Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Intraperitoneal Glucose Tolerance Test and Insulin Analysis
2.3. Plasma Lipids
2.4. Liver Enzyme Activities
2.5. Hepatic Gene Expression
2.6. Plasma Inflammatory Markers
2.7. Statistical Analysis
3. Results
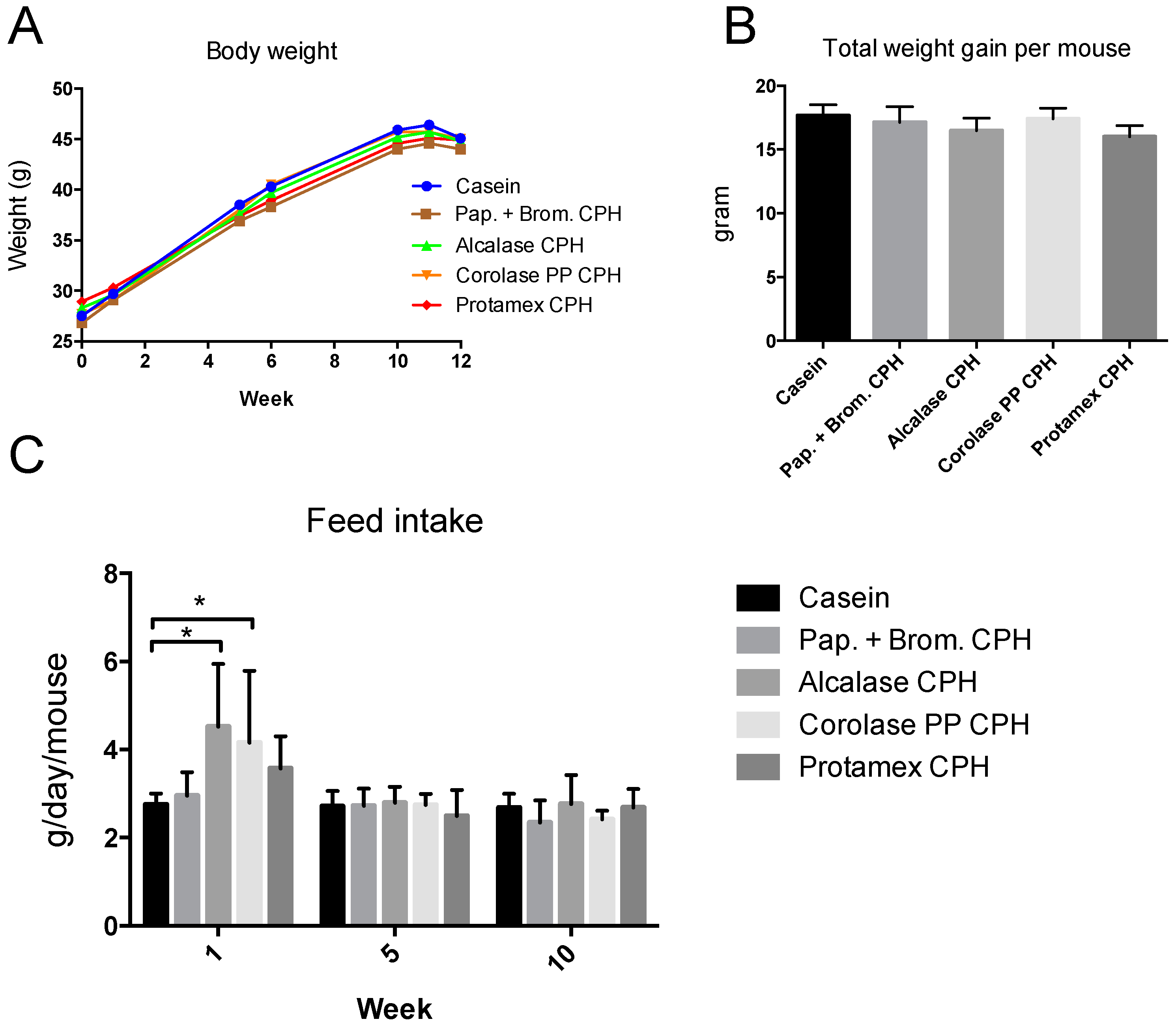
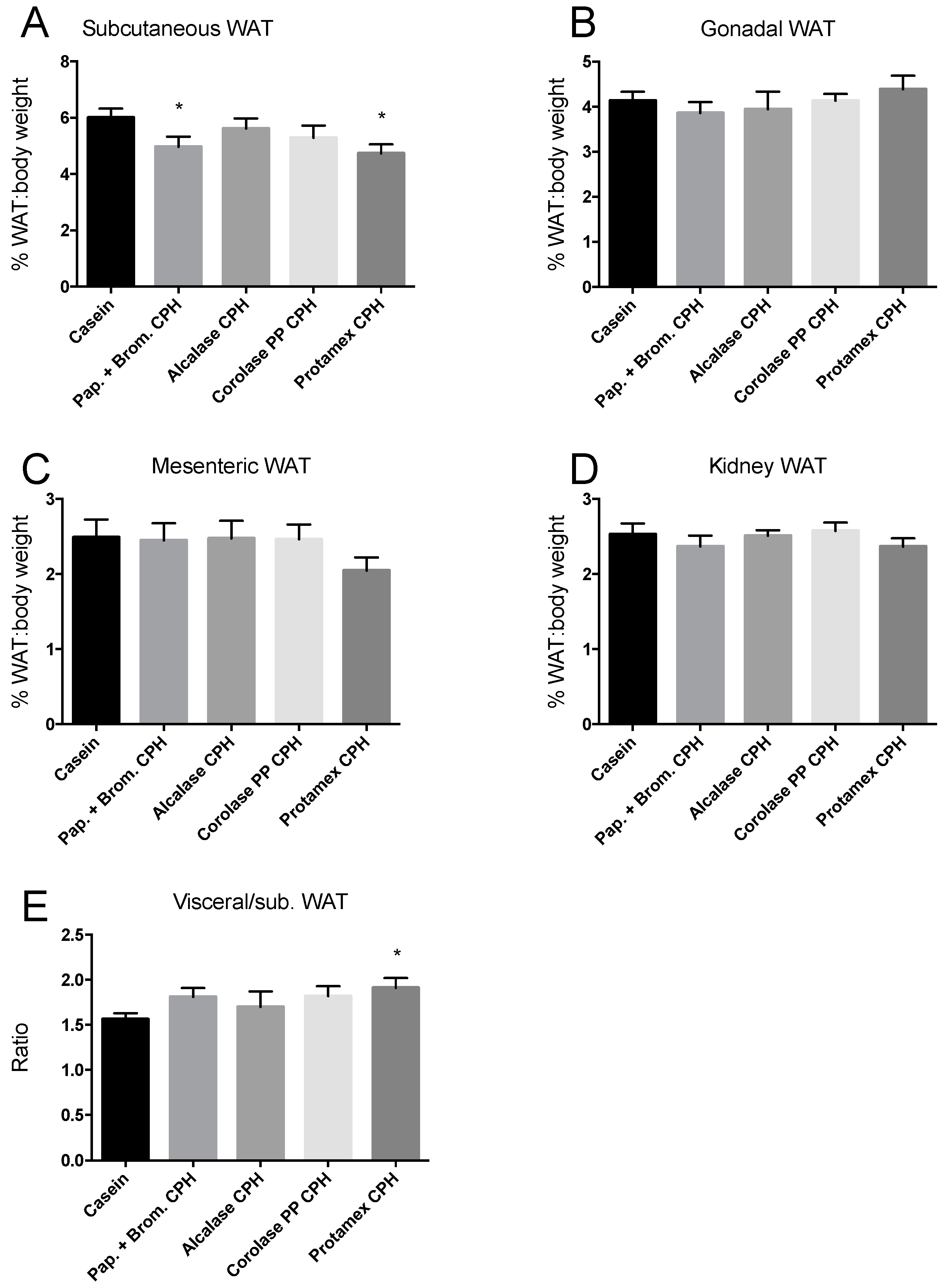
3.1. Weight Gain in C57BL/6 Mice after 12 Weeks on an Obesogenic Diet
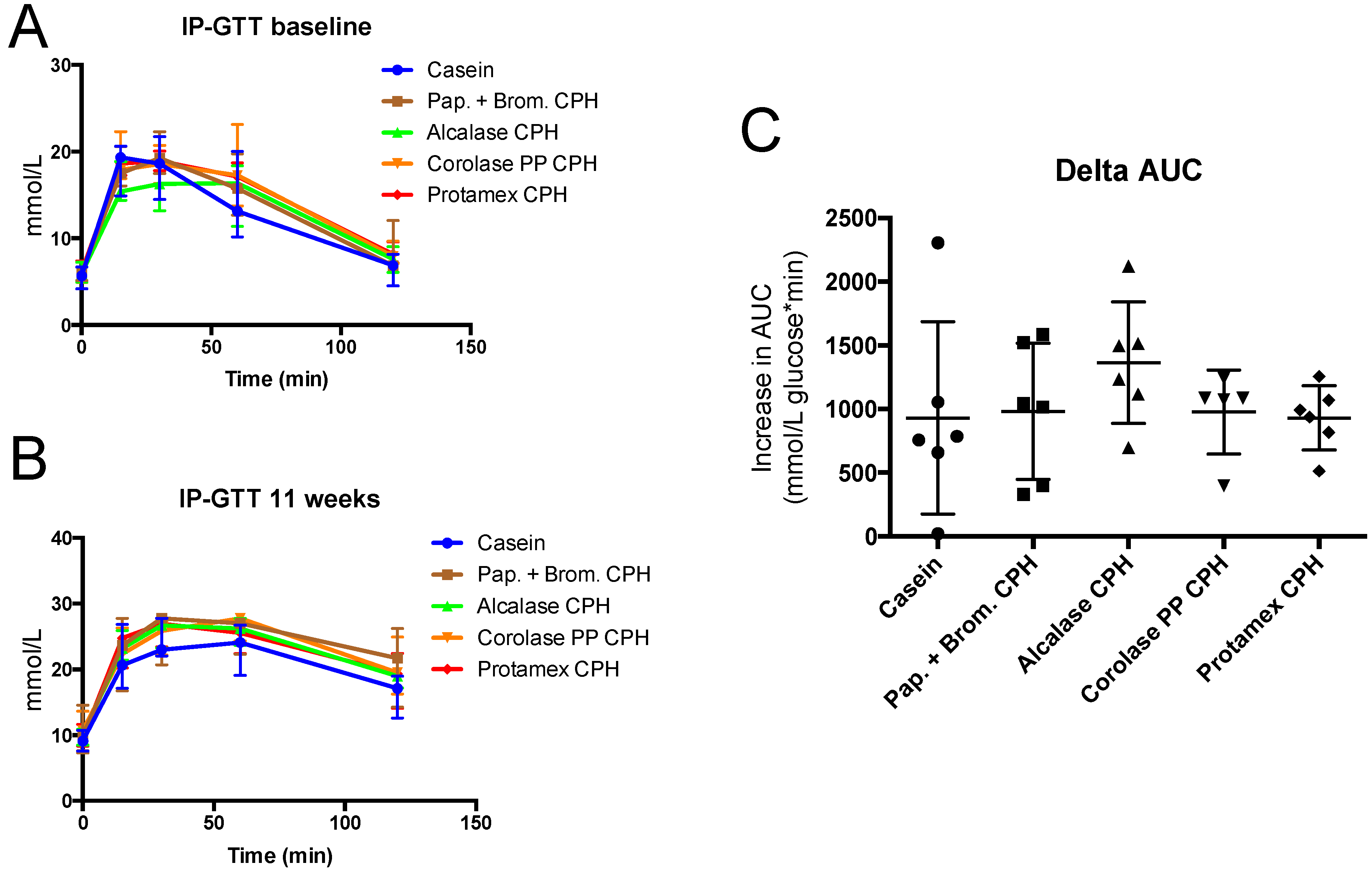
3.2. Intraperitoneal Glucose Tolerance Test
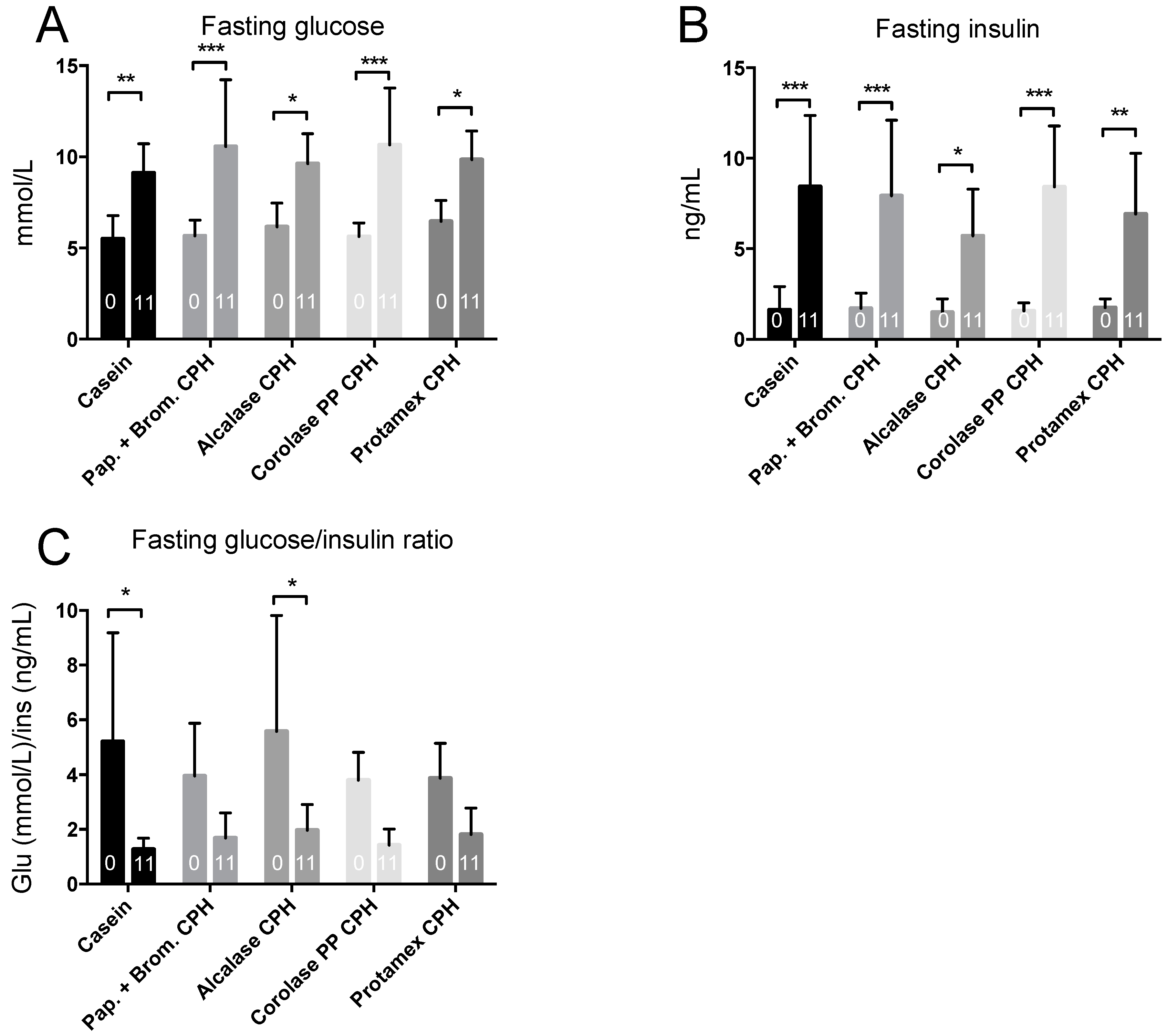
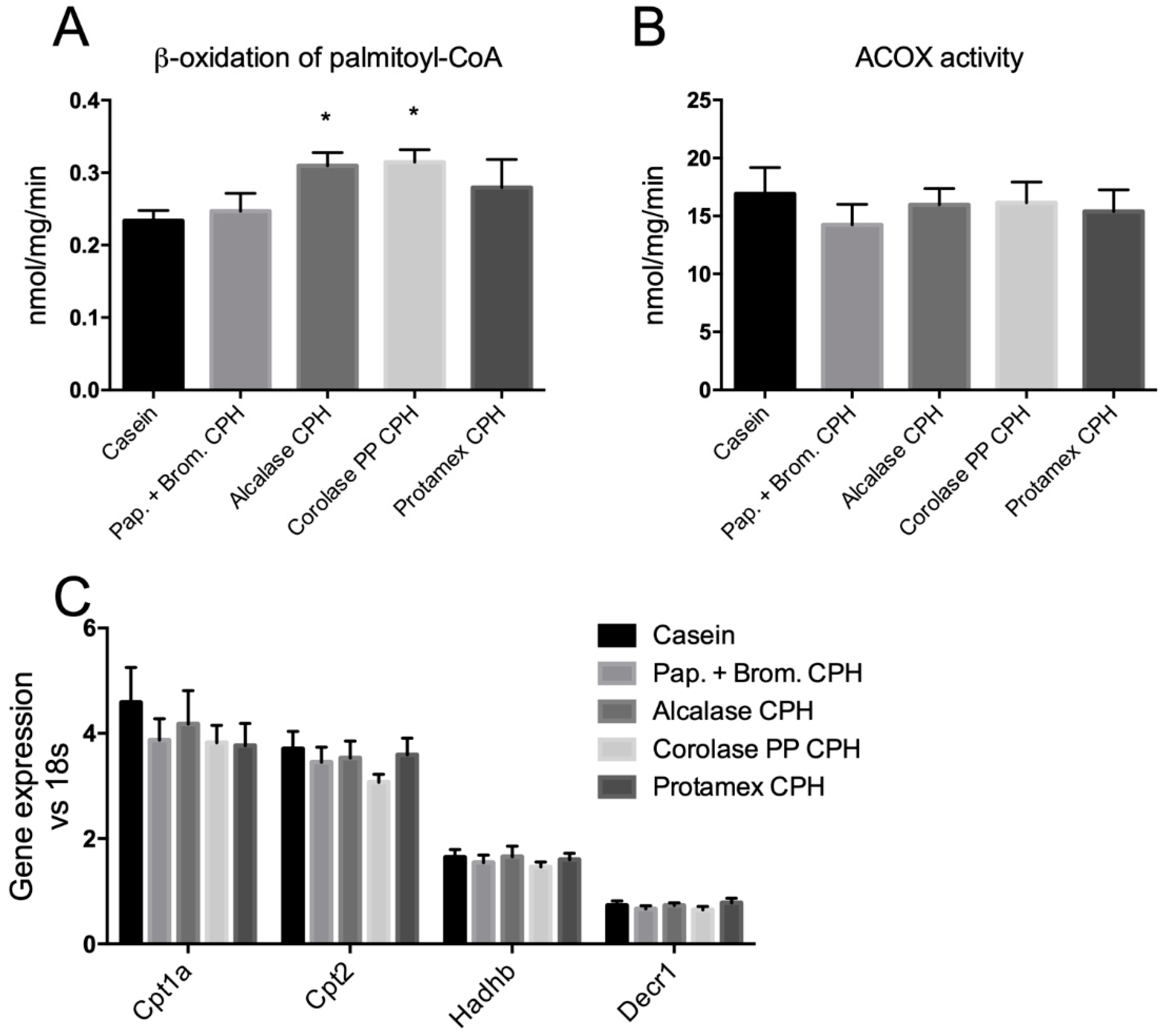
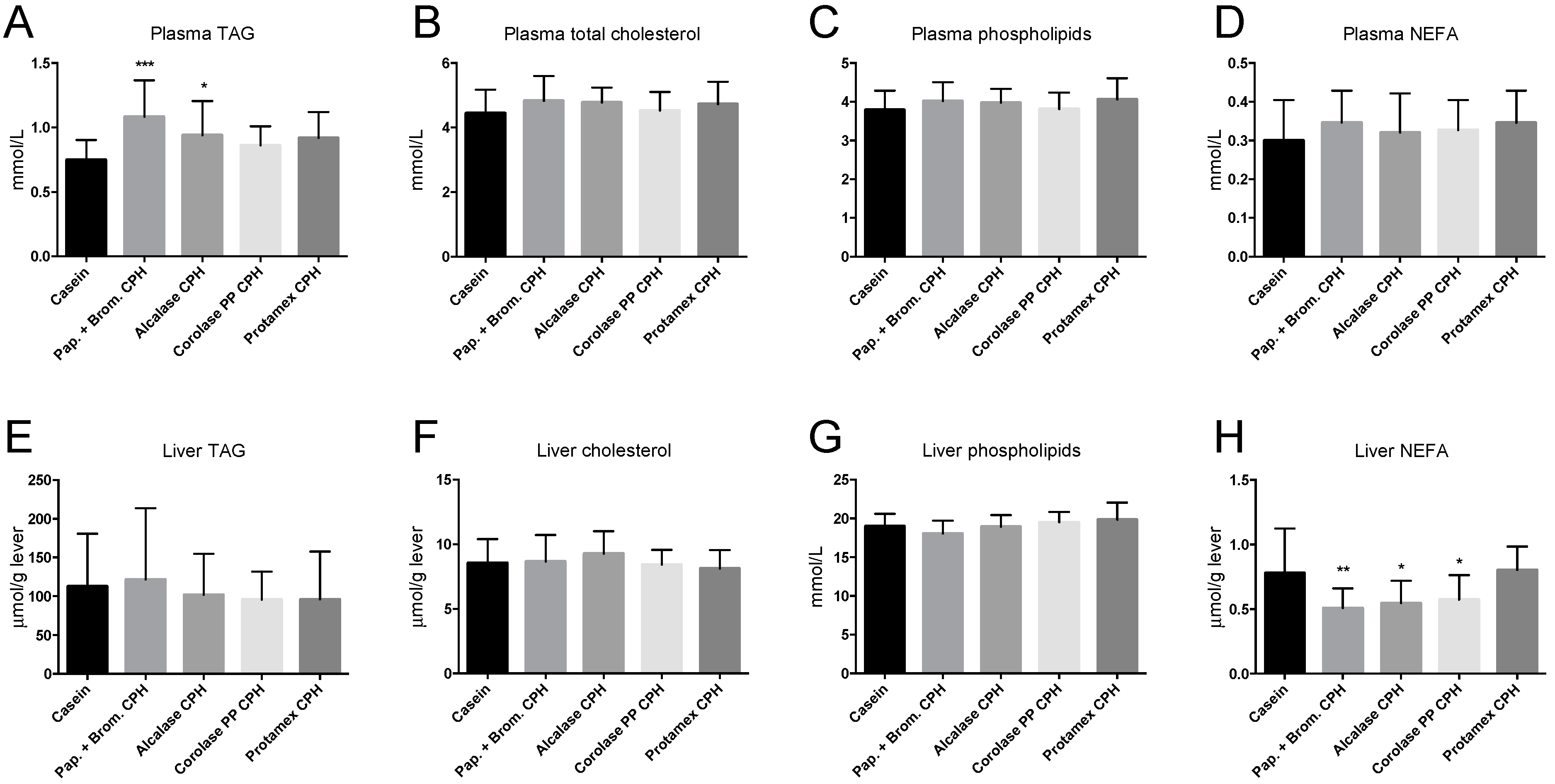
3.3. Hepatic Fatty Acid Oxidation and Plasma Lipid Levels
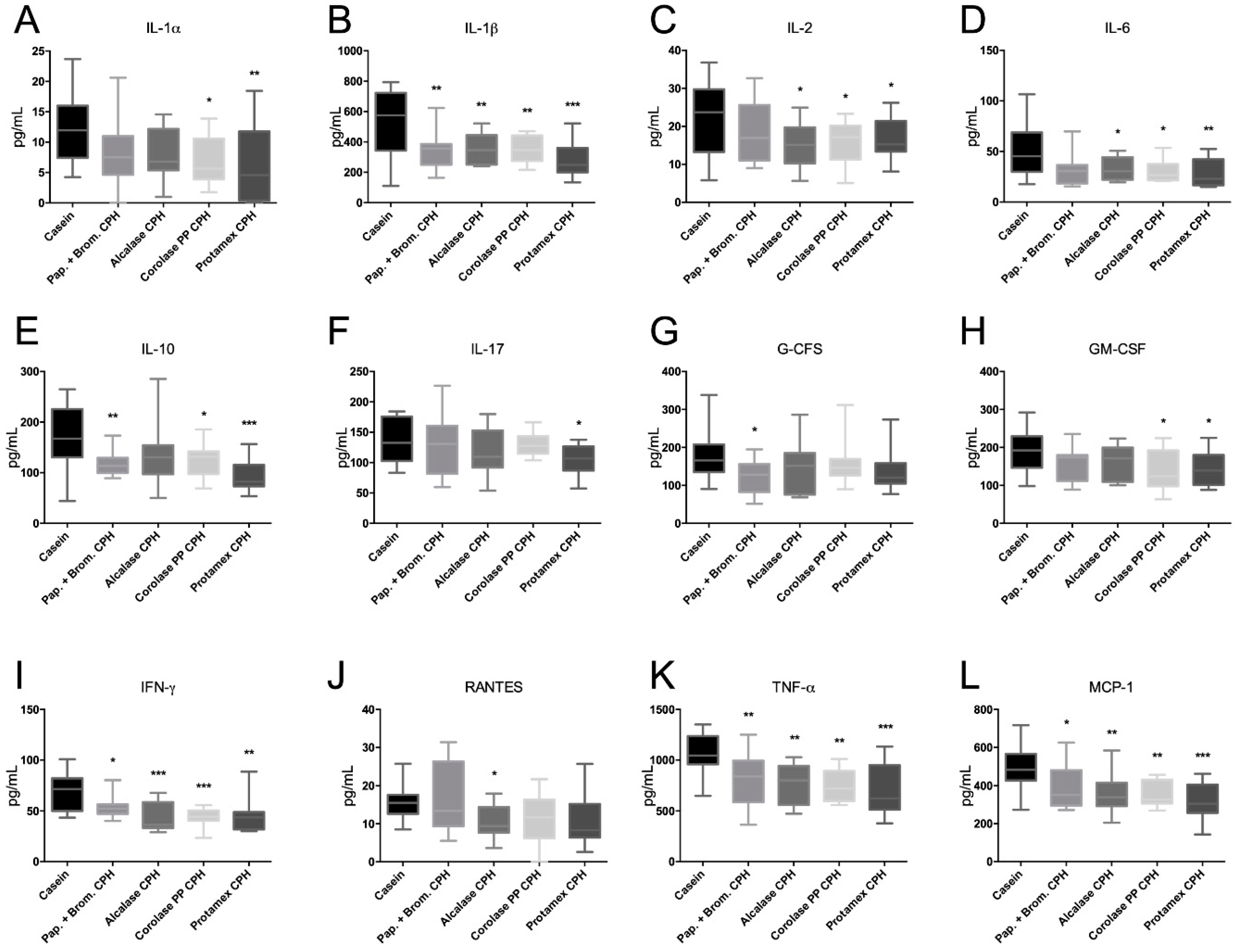
3.4. The Effect of Chicken Protein Hydrolysate Diets on Plasma Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Lee, Y.H. Non-Alcoholic Fatty Liver Disease: The Emerging Burden in Cardiometabolic and Renal Diseases. Diabetes Metab. J. 2017, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Drevon, C.A. Fatty acids and expression of adipokines. Biochim. Biophys. Acta 2005, 1740, 287–292. [Google Scholar] [CrossRef]
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 1993, 268, 26055–26058. [Google Scholar] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Anupama, N.; Sindhu, G.; Raghu, K.G. Significance of mitochondria on cardiometabolic syndromes. Fundam. Clin. Pharmacol. 2018, 32, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Botchlett, R.; Woo, S.L.; Liu, M.; Pei, Y.; Guo, X.; Li, H.; Wu, C. Nutritional approaches for managing obesity-associated metabolic diseases. J. Endocrinol. 2017, 233, R145–R171. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of chain length on absorption of biologically active peptides from the gastrointestinal tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta 2018, 1862, 81–196. [Google Scholar] [CrossRef]
- Jensen, I.J.; Maehre, H.K. Preclinical and Clinical Studies on Antioxidative, Antihypertensive and Cardioprotective Effect of Marine Proteins and Peptides—A Review. Mar. Drugs 2016, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Deussen, A. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit. Rev. Food Sci. Nutr. 2017, 1–20. [Google Scholar] [CrossRef]
- De Campos Zani, S.C.; Wu, J.; Chan, C.B. Egg and Soy-Derived Peptides and Hydrolysates: A Review of Their Physiological Actions against Diabetes and Obesity. Nutrients 2018, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Vik, R.; Tillander, V.; Skorve, J.; Vihervaara, T.; Ekroos, K.; Alexson, S.E.H.; Berge, R.K.; Bjørndal, B. Three differently generated salmon protein hydrolysates reveal opposite effects on hepatic lipid metabolism in mice fed a high-fat diet. Food Chem. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Vik, R.; Parolina, C.; Bjorndal, B.; Busnelli, A.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Halvorsen, B.; Aukrust, P.; et al. A salmon protein hydrolysate excerts lipid-independent anti-atherosclerotic activity in Apo E-deficient mice. Atherosclerosis 2014, 235, E188. [Google Scholar] [CrossRef]
- Johnson, P.R.; Hirsch, J. Cellularity of adipose depots in six strains of genetically obese mice. J. Lipid Res. 1972, 13, 2–11. [Google Scholar] [PubMed]
- Bjorndal, B.; Vik, R.; Brattelid, T.; Vigerust, N.F.; Burri, L.; Bohov, P.; Nygård, O.; Skorve, J.; Berge, R.K. Krill powder increases liver lipid catabolism and reduces glucose mobilization in tumor necrosis factor-alpha transgenic mice fed a high-fat diet. Metabolism 2012, 61, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Flatmark, T.; Osmundsen, H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur. J. Biochem. 1984, 141, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Hexeberg, S.; Skorve, J.; Lundquist, M.; Berge, R.K. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J. Lipid Res. 1993, 34, 13–22. [Google Scholar] [PubMed]
- Lindquist, C.; Bjorndal, B.; Rossmann, C.R.; Tusubira, D.; Svardal, A.; Rosland, G.V.; Tronstad, K.J.; Hallström, S.; Berge, R.K. Increased hepatic mitochondrial FA oxidation reduces plasma and liver TG levels and is associated with regulation of UCPs and APOC-III in rats. J. Lipid Res. 2017, 58, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Small, G.M.; Burdett, K.; Connock, M.J. A sensitive spectrophotometric assay for peroxisomal acyl-CoA oxidase. Biochem. J. 1985, 227, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Liaset, B.; Hao, Q.; Jorgensen, H.; Hallenborg, P.; Du, Z.Y.; Ma, T.; Marschall, H.U.; Kruhøffer, M.; Li, R.; Li, Q.; et al. Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J. Biol. Chem. 2011, 286, 28382–28395. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jeon, J.E.; Jang, S.Y.; Jeong, Y.J.; Jeon, S.M.; Park, H.J.; Choi, M.S. Differential effects of powdered whole soy milk and its hydrolysate on antiobesity and antihyperlipidemic response to high-fat treatment in C57BL/6N mice. J. Agric. Food Chem. 2011, 59, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR-/-/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Jeon, B.T.; Moon, S.H.; Lee, S.H. Enzymatic hydrolysate from velvet antler suppresses adipogenesis in 3T3-L1 cells and attenuates obesity in high-fat diet-fed mice. EXCLI J. 2017, 16, 328–339. [Google Scholar] [PubMed]
- Dong, L.; Li, Y.; Zhang, D.; Zhang, H.; Han, J.; Wang, Z.; Zhou, J.; Lu, C.; Su, X. Dietary Apostichopus japonicus Alleviates Diabetes Symptoms and Modulates Genes Expression in Kidney Tissues of db/db Mice. J. Agric. Food Chem. 2018, 66, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gaudel, C.; Nongonierma, A.B.; Maher, S.; Flynn, S.; Krause, M.; Murray, B.A.; Kelly, P.M.; Baird, A.W.; FitzGerald, R.J.; Newsholme, P. A whey protein hydrolysate promotes insulinotropic activity in a clonal pancreatic beta-cell line and enhances glycemic function in ob/ob mice. J. Nutr. 2013, 143, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.J.; Valentine, R.J.; Wilund, K.R.; Antao, N.; Baynard, T.; Woods, J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1164–E1171. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Healy, N.P.; Kirwan, A.M.; McArdle, M.A.; Holohan, K.; Nongonierma, A.B.; Keane, D.; Kelly, S.; Celkova, L.; Lyons, C.L.; McGillicuddy, F.C.; et al. A casein hydrolysate protects mice against high fat diet induced hyperglycemia by attenuating NLRP3 inflammasome-mediated inflammation and improving insulin signaling. Mol. Nutr. Food Res. 2016, 60, 2421–2432. [Google Scholar] [CrossRef]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjorndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in ApoE-deficient mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef]
- Han, Y.H.; Buffolo, M.; Pires, K.M.; Pei, S.; Scherer, P.E.; Boudina, S. Adipocyte-Specific Deletion of Manganese Superoxide Dismutase Protects From Diet-Induced Obesity Through Increased Mitochondrial Uncoupling and Biogenesis. Diabetes 2016, 65, 2639–2651. [Google Scholar] [CrossRef] [PubMed]
- Carraro, R.S.; Souza, G.F.; Solon, C.; Razolli, D.S.; Chausse, B.; Barbizan, R.; Victorio, S.C.; Velloso, L.A. Hypothalamic mitochondrial abnormalities occur downstream of inflammation in diet-induced obesity. Mol. Cell. Endocrinol. 2018, 460, 238–245. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | Casein | Papain + Bromelain CPH | Alcalase CPH | Corolase PP CPH | Protamex CPH |
|---|---|---|---|---|---|
| Protein (E%) | 19 | 19 | 19 | 19 | 19 |
| Casein (g) | 250 | 125 | 125 | 125 | 125 |
| CPH (g) | 125 | 125 | 125 | 125 | |
| Fat (E%) | 59 | 59 | 59 | 59 | 59 |
| Soy oil (g) | 35 | 35 | 35 | 35 | 35 |
| Lard (g) | 315 | 315 | 315 | 315 | 315 |
| Carbohydrate (E%) | 22 | 22 | 22 | 22 | 22 |
| Cornstarch (g) | 100 | 100 | 100 | 100 | 100 |
| Dyetrose (g) | 100 | 100 | 100 | 100 | 100 |
| Sucrose (g) | 100 | 100 | 100 | 100 | 100 |
| Fiber (g) | 50 | 50 | 50 | 50 | 50 |
| Micronutrients (E%) | 0 | 0 | 0 | 0 | 0 |
| AIN-93G-MX mineral mix (g) | 34 | 34 | 34 | 34 | 34 |
| AIN-93-VX vitamin mix (g) | 10 | 10 | 10 | 10 | 10 |
| L-Cysteine (g) | 3 | 3 | 3 | 3 | 3 |
| Choline bitartrate (g) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Tert-butyl-hydroquinone (g) | 014 | 014 | 014 | 014 | 014 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloysius, T.A.; Carvajal, A.K.; Slizyte, R.; Skorve, J.; Berge, R.K.; Bjørndal, B. Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines 2019, 6, 5. https://doi.org/10.3390/medicines6010005
Aloysius TA, Carvajal AK, Slizyte R, Skorve J, Berge RK, Bjørndal B. Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines. 2019; 6(1):5. https://doi.org/10.3390/medicines6010005
Chicago/Turabian StyleAloysius, Thomas A., Ana Karina Carvajal, Rasa Slizyte, Jon Skorve, Rolf K. Berge, and Bodil Bjørndal. 2019. "Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice" Medicines 6, no. 1: 5. https://doi.org/10.3390/medicines6010005
APA StyleAloysius, T. A., Carvajal, A. K., Slizyte, R., Skorve, J., Berge, R. K., & Bjørndal, B. (2019). Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines, 6(1), 5. https://doi.org/10.3390/medicines6010005




