. J. Pharm. 2011, 409, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Quaglia, F.; Borzacchiello, A.; Ambrosio, L.; La Rotonda, M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Choi, H.G.; Jung, J.H.; Ryu, J.M.; Yoon, S.J.; Oh, Y.K.; Kim, C.K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Dimer, F.A.; Pohlmann, A.R.; Guterres, S.S. Characterization of rheology and release profiles of olanzapine-loaded lipid-core nanocapsules in thermosensitive hydrogel. J. Nanosci. Nanotechnol. 2013, 13, 8144–8153. [Google Scholar] [CrossRef]
- Gu, D.; O’Connor, A.J.; Qiao, G.H.G.; Ladewig, K. Hydrogels with smart systems for delivery of hydrophobic drugs. Expert Opin. Drug Deliv. 2017, 14, 879–895. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubert, R.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Fresta, M. Innovative drug delivery systems for the administration of natural compounds. Curr. Bioact. Compd. 2007, 3, 262–277. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Gaspari, M.; Celano, M.; Wolfram, J.; Voce, P.; Puxeddu, E.; Filetti, S.; Celia, C.; Ferrari, M.; et al. Targeting the thyroid gland with thyroid-stimulating hormone (TSH)-nanoliposomes. Biomaterials 2014, 35, 7101–7109. [Google Scholar] [CrossRef] [Green Version]
- Cosco, D.; Federico, C.; Maiuolo, J.; Bulotta, S.; Molinaro, R.; Paolino, D.; Tassone, P.; Fresta, M. Physicochemical features and transfection properties of chitosan/poloxamer 188/poly(d,l-lactide-co-glycolide) nanoplexes. Int. J. Nanomed. 2014, 9, 2359–2372. [Google Scholar] [CrossRef]
- Cohen, R.; Kanaan, H.; Grant, G.J.; Barenholz, Y. Prolonged analgesia from Bupisome and Bupigel formulations: From design and fabrication to improved stability. J. Control. Release 2012, 160, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.C.; Kaye, A.D.; Urman, R.D. Liposomal bupivacaine and clinical outcomes. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Capasso, G.; Maestrelli, F.; Furlanetto, S. Optimization of formulation variables of benzocaine liposomes using experimental design. J. Liposome Res. 2008, 18, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Capasso, G.; González-Rodríguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. Effect of preparation technique on the properties and in vivo efficacy of benzocaine-loaded ethosomes. J. Liposome Res. 2009, 19, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.M.A.; Fraceto, L.F.; Santana, M.H.A.; Pertinhez, T.A.; Junior, S.O.; de Paula, E. Physico-chemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 2005, 39, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Maestrelli, F.; González-Rodríguez, M.L.; Michelacci, I.; Ghelardini, C.; Rabasco, A.M. Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur. J. Pharm. Biopharm. 2007, 67, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.; Kulkarni, V.; Murthy, R.S.R. Chitosan-based thermosensitive hydrogel containing liposomes for sustained delivery of cytarabine. Drug Dev. Ind. Pharm. 2009, 35, 49–56. [Google Scholar] [CrossRef]
- Li, X.; Fan, R.; Tong, A.; Yang, M.; Deng, J.; Zhou, L.; Zhang, X.; Guo, G. In situ gel-forming ap-57 peptide delivery system for cutaneous wound healing. Int. J. Pharm. 2015, 495, 560–571. [Google Scholar] [CrossRef]
- Caron, M.; Besson, G.; Etenna, S.L.-D.; Mintsa-Ndong, A.; Mourtas, S.; Radaelli, A.; Morghen, C.D.G.; Loddo, R.; La Colla, P.; Antimisiaris, S.G.; et al. Protective properties of non-nucleoside reverse transcriptase inhibitor (MC1220) incorporated into liposome against intravaginal challenge of rhesus macaques with RT-SHIV. Virology 2010, 405, 225–233. [Google Scholar] [CrossRef]
- Bochot, A.; Fattal, E.; Gulik, A.; Couarraze, G.; Couvreur, P. Liposomes dispersed within a thermosensitive gel: A new dosage form for ocular delivery of oligonucleotides. Pharm. Res. 1998, 15, 1364–1369. [Google Scholar] [CrossRef]
- Mura, P.; Mennini, N.; Nativi, C.; Richichi, B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur. J. Pharm. Biopharm. 2018, 122, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, Y.; Hou, X.; Li, G. Bleomycin a6-loaded anionic liposomes with in situ gel as a new antitumoral drug delivery system. Drug Deliv. 2016, 23, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Hsiao, W.L.; Pan, W.; Yang, Z. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Chen, G.; Wang, S. Thermosensitive hydrogel system with paclitaxel liposomes used in localized drug delivery system for in situ treatment of tumor: Better antitumor efficacy and lower toxicity. J. Pharm. Sci. 2016, 105, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.E.; Gentile, L.; Rossi, C.O.; Tavano, L.; Ranieri, G.A. Gels of pluronic f127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf. B Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef]
- Din, F.U.; Mustapha, O.; Kim, D.W.; Rashid, R.; Park, J.H.; Choi, J.Y.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Choi, H.G. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015, 94, 64–72. [Google Scholar] [CrossRef]
- Shen, M.; Xu, Y.Y.; Sun, Y.; Han, B.S.; Duan, Y.R. Preparation of a thermosensitive gel composed of a mpeg-plga-pll-crgd nanodrug delivery system for pancreatic tumor therapy. ACS Appl. Mater. Interfaces 2015, 7, 20530–20537. [Google Scholar] [CrossRef]
- Timur, S.S.; Sahin, A.; Aytekin, E.; Ozturk, N.; Polat, K.H.; Tezel, N.; Gursoy, R.N.; Calis, S. Design and in vitro evaluation of tenofovir-loaded vaginal gels for the prevention of HIV infections. Pharm. Dev. Technol. 2018, 23, 301–310. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Vaidya, B.; Prakash, A.; Rath, G.; Goyal, A.K. Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: Behavioral and biochemical assessment. J. Drug Target 2015, 23, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Wu, X.; Olson, V.; D’Souza, M.J. Characterization of rabies pDNA nanoparticulate vaccine in poloxamer 407 gel. Int. J. Pharm. 2018, 545, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pradhan, M.; Nag, M.; Singh, M.R. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artif. Cells Nanomed. Biotechnol. 2015, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Federico, C.; Morittu, V.M.; Britti, D.; Trapasso, E.; Cosco, D. Gemcitabine-loaded liposomes: Rationale, potentialities and future perspectives. Int. J. Nanomed. 2012, 7, 5423–5436. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leclair, G.; Hildgen, P.; Gupta, A.; Leroux, J.C. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J. Control. Release 2002, 82, 373–383. [Google Scholar] [CrossRef]
- Mourtas, S.; Fotopoulou, S.; Duraj, S.; Sfika, V.; Tsakiroglou, C.; Antimisiaris, S.G. Liposomal drugs dispersed in hydrogels. Effect of liposome, drug and gel properties on drug release kinetics. Colloids Surf. B Biointerfaces 2007, 55, 212–221. [Google Scholar] [CrossRef]
- Mourtas, S.; Haikou, M.; Theodoropoulou, M.; Tsakiroglou, C.; Antimisiaris, S.G. The effect of added liposomes on the rheological properties of a hydrogel: A systematic study. J. Colloid. Interface Sci. 2008, 317, 611–619. [Google Scholar] [CrossRef]
- Hurler, J.; Zakelj, S.; Mravljak, J.; Pajk, S.; Kristl, A.; Schubert, R.; Skalko-Basnet, N. The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int. J. Pharm. 2013, 456, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochot, A.; Fattal, E.; Grossiord, J.L.; Puisieux, F.; Couvreur, P. Characterization of a new ocular delivery system based on a dispersion of liposomes in a thermosensitive gel. Int. J. Pharm. 1998, 162, 119–127. [Google Scholar] [CrossRef]
- Fattal, E.; Bochot, A. Ocular delivery of nucleic acids: Antisense oligonucleotides, aptamers and siRNA. Adv. Drug Deliv. Rev. 2006, 58, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Collin, R.W.; Garanto, A. Applications of antisense oligonucleotides for the treatment of inherited retinal diseases. Curr. Opin. Ophthalmol. 2017, 28, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.E.; Maggisano, V.; Celano, M.; Cosco, D.; Mignogna, C. Anti-htert sirna-loaded nanoparticles block the growth of anaplastic thyroid cancer xenograft. Mol. Cancer Ther. 2018, 17, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Cilurzo, F.; Maiuolo, J.; Federico, C.; Di Martino, M.T.; Cristiano, M.C.; Tassone, P.; Fresta, M.; Paolino, D. Delivery of mir-34a by chitosan/plga nanoplexes for the anticancer treatment of multiple myeloma. Sci. Rep. 2015, 5, 17579. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobao, P.; Sousa Lobo, J.M. Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Expert Opin. Drug Deliv. 2013, 10, 1223–1237. [Google Scholar] [CrossRef]
- Mennini, N.; Mura, P.; Nativi, C.; Richichi, B.; Di Cesare Mannelli, L.; Ghelardini, C. Injectable liposomal formulations of opiorphin as a new therapeutic strategy in pain management. Future Sci. 2015, 1, FSO2. [Google Scholar] [CrossRef]
- Wisner, A.; Dufour, E.; Messaoudi, M.; Nejdi, A.; Marcel, A.; Ungeheuer, M.N.; Rougeot, C. Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc. Natl. Acad. Sci. USA 2006, 103, 17979–17984. [Google Scholar] [CrossRef] [Green Version]
- Yuba, E.; Osaki, T.; Ono, M.; Park, S.; Harada, A.; Yamashita, M.; Azuma, K.; Tsuka, T.; Ito, N.; Imagawa, T.; et al. Bleomycin-loaded pH-sensitive polymer–lipid-incorporated liposomes for cancer chemotherapy. Polymers 2018, 10, 74. [Google Scholar] [CrossRef]
- Liu, Y.; Yoo, S.D.; Li, L.; Fang, L.; Wen, Z.; Li, T. Formulation and characterization of boanmycin-loaded liposomes prepared by pH gradient experimental design. Drug Deliv. 2012, 19, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Russo, D.; Fresta, M. Liposomes as multicompartmental carriers for multidrug delivery in anticancer chemotherapy. Drug Deliv. Transl. Res. 2011, 1, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Montaseri, H.; Khalili, A.; Tafaghodi, M. Non-invasive endotracheal delivery of paclitaxel-loaded alginate microparticles. J. Chemother. 2016, 28, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Develiv. Ther. 2015, 9, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Muzzalupo, R.; Celia, C.; Citraro, R.; Caponio, D.; Picci, N.; Fresta, M. Novel peg-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed. Microdevices 2009, 11, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, D.; Abdel-Mageed, A.; Abdel-Hamid, F.; Ahmed, M. In vitro and In vivo Evaluation of Niosomal Gel Containing Aceclofenac for Sustained Drug Delivery. Int. J. Pharm. Sci. Res. 2014, 1, 11. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Ainbinder, D.; Paolino, D.; Fresta, M.; Touitou, E. Drug delivery applications with ethosomes. J. Biomed. Nanotechnol. 2010, 6, 558–568. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan lab® expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes(r) and transfersomes(r) containing linoleic acid: Physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 2012, 14, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Kandil, S.M.; Miniawy, H. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Di Marzio, L.; Marianecci, C.; Trapasso, E.; Paolino, D.; Celia, C.; Carafa, M.; Fresta, M. Colloidal supramolecular aggregates for therapeutic application in neuromedicine. Curr. Med. Chem. 2014, 21, 4132–4153. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Barrett, A.M.; Dehghani, F.; Foster, N.R. Increasing the dissolution rate of itraconazole processed by gas antisolvent techniques using polyethylene glycol as a carrier. Pharm. Res. 2008, 25, 1274–1289. [Google Scholar] [CrossRef]
- Koffi, A.A.; Agnely, F.; Ponchel, G.; Grossiord, J.L. Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. Eur. J. Pharm. Sci. 2006, 27, 328–335. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.P.; Guo, Y.S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.H.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Özgüney, I.; Kardhiqi, A.; Yıldız, G.; Ertan, G. In vitro-in vivo evaluation of in situ gelling and thermosensitive ketoprofen liquid suppositories. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.S.; Sah, H.; Jahng, Y.; Chang, H.W.; Son, J.K.; Lee, S.H.; Jeong, T.C.; Rhee, J.D.; Baek, S.H.; Kim, C.K.; et al. Physicochemical characterization of diclofenac sodium-loaded poloxamer gel as a rectal delivery system with fast absorption. Drug Dev. Ind. Pharm. 2003, 29, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.N.; Karakucuk, A.; Ilbasmis-Tamer, S.; Celebi, N. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. Eur. J. Pharm. Sci. 2018, 122, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Choong, P.F.M.; Dass, C.R. Recent developments in liposomes, microparticles and nanoparticles for protein and peptide drug delivery. Peptides 2010, 31, 184–193. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; De Angelis, F.; Cilurzo, F.; Celia, C.; Di Marzio, L.; Russo, D.; Tsapis, N.; Fattal, E.; Fresta, M. Aqueous-core peg-coated pla nanocapsules for an efficient entrapment of water soluble anticancer drugs and a smart therapeutic response. Eur. J. Pharm. Biopharm. 2015, 89, 30–39. [Google Scholar] [CrossRef]
- Palma, E.; Pasqua, A.; Gagliardi, A.; Britti, D.; Fresta, M.; Cosco, D. Antileishmanial activity of amphotericin b-loaded-plga nanoparticles: An overview. Materials (Basel) 2018, 11, E1167. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Cosco, D.; Fattal, E.; Fresta, M.; Tsapis, N. Perfluorocarbon-loaded micro and nanosystems for medical imaging: A state of the art. J. Fluor. Chem. 2015, 171, 18–26. [Google Scholar] [CrossRef]
- Haenisch, B.; Bönisch, H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 2011, 129, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Dibi, M.; Mohammad, A.; Srouji, A.E. Nanovaccines formulation and applications—A review. J. Drug Deliv. Sci. Technol. 2018, 44, 380–387. [Google Scholar] [CrossRef]
- Indelicato, G.; Burkhard, P.; Twarock, R. Classification of self-assembling protein nanoparticle architectures for applications in vaccine design. R. Soc. Open Sci. 2017, 4, 161092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Warade, S.; Singhavi, D.J. Improvement in ocular bioavailability and prolonged delivery of tobramycin sulfate following topical ophthalmic administration of drug-loaded mucoadhesive microparticles incorporated in thermosensitive in situ gel. J. Ocul. Pharmacol. Ther. 2018, 34, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Bandi, N.; Ayalasomayajula, S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting vegf expression. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1192–1201. [Google Scholar] [CrossRef]
- Chen, P.C.; Kohane, D.S.; Park, Y.J.; Bartlett, R.H.; Langer, R.; Yang, V.C. Injectable microparticle-gel system for prolonged and localized lidocaine release. II In vivo anesthetic effects. J. Biomed. Mater. Res. A 2004, 70, 459–466. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Z.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, H.; Zhang, Q. A novel localized co-delivery system with lapatinib microparticles and paclitaxel nanoparticles in a peritumorally injectable in situ hydrogel. J. Control. Release 2015, 220, 189–200. [Google Scholar] [CrossRef]
- Ménard, S.; Pupa, S.M.; Campiglio, M.; Tagliabue, E. Biologic and therapeutic role of her2 in cancer. Oncogene 2003, 22, 6570. [Google Scholar] [CrossRef]
- Bonde, G.V.; Yadav, S.K. Lapatinib nano-delivery systems: A promising future for breast cancer treatment. Expert Opin. Drug Deliv. 2018, 15, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for her2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mei, L.; Feng, S.-S. Paclitaxel drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 325–340. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Carlfors, J.; Petersson, R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur. J. Pharm. Sci. 1998, 6, 105–112. [Google Scholar] [CrossRef]
- Yoo, H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and pluronic for sustained release of human growth hormone (hGH). J. Biomater. Sci. Polym. Ed. 2007, 18, 1429–1441. [Google Scholar] [CrossRef]
- Chun, K.W.; Lee, J.B.; Kim, S.H.; Park, T.G. Controlled release of plasmid DNA from photo-cross-linked pluronic hydrogels. Biomaterials 2005, 26, 3319–3326. [Google Scholar] [CrossRef]
- Bonacucina, G.; Spina, M.; Misici-Falzi, M.; Cespi, M.; Pucciarelli, S.; Angeletti, M.; Palmieri, G.F. Effect of hydroxypropyl beta-cyclodextrin on the self-assembling and thermogelation properties of Poloxamer 407. Eur. J. Pharm. Sci. 2007, 32, 115–122. [Google Scholar] [CrossRef]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974p designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.-Z.; Rhee, J.-D.; Kim, C.-K.; Lim, S.-J.; Kim, K.-M.; Oh, P.-S.; Choi, H.-G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
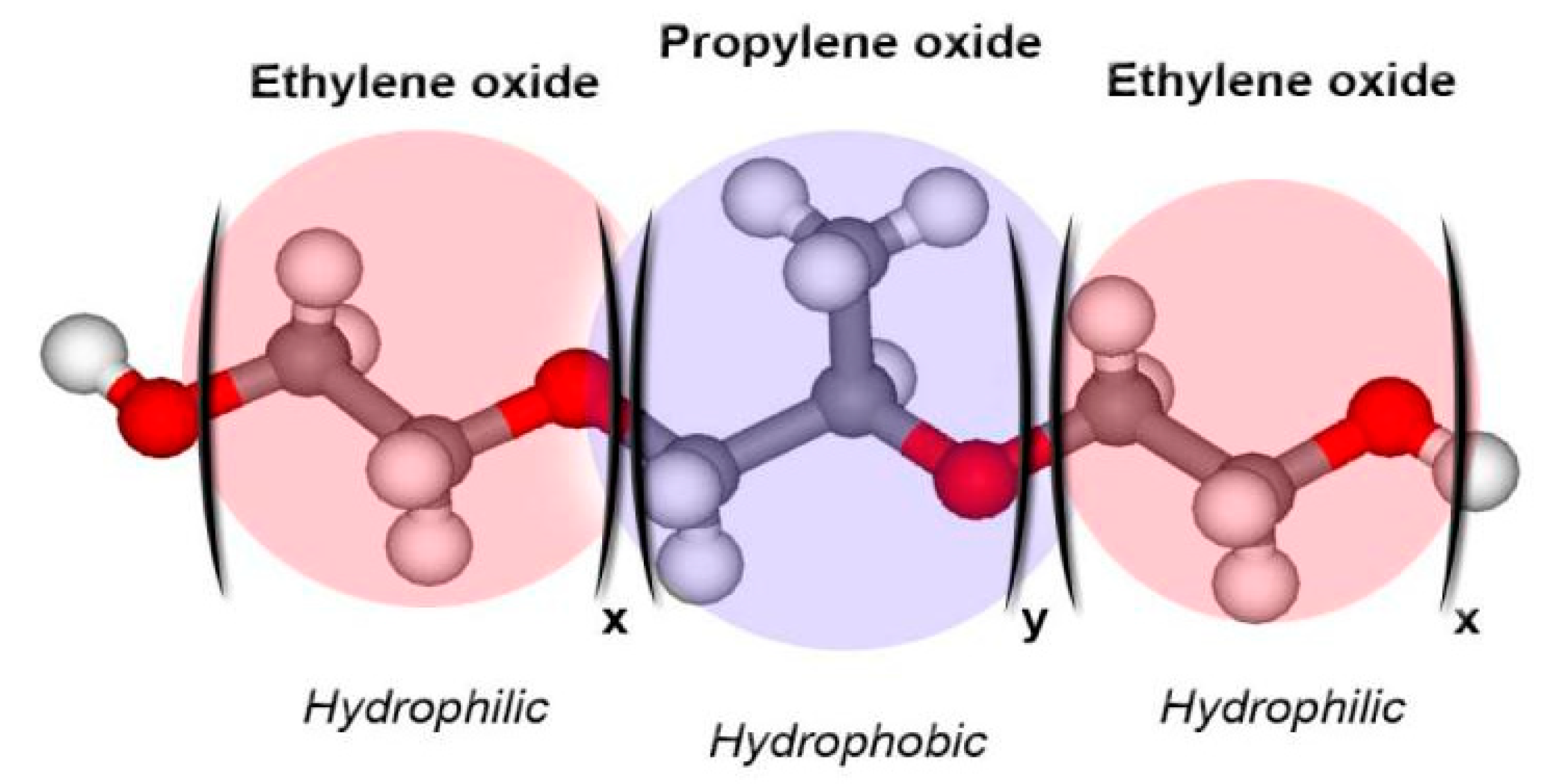
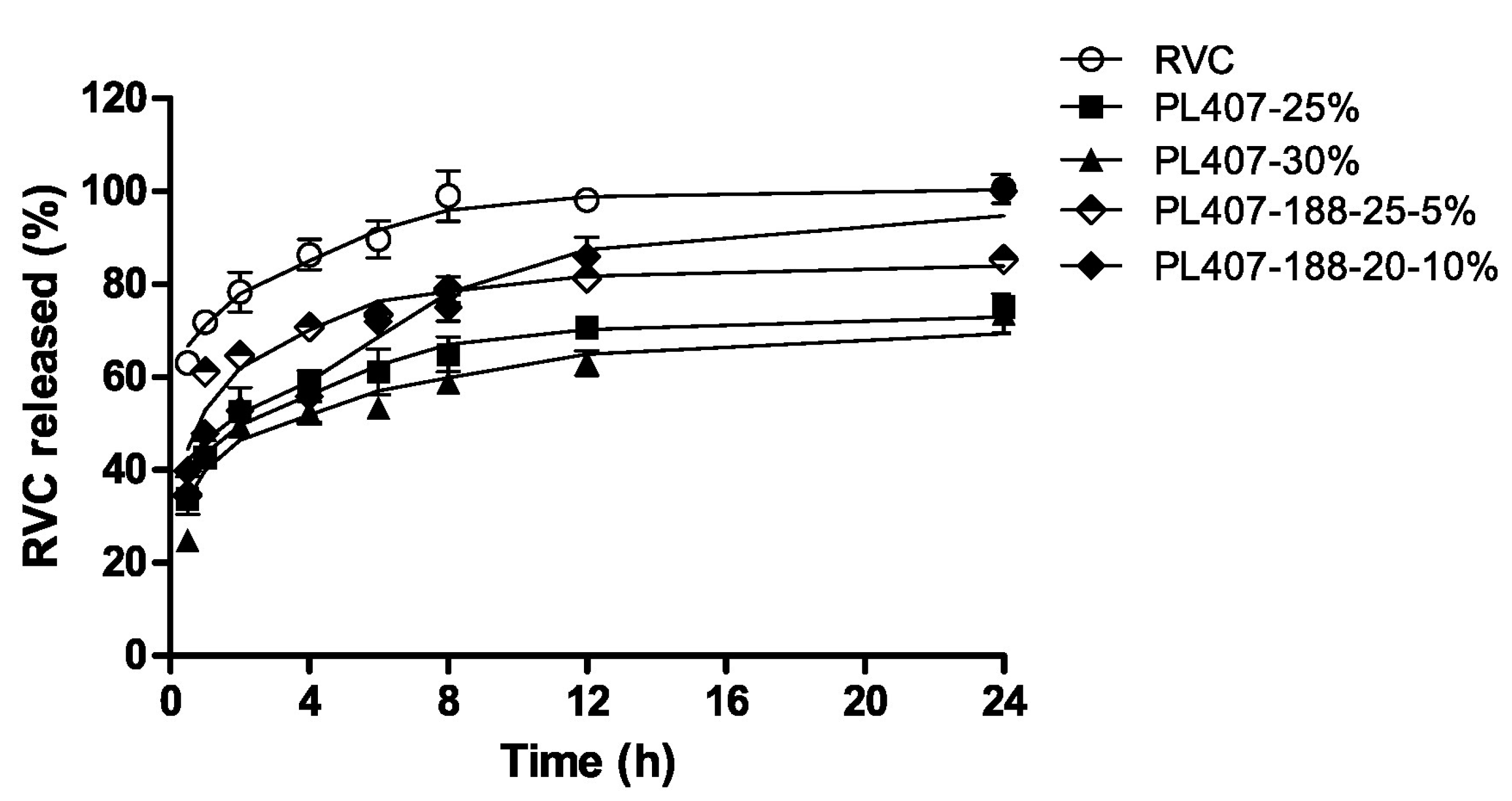
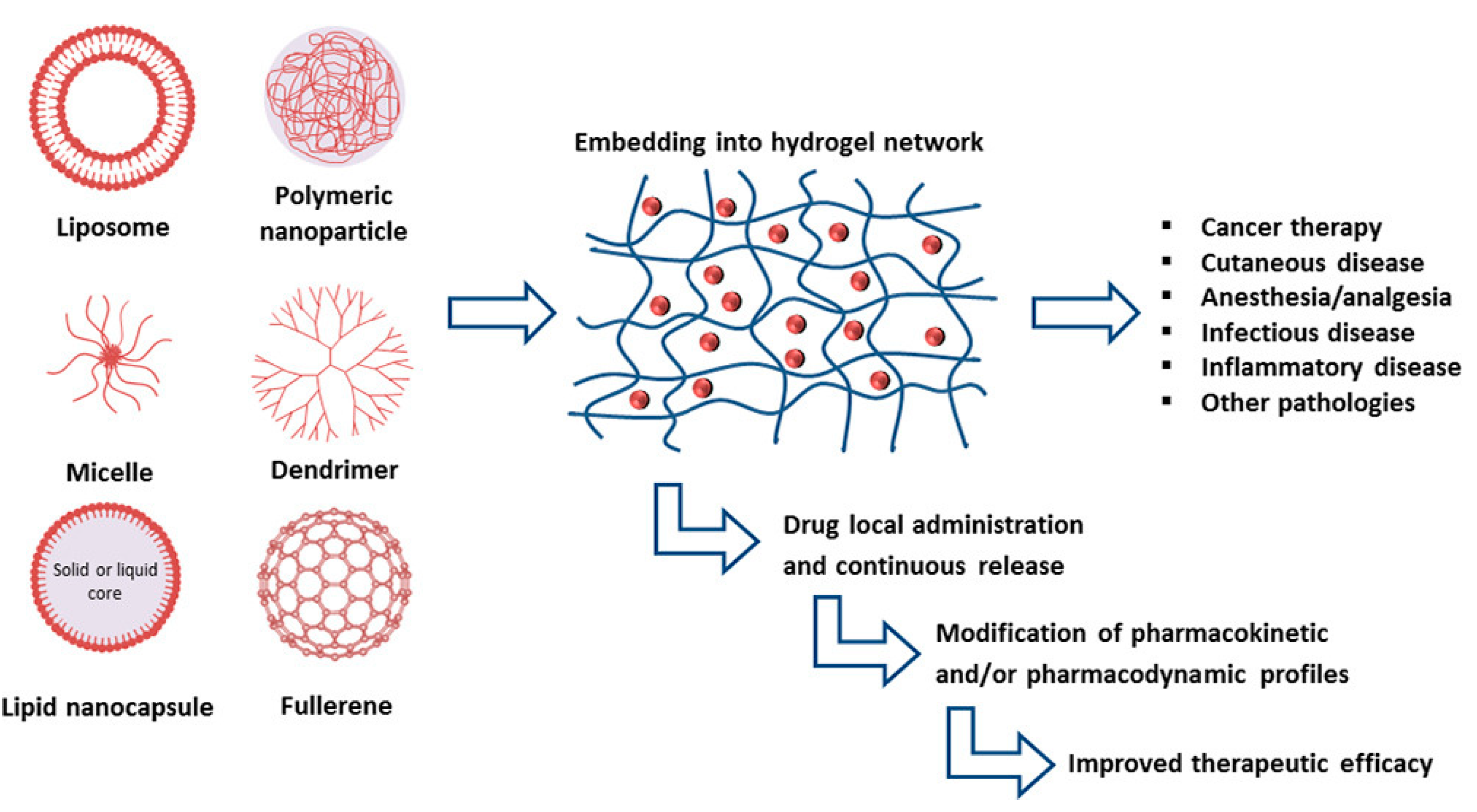
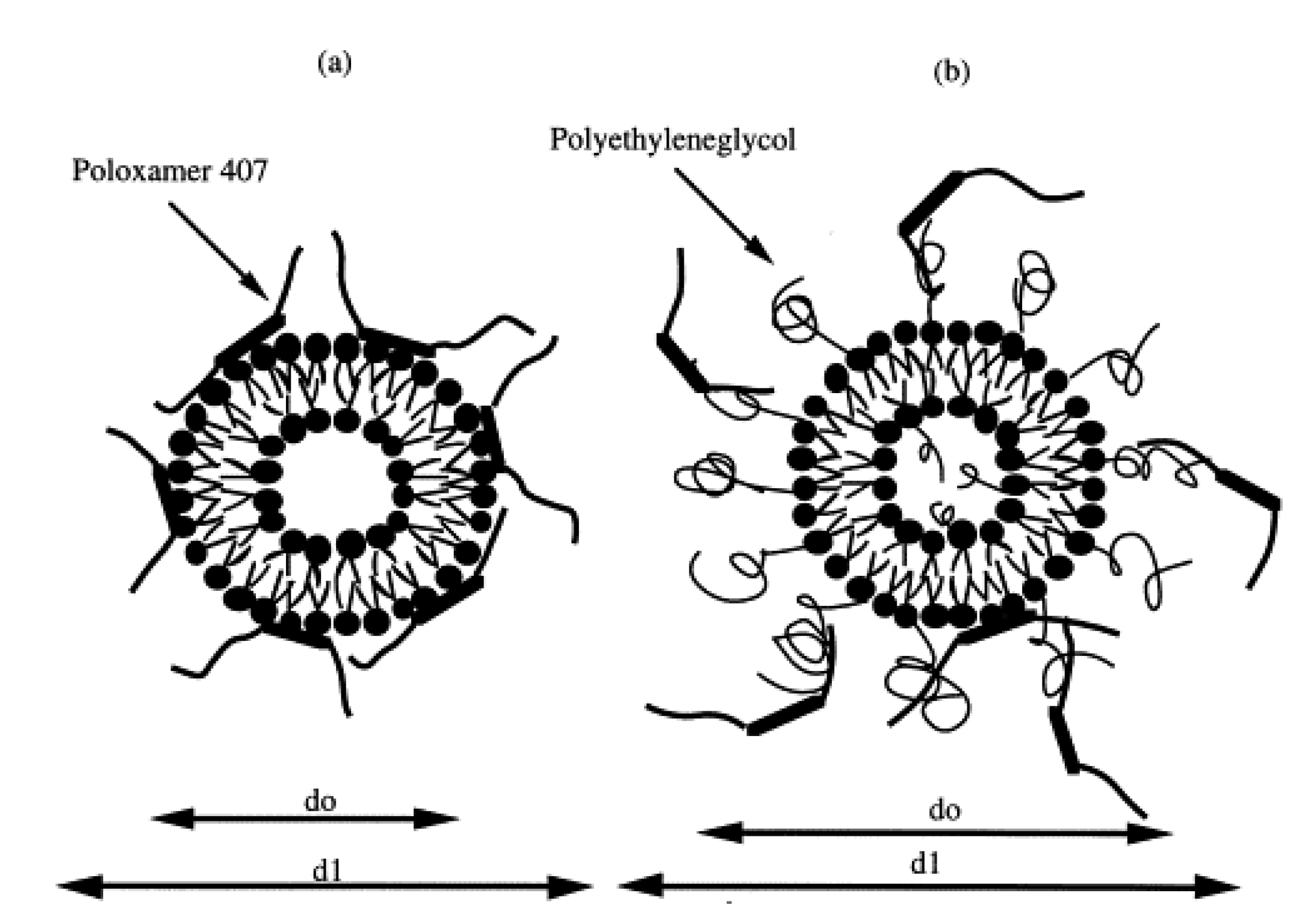
| Type of Nanocarrier | Formulation | Composition of Nanocarrier | Gel Composition | Reference |
|---|---|---|---|---|
| Vesicular Systems | ||||
| Liposomes | pdT16-loaded liposomes dispersed in a P407-based hydrogel as innovative ocular controlled release formulation. | SPC 1, CHOL 2, PEG-DSPE 3 | P407 4 | [70] |
| Liposomal mucoadhesive thermo-sensitive gel for the nasal delivery of opiorphin (OPI). | EPC 5, CHOL, SA 6, PEG-DSPE | P407, Carbopol 934P | [71] | |
| Bleomycin A6 (BLM A6)-loaded liposomes dispersed within thermo-sensitive in-situ gel for a sustained drug release. | PC S100 7, DPPG 8, CHOL | P407, P188 9 | [72] | |
| Paclitaxel (PTX)-loaded liposomes embedded in P407 gel for a controlled drug release and an improved antitumor efficiency. | SPC | P407 | [73] | |
| Thermo-sensitive hydrogel system containing PTX-liposomes for the local treatment of tumors. | SPC, CHOL | P407, P188 | [74] | |
| Niosomes | Tween 60-based vesicles embedded in P407 hydrogel as a transdermal drug delivery system. | Tween 60 10 | P407 | [75] |
| Ethosomes | P407-based thermo-sensitive hydrogel containing zolmitriptan (ZMT)-loaded ethosomes for an efficacious brain targeting and a sustained drug release. | Soya lecithin, ethanol | P407, Carbopol 934, HPMC 11 K100 | [76] |
| Solid lipid nanoparticles (SLNs) | ||||
| SLNs dispersed in a P407-based gel as delivery system for itraconazole. | Stearic acid, Compritol 888, Compritol E ATO | P407, Carbopol 934 | [77] | |
| SLNs-loaded dual-reverse thermo-sensitive hydrogel for rectal administration of flurbiprofen. | Tricaprin, triethanolamine | P407, P188 | [78] | |
| Polymeric nanoparticles (NPs) | ||||
| Thermo-sensitive gel containing paclitaxel-loaded NPs as delivery system for pancreatic tumor therapy. | mPEG-PLGA-PLL-cRGD 12 | P407, P188, HPMC, MC 13, Sodium Alginate | [79] | |
| Tenofovir–loaded NPs embedded in vaginal gels for the prevention of HIV infections. | Chitosan, TPP 14 | P407 | [80] | |
| Intranasal in-situ gel with nanoparticulated polymeric carriers for brain delivery of tramadol. | Chitosan, TPP | P407, HPMC K15M | [81] | |
| Rabies pDNA nanoparticulate vaccine in P407 hydrogel. | PLGA 15 | P407 | [82] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2019, 6, 7. https://doi.org/10.3390/medicines6010007
Giuliano E, Paolino D, Fresta M, Cosco D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines. 2019; 6(1):7. https://doi.org/10.3390/medicines6010007
Chicago/Turabian StyleGiuliano, Elena, Donatella Paolino, Massimo Fresta, and Donato Cosco. 2019. "Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations" Medicines 6, no. 1: 7. https://doi.org/10.3390/medicines6010007







