The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria
Abstract
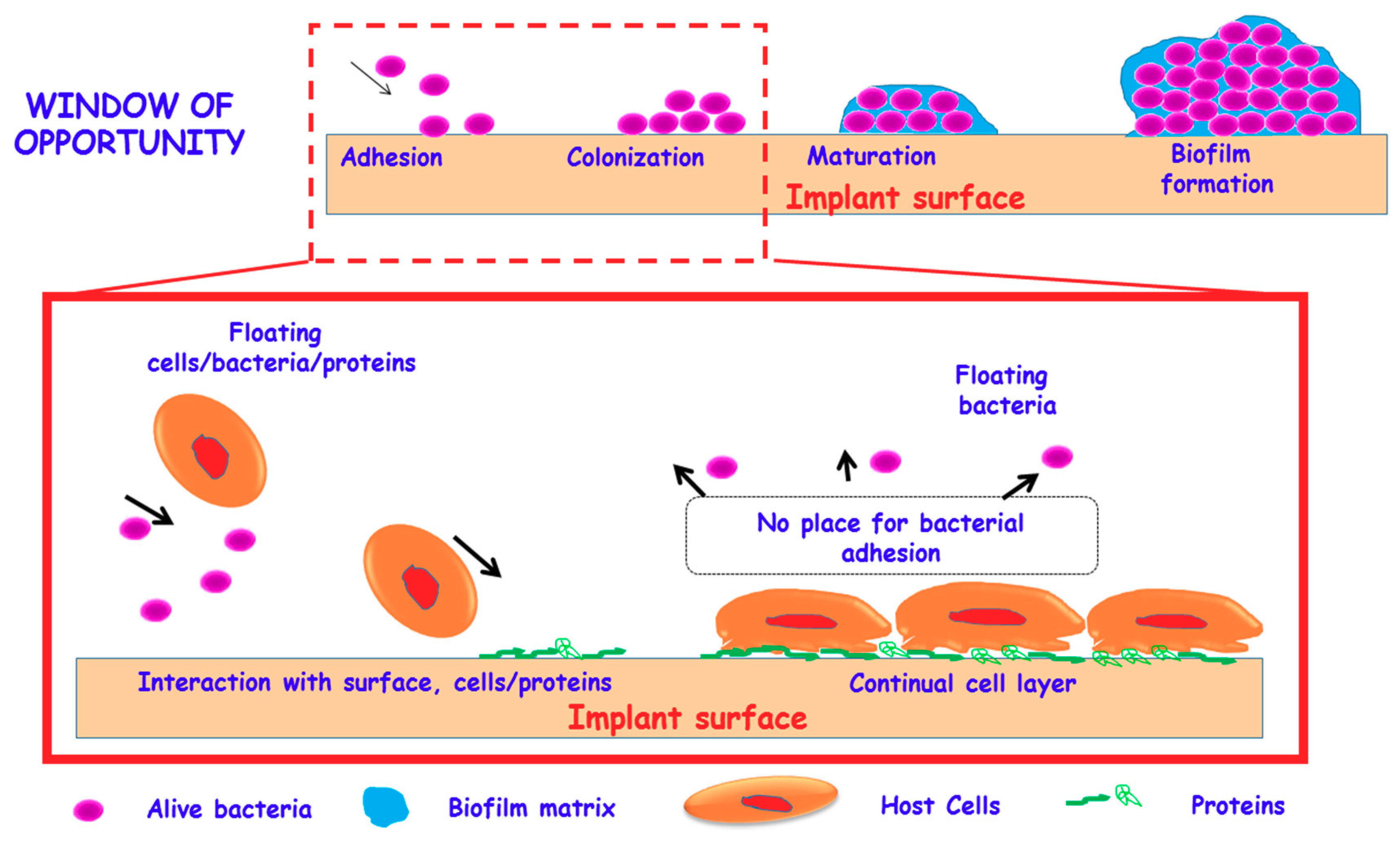
1. Introduction
2. Tuning the Surface Properties of Biomaterials
3. Significance of Zwitterionization of Biomaterials
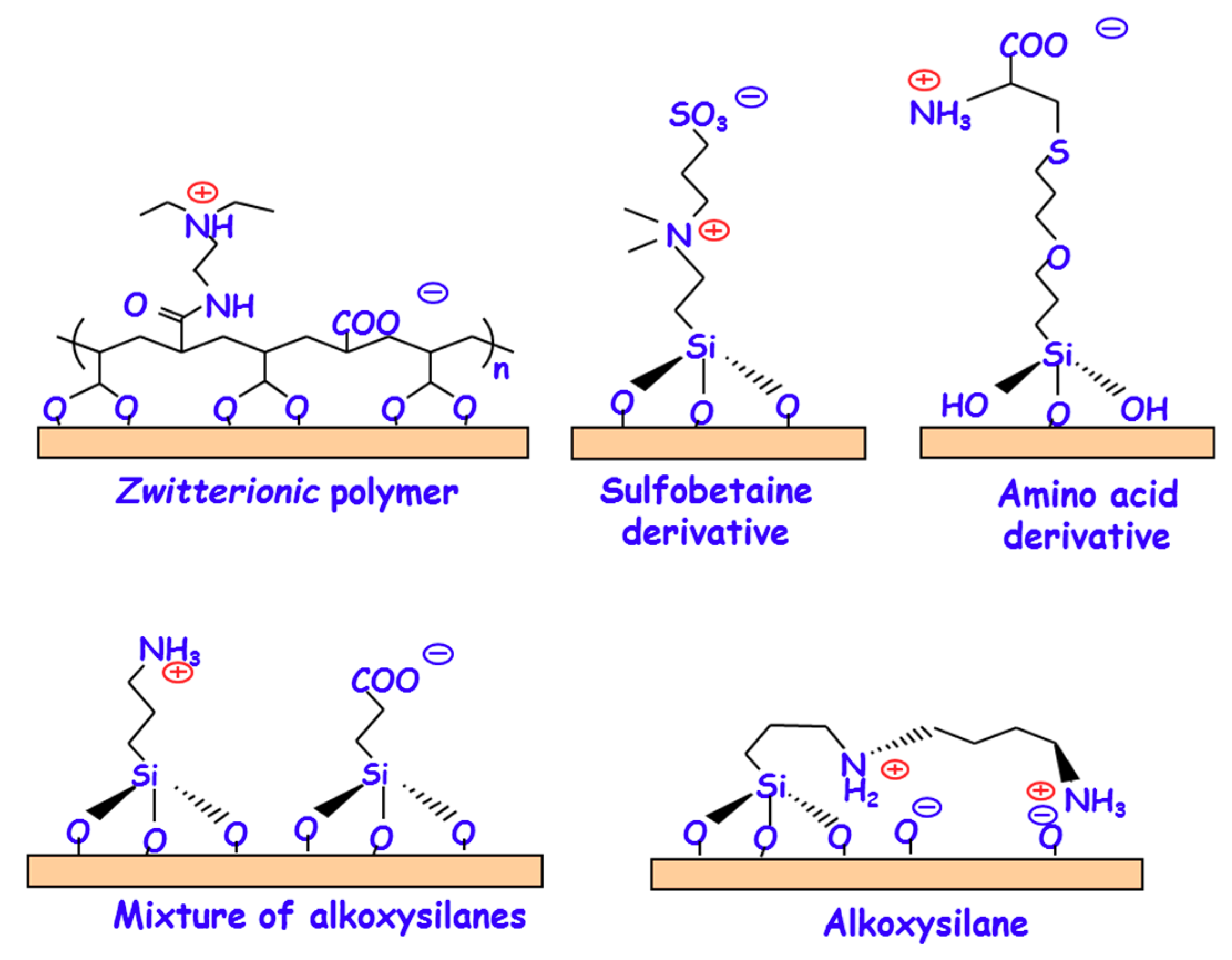
3.1. Chemical Strategies for the Zwitterionization of Biomaterials
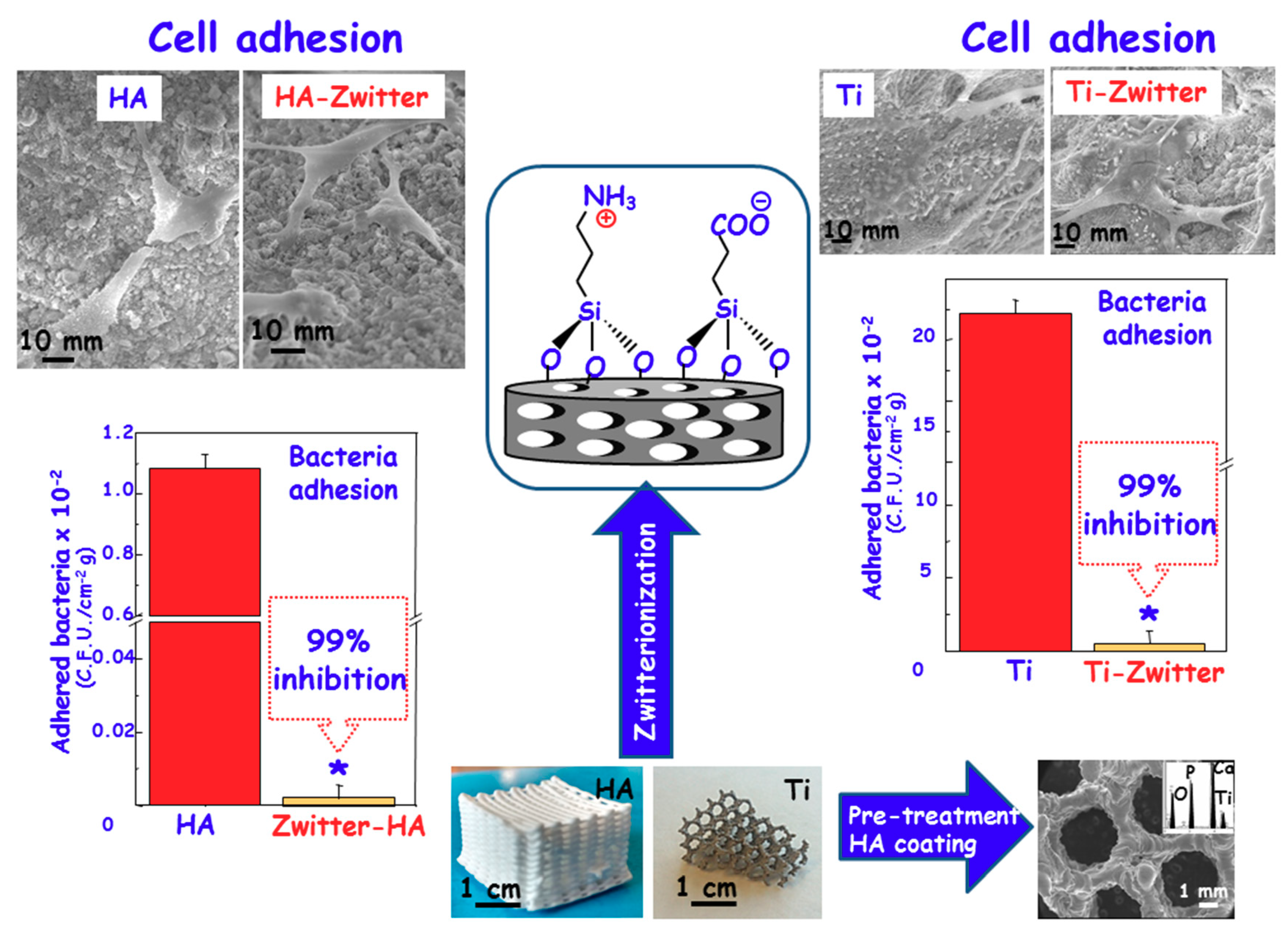
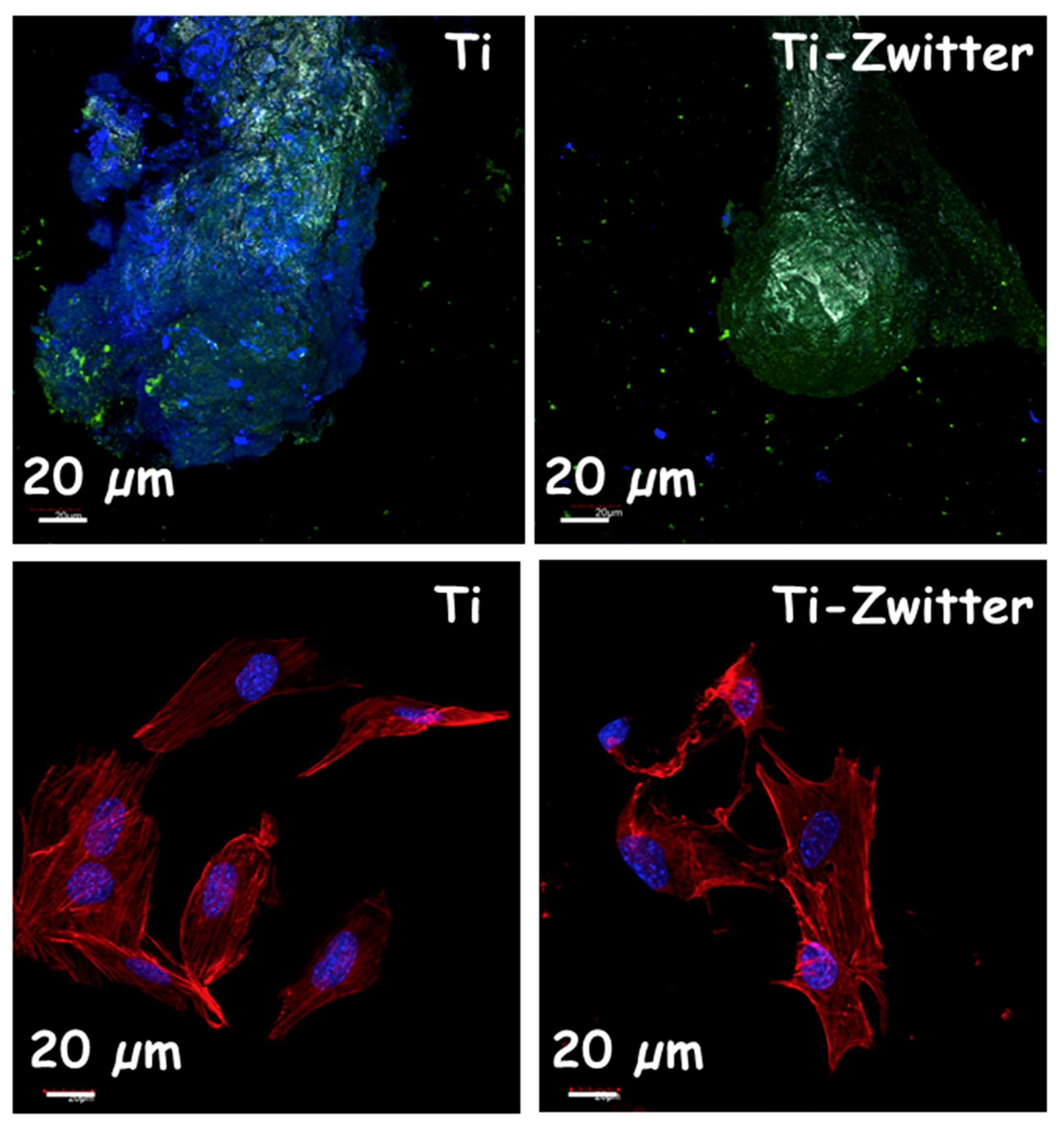
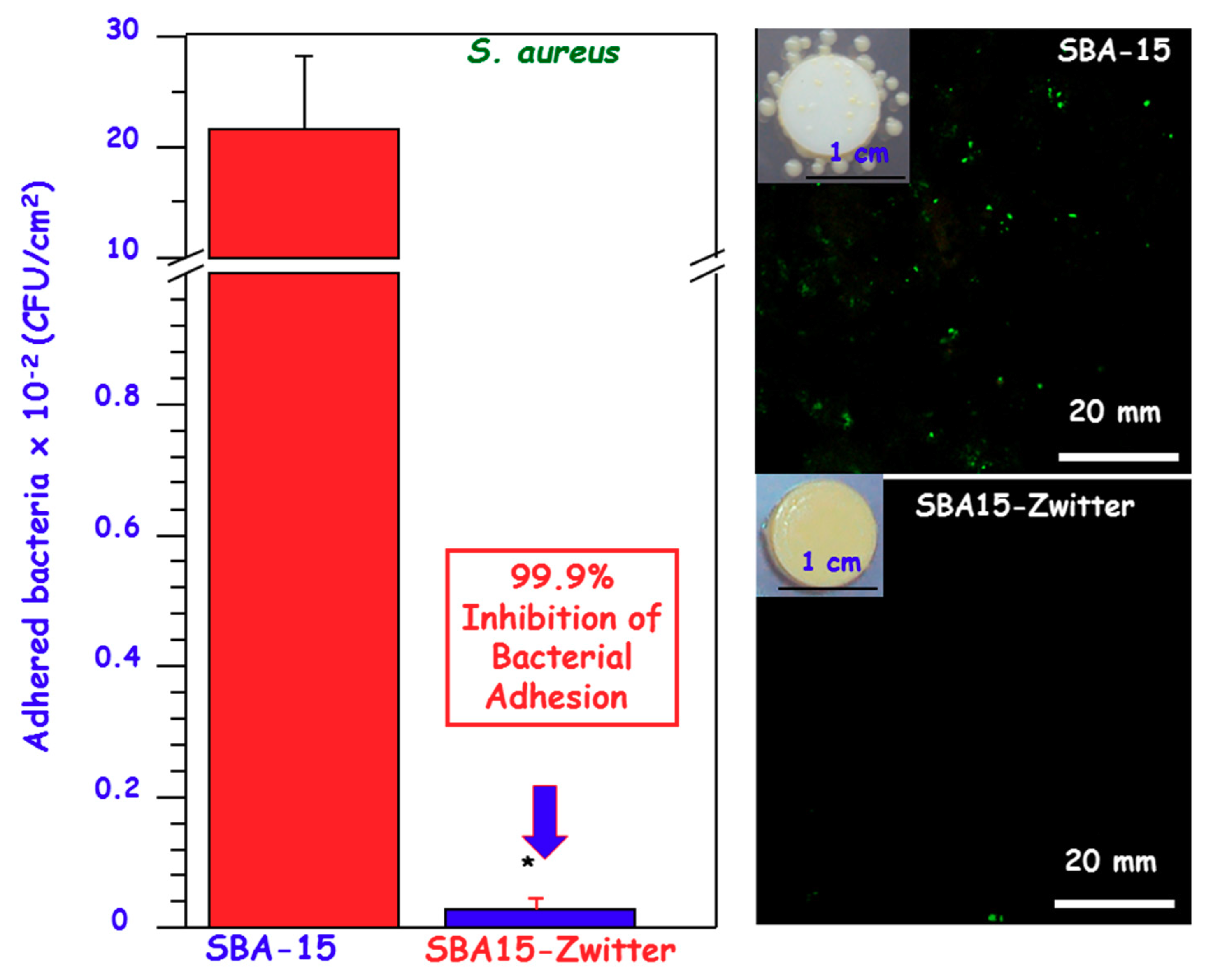
3.2. Zwitterionization of Biomaterials to Prevent Bacterial Infection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ofori-Asenso, R. “When the Bug Cannot Be Killed”—The Rising Challenge of Antimicrobial Resistance. Medicines 2017, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Kontopidis, I.; Gkegkes, I.D.; Rafailidis, P.I.; Falagas, M.E. Incidence, characteristics, and outcomes of patients with bone and joint infections due to community-associated methicillin-resistant Staphylococcus aureus: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; García-Martín, J.M.; Álvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprinting 2015, 2, 20. [Google Scholar] [CrossRef]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef] [PubMed]
- Truonga, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Zwitterionic ceramics for biomedical applications. Acta Biomater. 2016, 40, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet (Lond. Engl.) 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van der Mei, H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012, 8, e1002440. [Google Scholar] [CrossRef] [PubMed]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; Demeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Chagnot, C.; Zorgani, M.A.; Astruc, T. Proteinaceous determinants of surface colonization in bacteria: Bacterial adhesion and biofilm formation from a protein secretion perspective. Front. Microbiol. 2013, 4, 303. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Ponche, A.; Anselme, K. Bacteria/material interfaces: Role of the material and cell wall properties. J. Adhes. Sci. Technol. 2010, 24, 2165–2201. [Google Scholar] [CrossRef]
- Bohmler, J.; Ponche, A.; Anselme, K.; Ploux, L. Self-assembled molecular platforms for bacteria/material biointerface studies: Importance to control functional group accessibility. ACS Appl. Mater. Interfaces 2013, 5, 10478–10488. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. Biomolecular mechanisms of Staphylococcal biofilm formation. Future Microbiol. 2013, 8, 509–524. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.E.; Ginsberg, M.H.; Plow, E.F. Arginyl-glycyl-aspartic acid (RGD): A cell adhesion motif. Trends Biochem. Sci. 1991, 16, 246–250. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; Van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: ‘The race for the surface’ experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.G.; Hueso, J.L.; Vallet-Regí, M. Synthesis and characterization of zwitterionic SBA-15 nanostructured materials. Chem. Mater. 2010, 23, 6459–6466. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolysable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Colilla, M.; Feito, M.J.; Ramírez-Santillán, C.; Portolés, M.T.; Vallet-Regí, M. Inhibition of bacterial adhesion on biocompatible zwitterionic SBA-15 mesoporous materials. Acta Biomater. 2011, 7, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, P.S. Understanding Bacteria; Kluwer Academic: Dordrecht, The Netherland, 2003. [Google Scholar]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. Int. Ed. 2008, 47, 8831–8834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, G.; Xue, H.; Chena, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Lalani, R.; Liu, L. Electrospun zwitterionic poly(sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Mao, J.; Yang, M.; Wang, D.; Bo, S.; Ji, X. Phase Behavior of Poly(sulfobetaine methacrylate)-Grafted Silica Nanoparticles and Their Stability in Protein Solutions. Langmuir 2011, 27, 15282–15291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Murou, M.; Kitano, H.; Ohno, K.; Saruwatari, Y. Silica particles coated with zwitterionic polymer brush: Formation of coloidal crystals and anti-biofouling properties in aqueous medium. Colloids Surf. B Biointerfaces 2011, 84, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Cao, Z.; Xue, H.; Xu, Y.; Jiang, S. Novel zwitterionic-polymer-coated silica nanoparticles. Langmuir 2009, 25, 3196–3199. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Yu, Z.Q.; Hong, C.Y.; Pan, C.Y. Biocompatible zwitterionic sulfobetaine copolymer-coated mesoporous silica nanoparticles for temperature-responsive drug release. Macromol. Rapid Commun. 2012, 14, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, H.; Gao, C.; Carr, L.; Wang, J.; Chu, B.; Jiang, S. Imaging and cell targeting characteristics of magnetic nanoparticles modified by a functionalizable zwitterionic polymer with adhesive 3,4-dihydroxyphenyl-l-alanine linkages. Biomaterials 2010, 31, 6582–6588. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Lin, J.; Li, M.; Ma, Y.; Chen, Y.; Zhang, C.; Li, D.; Gu, H. Prolonged in vivo circulation time by zwitterionic modification of magnetite nanoparticles for blood pool contrast agents. Contrast Media Mol. Imaging 2012, 7, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.E.; Gu, F.X. Surface functionalization of silica nanoparticles with cysteine: A low-fouling zwitterionic surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.; Garcia-Uriostegui, L.; Rodríguez, O.; Izquierdo-Barba, I.; Salinas, A.J.; Toriz, G.; Vallet-Regí, M.; Delgado, E. Lysine-Grafted MCM-41 Silica as An Antibacterial Biomaterial. Bioengineering 2017, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; García, A.; Vallet-Regí, M. Prevention of bacterial adhesion to zwitterionic biocompatible mesoporous glasses. Acta Biomater. 2017, 57, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Jaber, J.A.; Schlenoff, J.B. Zwitterion-stabilized silica nanoparticles: Toward nonstick nano. Langmuir 2010, 26, 16884–16889. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration as an alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Estephan, Z.G.; Hariri, H.H.; Schlenoff, J.B. One-pot, exchange-free, room-temperature synthesis of sub-10 nm aqueous, noninteracting, and stable zwitterated iron oxide nanoparticles. Langmuir 2008, 29, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Lee, N.I.J.; Han, H.-S.; Cordero, J.M.; Liu, W.; Bawendi, M.G. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 2012, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bruns, O.T.; Chen, O.; Bawendi, M.G. Compact zwitterion-coated iron oxide nanoparticles for in vitro and in vivo imaging. Integr. Biol. 2013, 5, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Chi, X.; Bao, J.; Yang, L.; Zhao, W.; Chen, Z.; Wang, X.; Chen, X.; Gao, J. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano 2013, 7, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Martínez-Carmona, M.; Sánchez-Salcedo, S.; Ruiz-González, M.L.; González-Calbet, J.M.; Vallet-Regí, M. A novel zwitterionic bioceramic with dual antibacterial capability. J. Mater. Chem. B 2014, 2, 5639–5651. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Design and preparation of biocompatible zwitterionic hydroxyapatite. J. Mater. Chem. B 2013, 1, 1595–1606. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Bio-Ceramics with Clinical Applications; John Wiley & Sons Ltd.: Chichester, UK, 2014. [Google Scholar]
- Arcos, D.; Boccaccini, A.R.; Bohner, M.; Díez-Pérez, A.; Epple, M.; Gómez-Barrena, E.; Herrera, A.; Planell, J.A.; Rodríguez-Mañas, L.; Vallet-Regí, M. The relevance of biomaterials to the prevention and treatment of osteoporosis. Acta Biomater. 2014, 10, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- García, A.; Izquierdo-Barba, I.; Colilla, M.; López de Laorden, C.; Vallet-Regí, M. Preparation of 3D scaffolds in the SiO2–P2O5 system with tailored hierarchical mesomacroporosity. Acta Biomater. 2011, 7, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Malstem, M.; Doadrio, J.C.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Tailoring hierarchical meso-macroporous 3D scaffolds: From nano to macro. J. Mater. Chem. B 2014, 2, 49–58. [Google Scholar] [CrossRef]
- Quan, Y.J.; Drescher, P.; Zhang, F.M.; Burkel, E.; Seitz, H. Cellular Ti6Al4V with carbon nanotube-like structures fabricated by selective electron beam melting. Rapid Prototyp. J. 2014, 20, 541. [Google Scholar] [CrossRef]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies forengineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Charlotte, L.H.; Wei, L.L.; Joachim, S.C.L. Drug-eluting scaffolds for bone and cartilage regeneration. Drug Discov. Today 2014, 19, 714–724. [Google Scholar]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar]
- Rodríguez-Palomo, A.; Monopoli, D.; Afonso, H.; Izquierdo-Barba, I.; Vallet-Regí, M. Surface zwitterionization of customized 3D Ti6Al4V scaffolds: A promising alternative to eradicate bone infection. J. Mater. Chem. B 2016, 4, 4356–4365. [Google Scholar] [CrossRef]
- Lanone, S.; Boczkowski, J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr. Mol. Med. 2006, 6, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotech. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chem. A Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. J. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M. Fascinating properties of bioactive templated glasses: A new generation of nanostructured bioceramics. Solid State Sci. 2011, 13, 773–783. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. https://doi.org/10.3390/medicines5040125
Colilla M, Izquierdo-Barba I, Vallet-Regí M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines. 2018; 5(4):125. https://doi.org/10.3390/medicines5040125
Chicago/Turabian StyleColilla, Montserrat, Isabel Izquierdo-Barba, and María Vallet-Regí. 2018. "The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria" Medicines 5, no. 4: 125. https://doi.org/10.3390/medicines5040125
APA StyleColilla, M., Izquierdo-Barba, I., & Vallet-Regí, M. (2018). The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines, 5(4), 125. https://doi.org/10.3390/medicines5040125






