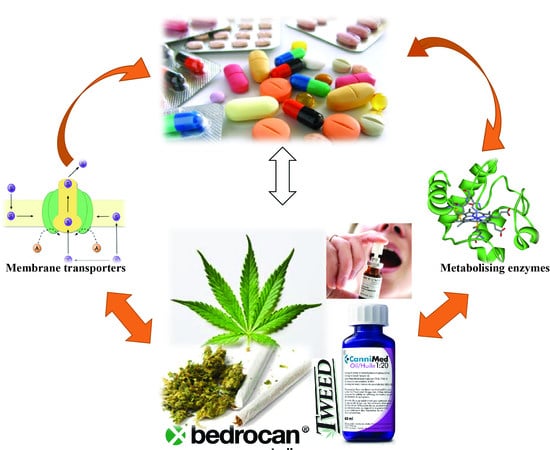
Medicinal Cannabis—Potential Drug Interactions
Abstract
:1. Introduction
2. Potential Drug Interactions
3. Effects of Cannabis on Drug Metabolizing Enzymes and Related Drug Interactions
4. Other Potential Drug Interactions
5. Conclusions
Funding
Conflicts of Interest
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018. [Google Scholar] [CrossRef]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef]
- Brown, A. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. Non-CB 1, non-CB 2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: Focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol. 2010, 5, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.; Abood, M.E.; Alexander, S.; Di Marzo, V.; Elphick, M.; Greasley, P.; Hansen, H.S.; Kunos, G.; Mackie, K. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Peres, F.F.; Diana, M.C.; Levin, R.; Suiama, M.A.; Almeida, V.; Vendramini, A.M.; Santos, C.M.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A. Cannabidiol administered during peri-adolescence prevents behavioral abnormalities in an animal model of schizophrenia. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Saravia, R.; Ten-Blanco, M.; Julià-Hernández, M.; Gagliano, H.; Andero, R.; Armario, A.; Maldonado, R.; Berrendero, F. Concomitant THC and stress adolescent exposure induces impaired fear extinction and related neurobiological changes in adulthood. Neuropharmacology 2018, 144, 345–357. [Google Scholar] [CrossRef]
- Wade, N.E.; Wallace, A.L.; Swartz, A.M.; Lisdahl, K.M. Aerobic Fitness Level Moderates the Association Between Cannabis Use and Executive Functioning and Psychomotor Speed Following Abstinence in Adolescents and Young Adults. J. Int. Neuropsychol. Soc. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, V.; Mrdjanovic, J.; Borisev, I. A Review of the Therapeutic Antitumor Potential of Cannabinoids. J. Altern. Compl. Med. 2017, 23, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Vuger, A.T.; Šeparović, R.; Silovski, T.; Pavlović, M.; Pavlica, V.; Knežević, S.V. Cannabis in oncology. Libri Oncol. 2016, 44, 51–57. [Google Scholar]
- Davis, M.P. Cannabinoids for Symptom Management and Cancer Therapy: The Evidence. J. Natl. Comprehen. Cancer Netw. 2016, 14, 915–922. [Google Scholar] [CrossRef]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bouquié, R.; Deslandes, G.; Mazaré, H.; Cogné, M.; Mahé, J.; Grégoire, M.; Jolliet, P. Cannabis and anticancer drugs: Societal usage and expected pharmacological interactions—A review. Fundam. Clin. Pharmacol. 2018, 32, 462–484. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, L.; Kinnings, S.L.; Bourne, P.E. Novel computational approaches to polypharmacology as a means to define responses to individual drugs. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Zhou, W.; Wang, Y.; Yang, L. Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice. J. Ethnopharmacol. 2013, 146, 773–793. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Abd-Elsalam, W.H.; El badawy, S.A.; Taher, E.; Fares, M.; Torres, A.; Chang, D.; Guang Li, C. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2018. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Ezzat, S.M.; Elsakhawy, F.S.; Kamel, G.M.; Abdel-Kawy, M.A. Impact of certain Solanum speciess natural products as potent cytotoxic and anti-Inflammatory agents. J. Med. Plants Res. 2015, 9, 779–786. [Google Scholar]
- Ho, T.T.; Tran, Q.T.; Chai, C.L. The polypharmacology of natural products. Futur. Med. Chem. 2018, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Meiri, E.; Jhangiani, H.; Vredenburgh, J.J.; Barbato, L.M.; Carter, F.J.; Yang, H.-M.; Baranowski, V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007, 23, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.J.; Knoderer, H.M. Characterization of dronabinol usage in a pediatric oncology population. J. Pediatr. Pharmacol. Ther. 2015, 20, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Polito, S.; MacDonald, T.; Romanick, M.; Jupp, J.; Wiernikowski, J.; Vennettilli, A.; Khanna, M.; Patel, P.; Ning, W.; Sung, L.; et al. Safety and efficacy of nabilone for acute chemotherapy-induced vomiting prophylaxis in pediatric patients: A multicenter, retrospective review. Pediatr. Blood Cancer 2018, 65, e27374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symp. Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T.; Albert Lux, E.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.H.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. J. Pain 2017, 11, 119–133. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symp. Manag. 2018, 55, 179–188. [Google Scholar] [CrossRef]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain Symp. Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain Off. J. Am. Pain Soc. 2012, 13, 438–449. [Google Scholar] [CrossRef]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A Double-Blind, Placebo-Controlled, Crossover Pilot Trial With Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symp. Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.I.; Couey, P.; Shade, S.B.; Kelly, M.E.; Benowitz, N.L. Cannabinoid–Opioid Interaction in Chronic Pain. Clin. Pharmacol. Ther. 2011, 90, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Yang, L.; Zhou, S.-F. Interactions between Chinese herbal medicines and drugs. Aust. J. Acupunct. Chin. Med. 2007, 2, 17. [Google Scholar]
- Damkier, P.; Lassen, D.; Christensen, M.M.H.; Madsen, K.G.; Hellfritzsch, M.; Pottegård, A. Interaction between warfarin and cannabis. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Lau, D.; Allen, J.; Arnold, J. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Holcberg, G. Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: An ex vivo study. Am. J. Obstet. Gynecol. 2013, 209, 573.e1–573.e15. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Panetta, J.; Hoskins, J.; Bebawy, M.; Roufogalis, B.; Allen, J.; Arnold, J. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem. Pharmacol. 2006, 71, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-J.; Wang, J.-S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; DeVane, C.L. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef]
- Tournier, N.; Chevillard, L.; Megarbane, B.; Pirnay, S.; Scherrmann, J.-M.; Decleves, X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int. J. Neuropsychopharmacol. 2010, 13, 905–915. [Google Scholar] [CrossRef]
- Arnold, J.C.; Hone, P.; Holland, M.L.; Allen, J.D. CB2 and TRPV1 receptors mediate cannabinoid actions on MDR1 expression in multidrug resistant cells. Pharmacol. Rep. 2012, 64, 751–757. [Google Scholar] [CrossRef]
- Feinshtein, V.; Erez, O.; Ben-Zvi, Z.; Erez, N.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Huleihel, M.; Holcberg, G. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ 2013, 1, e153. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Van Bambeke, F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 2011, 12, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Wittgen, H.G.; van den Heuvel, J.J.; van den Broek, P.H.; Dinter-Heidorn, H.; Koenderink, J.B.; Russel, F.G. Cannabinoid CB1 receptor antagonists modulate transport activity of multidrug resistance-associated proteins MRP1, MRP2, MRP3, and MRP4. Drug Metab. Dispos. 2011. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Allen, J.D.; Arnold, J.C. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur. J. Pharmacol. 2008, 591, 128–131. [Google Scholar] [CrossRef]
- Engels, F.K.; De Jong, F.A.; Sparreboom, A.; Mathot, R.A.; Loos, W.J.; Kitzen, J.J.; De Bruijn, P.; Verweij, J.; Mathijssen, R.H. Medicinal cannabis does not influence the clinical pharmacokinetics of irinotecan and docetaxel. Oncologist 2007, 12, 291–300. [Google Scholar] [CrossRef]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; Program, U.C. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Manini, A.F.; Yiannoulos, G.; Bergamaschi, M.M.; Hernandez, S.; Olmedo, R.; Barnes, A.J.; Winkel, G.; Sinha, R.; Jutras-Aswad, D.; Huestis, M.A. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J. Addict. Med. 2015, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Arellano, A.L.; Papaseit, E.; Romaguera, A.; Torrens, M.; Farre, M. Neuropsychiatric and General Interactions of Natural and Synthetic Cannabinoids with Drugs of Abuse and Medicines. CNS Neurol. Disord. Drug Targets 2017, 16, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtelová, G.; Nosková, K.; Turjap, M.; Sulcová, A.; Hanus, L.; Jurica, J. Cannabinoids and cytochrome P450 interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Carmona, N.E.; Lee, Y.L.; Ragguett, R.M.; Pan, Z.; Rosenblat, J.D.; Subramaniapillai, M.; Shekotikhina, M.; Almatham, F.; Alageel, A.; et al. Drug-drug interactions as a result of co-administering Delta(9)-THC and CBD with other psychotropic agents. Expert Opin. Drug Saf. 2018, 17, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D.; Chan, L.N. Pharmacokinetic Drug Interactions with Tobacco, Cannabinoids and Smoking Cessation Products. Clin. Pharm. 2016, 55, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Szaflarski, J.P. Cannabis for the Treatment of Epilepsy: An Update. Curr. Neurol. Neurosci. Rep. 2018, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.Y.; Kim, J.H.; Kim, D.K.; Lee, H.S. Synthetic cannabinoids are substrates and inhibitors of multiple drug-metabolizing enzymes. Arch. Pharm. Res. 2018, 41, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Fantegrossi, W.E. Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites. In Neuropharmacology of New Psychoactive Substances (NPS): The Science Behind the Headlines; Baumann, M.H., Glennon, R.A., Wiley, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 249–262. [Google Scholar] [CrossRef]
- Haroutounian, S.; Ratz, Y.; Ginosar, Y.; Furmanov, K.; Saifi, F.; Meidan, R.; Davidson, E. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain. Clin. J. Pain 2016, 32, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Wall, M.M.; Liu, S.-M.; Blanco, C. Cannabis use and risk of prescription opioid use disorder in the United States. Am. J. Psychiatry 2017, 175, 47–53. [Google Scholar] [CrossRef]
- Ronen, A.; Chassidim, H.S.; Gershon, P.; Parmet, Y.; Rabinovich, A.; Bar-Hamburger, R.; Cassuto, Y.; Shinar, D. The effect of alcohol, THC and their combination on perceived effects, willingness to drive and performance of driving and non-driving tasks. Accid. Anal. Prev. 2010, 42, 1855–1865. [Google Scholar] [CrossRef]
- Hartman, R.L.; Brown, T.L.; Milavetz, G.; Spurgin, A.; Gorelick, D.A.; Gaffney, G.; Huestis, M.A. Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without alcohol. Clin. Chem. 2015, 61, 850–869. [Google Scholar] [CrossRef]
- Atwal, N.; Casey, S.L.; Mitchell, V.A.; Vaughan, C.W. THC and gabapentin interactions in a mouse neuropathic pain model. Neuropharmacology 2019, 144, 115–121. [Google Scholar] [CrossRef]
- Russo, E.B. Current therapeutic cannabis controversies and clinical trial design issues. Front. Pharmacol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
| Cannabis Based Treatment | Study Type/Location/n | Dosage/Administration | Efficacy, Tolerability and Notes | References |
|---|---|---|---|---|
| Chemotherapy Induced Nausea and Vomiting (CINV) | ||||
| -Dronabinol [Marinol®; (-) trans Δ9-THC) alone or in combination with ondansetron (8–15 mg IV] | -Interventional (Placebo controlled). -n = 64. -USA. | -Capsule (2.5–20 mg). -Oral. | -Both were effective in CINV and well tolerated while dronabinol was more effective. -Combination is not more effective. | [23] |
| -Dronabinol [Marinol®; (-) trans Δ9-THC] | -Interventional (retrospective). -Children with malignancy. | -Solution administered orally (2.5–5 mg/m2 body surface every 6 h as needed). | -Positive response were reported for 60% of patients. -Prospective trial would be needed to confirm the dronabinol effect in CINV therapy. | [24] |
| -Nabilone with 5HT3 antagonist | -Interventional (retrospective) -n = 110 with median age 14 years with malignancy. | -Oral. | -Adverse effect was reported with minor clinical significance. -Poor nausea control in nabilone treated group. | [25] |
| Cancer Pain | ||||
| -Sativex® (Δ9-THC: CBD at a ratio of 27:25 mg/mL) -THC (27 mg/mL) | -Interventional (Double Blind, Randomized, Parallel Group, Placebo Controlled), n = 177. -Phase 3. -UK. | -Oromucodal spray with maximum Δ9-THC: CBD (130:120 mg/day) or 130 mg/day Δ9-THC alone Each actuation is 100 μL. | -Compared with the placebo, the Sativex treated group showed significant pain relief unlike the Δ9-THC which was non-significant. -Reported adverse effects including dizziness, gastrointestinal disorders and confusion. | [26] |
| -Sativex® (Δ9-THC: CBD at a ratio of 27:25 mg/mL) | -Interventional (single group assignment) -Phase 3. -UK. | -Oromucodal spray with maximum 130:120 mg/day of Δ9-THC: CBD. | -The long-term use is well tolerated without losing pain-relieving effects in terminal cancer-related pain refectory to opioids. -Adverse effects and tolerability assessed at the RCT withdrawal visit, 7–10 days later, then monthly, and at the withdrawal or completion of the study. | [27] |
| - Sativex® (Δ9-THC: CBD at a ratio of 27:25 mg/mL) | -Interventional (Double Blind, Randomized, Parallel Group, Placebo Controlled). -Phase 3. -Multicentric. -n = 399. | -Oromucodal spray (100 μL per actuation twice daily in the morning and evening with a maximum of 10 sprays for 5 weeks). | -No significant difference was reported in advanced cancer patients with chronic pain (unalleviated with opioids). -Nabiximol still beneficial to secondary endpoints. -No evidence of abuse or misuse was reported. | [28] |
| -No significant difference was reported in advanced cancer patients with chronic uncontrolled pain. | [29] | |||
| -Nabiximols (Sativex®; Δ9-THC: CBD at a ratio of 27:25 mg/mL) | -Interventional (Double Blind, Randomized, Parallel Group, Placebo Controlled). -Phase 2. -USA. -n = 360. | -Oromucodal spray in low (1–4 sprays/day), medium (6–10 sprays/day) and high (11–16 sprays/day) doses. | -Efficacy and safety were reported at low and medium doses against advanced cancer pain. -The adverse effects at high doses. | [30] |
| -Nabiximols (Sativex®, Δ9-THC: CBD at a ratio of 27:25 mg/mL) | -Interventional (Double-Blind, Placebo controlled, Crossover Pilot trial). -n = 16. | -Sublingual spray (7.5–30 mg/day). | -No significant difference was reported against chemotherapy-induced neuropathy. -Two-fold reduction of the pain in the responder group with adverse effects. | [31] |
| Cannabis cigarettes (3.56% Δ9-THC) in combination with opiates | -Interventional (open label). | -Pulmonary administration for chronic pain, including cancer patients. | -Declined chronic pain around 27% in patients receiving oxycodone or morphine analgesics. -No serious adverse effects were reported. | [32] |
| Cannabinoid Based Treatment and Interactions | Affected Transporters and/or Metabolic Enzymes | Experimental Results, Notes and Outcomes | References |
|---|---|---|---|
| Cannabis, THC, CBD, CBN with either chemotherapies, abuse drugs or medications | -Membrane transporters ABC super family (glycoprotein P; P-gp, Breast cancer-resistance protein; BCRP, and multidrug resistance protein; MRP1, 2, 3 and 4) -Cytochrome P450 (3A, 2D6, 2C9, 1A1, 1A2, 1B1, 2B6 and 2C8) -UDP-glucuronosyltransferases (UGTs) | -P-gp, BCRP, and MRP1-4 transporters expression were dysregulated by cannabinoids, but in higher concentrations than that usually measured in cannabis smokers. -CYP3A was competitively inhibited by THC, CBD and CBN, with CBD being the most potent in a concentration compatible with that in usual cannabis inhalation. -CYP2D6 was inhibited by THC, CBD and CBN, with CBD being the most potent in a higher concentration than that in usual cannabis consumption. -CYP2C9 was inhibited by THC, CBD and CBN, with CBD inhibitory effect being dependent on the used substrates. -CYP1A1, 1A2, 1B1, 2B6, 2C19, 3A4 and 2C8 were strongly inhibited by CBD. -UGT1A9, and 2B7 were inhibited by CBD. -UGT1A7, 1A8, and 1A9 were inhibited by CBN. -UGT2B7 was activated by CBN.
| [17,50,51] |
| Δ9-THC, CBD and marijuana inhalation with psychotropic agents | -Cytochrome P450 | -CYP2C9 and CYP3A4 were inhibited by Δ9-THC. -CYP2C19 and CYP3A4 were inhibited by CBD. -CYP1A1 and CYP1A2 were induced by marijuana inhalation.
| [52] |
| Cannabinoids on other drugs | Cytochrome P450 | -CYP3A4 inhibitors and stimulators affect the elimination of Δ9-THC and CBD.
| [53] |
| CBD with antiepileptic drugs | Cytochrome P450 or unknown | Clinical studies of DDI: -Non-significant increase of the clobazam plasma level administered with CBD (n = 13 children) due to potent inhibition of CYP2C19. -Significant change of plasma level of N-desmethylclobazam by CBD co-administration while no significant change in the level of valproate, stiripentol and levetiracetam (n = 24 open label trial). -All patients showed significant changes of the plasma levels of clobazam, N-desmethylclobazam, rufinamide, and topiramate by increasing CBD doses. The mean therapeutic range was exceeded for clobazam and N-desmethylclobazam; the plasma levels of eslicarbazepine and zonisamide were increased in adults only (n = 39 adults and 42 children).
| [47,54] |
| Synthetic and Phyto-cannabinoids | -Cytochrome P450 -UGTs | -CYP1A catalysed MROD activity was weakly inhibited by MAM-2201, JWH-019, STS-135, and UR-144. -CYP2C8 catalysed amodiaquine N-deethylase was strongly inhibited by AM-2201, MAM-2201, and EAM-2201. -CYP2C9 catalysed diclofenac hydroxylation and CYP3A-catalyzed midazolam 1′-hydroxylation were inhibited by AM-2201 and MAM-2201. -CYP2C9 catalysed diclofenac 4′-hydroxylation, CYP2C19-catalyzed [S] -mephenytoin 4′-hydroxylation, and CYP3A-catalyzed midazolam 1′ hydroxylation were strongly inhibited by EAM-2201 (time-dependent inhibition). -CYP2B6 and CYP2C9 were strongly inhibited by THC, CBN and CBD. -CYP2A6 was inhibited by THC and CBN (mechanism-based inhibition). -CYP2D6 was competitively inhibited by CBD. -CYP1A1 mRNA expression was increased by THC in Hepa-1 cells, but EROD activity in CYP1A1 supersomes was inhibited by THC. -CYP1A1, CYP1A2, and CYP1B1 were strongly inhibited by CBD (mechanism-based inhibition). -CYP3A was inhibited by CBD in human liver microsomes. -CYP2C19-catalyzed [S] -mephenytoin hydroxylation was inhibited by (CBD and THC (Mixed-type inhibition). -UGT1A9- and UGT2B7 catalysed ethanol glucuronidation were non-competitively inhibited by CBD, and unlike the inclined ethanol glucuronidation in human liver microsome by CBN (dose dependent). -UGT1A3 catalysed chenodeoxycholic acid 24-acylglucuronidation was strongly competitively inhibited by AM-2201, MAM-2201, and EAM-2201. -UGT2B7-mediated naloxone 3β-D-glucuronidation was competitively inhibited by AM-2201.
| [55,56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsherbiny, M.A.; Li, C.G. Medicinal Cannabis—Potential Drug Interactions. Medicines 2019, 6, 3. https://doi.org/10.3390/medicines6010003
Alsherbiny MA, Li CG. Medicinal Cannabis—Potential Drug Interactions. Medicines. 2019; 6(1):3. https://doi.org/10.3390/medicines6010003
Chicago/Turabian StyleAlsherbiny, Muhammad A., and Chun Guang Li. 2019. "Medicinal Cannabis—Potential Drug Interactions" Medicines 6, no. 1: 3. https://doi.org/10.3390/medicines6010003
APA StyleAlsherbiny, M. A., & Li, C. G. (2019). Medicinal Cannabis—Potential Drug Interactions. Medicines, 6(1), 3. https://doi.org/10.3390/medicines6010003