Anti-Plasmodium falciparum Activity of Extracts from 10 Cameroonian Medicinal Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Extraction of Plant Materials
2.2. Plasmodium Falciparum Culture and Maintenance
2.3. In Vitro Anti-Plasmodial Assay
2.4. Cytotoxicity Study of the Selected Extracts Using MTT Assay
3. Results and Discussion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. World Malaria Report; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Wells, T.N. Discovering and developing new medicines for malaria control and elimination. Infect. Disord. Drug Targets 2013, 13, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Culleton, R.; Zhang, M.; Ramaprasad, A.; von Seidlein, L.; Zhou, H.; Zhu, G.; Tang, J.; Liu, Y.; Wang, W.; et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N. Engl. J. Med. 2017, 376, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Craigg, G.M.; Newman, D.G.; Snader, K.M. Natural products drug discovery and development. J. Nat. Prod. 1997, 26, 524–528. [Google Scholar]
- Boyom, F.F.; Tsouh, F.P.V.; Tchokouaha, Y.L.R.; Ngoutane, M.A.; Madiesse, K.A.E.; Mbacham, F.W.; Tsamo, E.; Amvam, Z.P.H.; Jiri, G.; Rosenthal, P.J. Potent antiplasmodial extracts from Cameroonian Annonaceae. J. Ethnopharmacol. 2011, 134, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Tsabang, N.; Tsouh, F.P.V.; Yamthe, T.L.R.; Noguem, B.; Bakarnga-Via, I.; Dongmo, N.M.S.; Nkongmeneck, B.A.; Boyom, F.F. Ethnopharmacological survey of Annonaceae medicinal plants used to treat malaria in four areas of Cameroon. J. Ethnopharmacol. 2012, 139, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D. Natural products as drugs. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 17–19. [Google Scholar] [CrossRef]
- Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. 2000, 14, 478–488. [Google Scholar] [CrossRef]
- Aguiar, A.C.C.; da Roche, E.M.M.; de Souza, N.B.; França, T.C.C.; Krettli, A.U. New approaches in antimalarial drug discovery and development—A review. Mem. Inst. Oswaldo Cruz 2012, 107, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Sebisubi, F.M.; Tan, G.T. Phytochemistry and Pharmacognosy: Natural Products with Antimalarial Activity; UNESCO-EOLSS: Paris, France, 2016. [Google Scholar]
- Boullard, B. Plantes médicinales du monde. Réalités et croyances; Editions ESTEM: Paris, France, 2001. [Google Scholar]
- Thomson, L.A.J.; Evans, B. Species Profiles for Pacific Island Agroforesty; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2006. [Google Scholar]
- Adeloye, A.O.; Aderogba, M.A.; Idowu, T.O.; Obuotor, E.M.; Ogundaini, A.O. Investigation of the antioxidant activity of Alchornea laxiflora (Benth) and its constituents. J. Food Technol. 2005, 3, 365–369. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Jiofack, T.; Ayissi, I.; Fokunang, C.; Guedje, N.; Kemeuze, V. Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. Afr. J. Pharm. Pharmacol. 2009, 3, 144–150. [Google Scholar]
- Abiodun, O.O.; Gbotosho, G.O.; Ajaiyeoba, E.O.; Happi, C.T.; Hoefer, S.; Wittlin, S.; Sowunmi, A.; Brun, R.; Oduola, A.M.J. Comparison of SYBR Green I-, PicoGreen-, and [3H]-hypoxanthine-based assays for in vitro antimalarial screening of plants from Nigerian ethnomedicine. Parasitol. Res. 2010, 106, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.A. Annona senegalensis Persoon: A multipurpose shrub, its phytotherapic, phytopharmacological and phytomedicinal uses. Int. J. Sci. Technol. 2013, 2, 862–865. [Google Scholar]
- Wele, M.; Kirkman, L.; Diarra, N.; Goita, Y.; Doumbia, M.; Traore, K.; Diallo, D. Antiplasmodial potential and phytochemical screening of ten plants used as antimalarial in Mali. Eur. J. Med. Plants 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Hayat, M.M.; Amandeep, S.; Hardeep, K. Bioassay guided fractionation and in vitro antiplasmodial activity of Ficus deltoidea and Ficus benzamine. Pharmacogn. J. 2018, 10, 235–240. [Google Scholar]
- Kayembe, J.; Taba, K.; Ntumba, K.; Tshiongo, M.; Kazadi, T. In vitro anti-malarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J. Med. Plant Res. 2010, 4, 991–994. [Google Scholar]
- Abiodun, O.; Gbotosho, G.; Ajaiyeoba, E.; Happi, T.; Falade, M.; Wittlin, S.; Sowunmi, A.; Brun, R.; Oduola, A. In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharm. Biol. 2011, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Bagavan, A.; Rahuman, A.A.; Zahir, A.A.; Kamaraj, C.; Elango, G.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar. J. 2015, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Nkemnele, B.A.; Mohanakrishnan, D. Antimalarial, antiplasmodial and analgesic activities of root extract of Alchornea laxiflora. Pharm. Biol. 2017, 55, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Mbouna, C.D.J.; Kouipou, T.R.M.; Keumoe, R.; Tchokouaha, Y.L.R.; Fokou, T.P.V.; Tali, M.T.B.; Sahal, D.; Boyom, F.F. Potent antiplasmodial extracts and fractions from Terminalia mantaly and Terminalia superba. Malar. J. 2018, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Ogundipe, O.O.; Moody, J.O.; Houghton, P.J.; Odelola, H.A. Bioactive chemical constituents from Alchornea laxiflora (Benth) pax and Hoffman. J. Ethnopharmacol. 2001, 74, 275–280. [Google Scholar] [CrossRef]
- Mapi, J. Contribution à L’étude Ethnobotanique et Analyse chimique de Quelques Plantes Utilisées en Médecine Traditionnelle dans la Région de Nkongsamba (Cameroun). Ph.D. Thesis, University of Yaoundé, Yaoundé, Cameroon, 1988.
- Dalziel, J.M. The useful plants of west tropical Africa; The Crown Agents for the colonies: London, UK, 1937. [Google Scholar]
- Sagnia, B.; Fedeli, D.; Casetti, R.; Montesano, C.; Falcioni, G.; Colizzi, V. Antioxidant and anti-inflammatory activities of extracts from Senna alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLoS ONE 2014, 9, e103999. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fuorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Met. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bagavan, A.; Rahuman, A.A.; Kaushik, N.K.; Sahal, D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol. Res. 2011, 108, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Okpekon, T.; Yolou, S.; Gleye, C.; Roblot, F.; Loiseau, P.; Bories, C.; Grellier, P.; Frappier, F.; Laurens, A.; Hocquemiller, R. Antiparasitic activities of medicinal plants used in Ivory Coast. J. Ethnopharmacol. 2004, 90, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shuaibu, M.N.; Wuyep, P.A.; Yanagi, T.; Hirayama, K.; Tanaka, T.; Kouno, I. The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol. Res. 2008, 102, 1119–11127. [Google Scholar] [CrossRef] [PubMed]
- Ngbolua, K.N.; Mudogo, V.; Mpiana, P.T.; Tshibangu, D.S.T.; Tshilanda, D.D.; Maseng, C.A. In vitro and in vivo anti-malarial and cytotoxic activities of ethanolic extracts of Annona senegalensis Pers (Annonaceae) from Democratic Republic of the Congo. J. Mod. Drug Discov. Drug Deliv. Res. 2014, 3, 1–7. [Google Scholar]
- Ndjonka, D.; Bergmann, B.; Agyare, C.; Zimbres, M.F.; Luersen, K.; Hensel, A.; Wrenger, C.; Liebau, E. In vitro activity of extracts and isolated polyphenols from west African medicinal plants against Plasmodium falciparum. Parasitol. Res. 2012, 111, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zirihi, G.N.; Mambu, L.; Guede-Guina, F.; Bodo, B.; Grellier, P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005, 98, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Poupin, J.; Tran, H.; Tran, H. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietman. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Muregi, F.W.; Ishih, A.; Miyase, T.; Suzuki, T.; Kino, H.; Amano, T.; Mkoji, G.M.; Terada, M.A. Antimalarial activity of methanolic extracts from plants used in Kenyan ethnomedicine and their interactions with chloroquine (CQ) against a CQ-tolerant rodent parasite, in mice. J. Ethnopharmacol. 2007, 111, 190–195. [Google Scholar] [CrossRef] [PubMed]

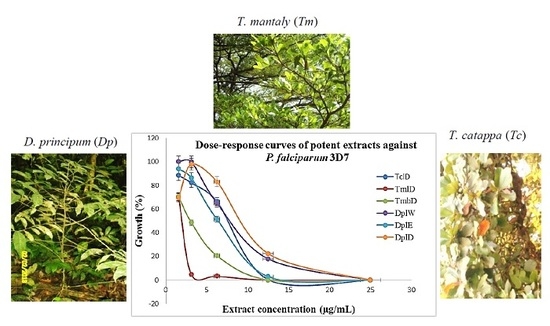
| Names of Plant Species (Family) and Voucher Specimen Number | Local Name | Collection Site in CAMEROON (Year) | Main Traditional Uses | Part Used | Extract Code | Extraction Yield (% w/w) # | |
|---|---|---|---|---|---|---|---|
| 1 | Alchornea lacifolia (Euphorbiaceae) 601610/HNC | Eboe | Mount Kalla (2014) | Malaria, inflammation, and infectious diseases [16,28] | Leaf | All E | 5.90 |
| All D | 4.50 | ||||||
| Twig | Altw W | 9.70 | |||||
| Altw E | 5.43 | ||||||
| Altw D | 6.20 | ||||||
| Stem | Alst W | 8.31 | |||||
| Alst E | 6.81 | ||||||
| Alst D | 7.91 | ||||||
| Trunk | Altr W | 7.98 | |||||
| Altr D | 8.95 | ||||||
| 2 | Annona senegalensis (Annonaceae) 32071/HNC | African custard apple | Bafia (2015) | Fever, diarrhea, joints and respiratory diseases, conjunctivitis, wounds, trypanosomiasis, jaundice, hemorrhoids, convulsions, ovarian cancer, and asthenia [20] | Bark | Asb W | 6.80 |
| Asb Et | 7.00 | ||||||
| Asb WEt | 8.20 | ||||||
| Asb D | 7.82 | ||||||
| Leaf | Asl WEt | 8.70 | |||||
| Asl W | 10.00 | ||||||
| Asl Et | 16.70 | ||||||
| Asl D | 15.59 | ||||||
| Twig | Astw WEt | 6.10 | |||||
| Astw Et | 9.20 | ||||||
| Astw W | 4.30 | ||||||
| Astw D | 3.40 | ||||||
| Stem | Asst WEt | 2.00 | |||||
| Asst Et | 4.70 | ||||||
| Asst W | 1.10 | ||||||
| Asst D | 2.12 | ||||||
| 3 | Cananga odorata (Annonaceae) 42250/HNC | Ylang ylang | Yaoundé (2014) | Fever, malaria, hepatitis, anxiety, itches, tension, shock, fear and panic [17] | Flower | Cofl D | 20.02 |
| 4 | Drypetes principum (Euphorbiaceae) 52007/HNC | ND | Mount Kalla (2014) | ND | Leaf | Dpl W | 12.19 |
| Dpl D | 19.28 | ||||||
| Dpl E | 10.21 | ||||||
| Twig | Dptw W | 9.76 | |||||
| Dptw E | 8.90 | ||||||
| Dptw D | 7.47 | ||||||
| Stem | Dpst W | 7.65 | |||||
| Dpst D | 8.43 | ||||||
| Dpst E | 6.56 | ||||||
| 5 | Ficus benjamina (Moraceae) 65599/HNC | ND | Yaoundé (2015) | Malaria and other parasitic diseases [22] | Fruit | Fbfr WEt | 19.37 |
| Fbfr W | 29.17 | ||||||
| Fbfr D | 23.21 | ||||||
| Leaf | Fbl WEt | 21.45 | |||||
| Fbl W | 26.71 | ||||||
| Fbl D | 24.54 | ||||||
| Stem | Fbst WEt | 10.18 | |||||
| Fbst W | 7.33 | ||||||
| Fbst D | 6.45 | ||||||
| 6 | Ficus exasperata (Moraceae) 19095/HNC (YA) | Lewoua | Yaoundé (2015) | Malaria, dysentery, hemorrhoids, and urinary infections [18] | Leaf | Fel W | 19.23 |
| Fel WEt | 20.45 | ||||||
| Fel D | 19.56 | ||||||
| Stem | Fest W | 8.10 | |||||
| Fest WEt | 11.89 | ||||||
| Fest D | 9.36 | ||||||
| 7 | Occimum gratissimum (Lamiaceae) 5817/SRF/Cam | Messep | Yaoundé (2015) | Headaches, giddiness, cold and cough, headache, fever, ophthalmic, skin diseases, and pneumonia, diarrhea, dysentery, piles, and convulsions [29,30] | Leaf | Ogl Et | 8.87 |
| Ogl M | 10.21 | ||||||
| Root | Ogr Et | 9.11 | |||||
| Ogr M | 8.32 | ||||||
| Stem | Ogst Et | 7.69 | |||||
| Ogst M | 10.80 | ||||||
| 8 | Senna alata (Fabaceae) 1871/HNC (YA) | Ngom-Ntam Ndawolo | Yaoundé (2015) | Yellow fever, malaria, diabetes, constipation, hemorrhoids, inguinal hernia, blennorrhagia, and syphilis [31] | Leaf | Cal Et | 12.10 |
| Cal D | 10.34 | ||||||
| Stem | Cast W | 9.20 | |||||
| Cast D | 7.90 | ||||||
| Twig | Catw Et | 7.50 | |||||
| Catw W | 10.01 | ||||||
| Catw D | 9.15 | ||||||
| 9 | Terminalia catappa (Combretaceae) 51244/HNC | Tropical almond | Yaoundé (2015) | Fever, diaphoretic, amoebiasis, mouth infections, leprosy, headaches, wounds, gonorrhea and anemia [14,15] | Leaf | Tcl D | 20.27 |
| 10 | Terminalia mantaly (Combretaceae) 64212/HNC | - | Yaoundé (2015) | Gastroenteritis, hypertension, diabetes, oral and skin conditions, oral and genital candidiasis [17,27] | Leaf | Tml D | 27.70 |
| Bark | Tmb D | 23.10 |
| Plant Species (Family) | Extracts | P. falciparum (IC50 µg/mL) | 4 CC50 (µg/mL) | 5 SI (CC50/IC50) | |||
|---|---|---|---|---|---|---|---|
| 1Pf3D7 | 2PfINDO | 3 RI | Pf3D7 | PfINDO | |||
| Alchornea Lacifolia (Euphorbiaceae) 601610/HNC | Alst E | 14.88 ± 0.12 | 15.64 ± 0.63 | 1.05 | >200 | >13.44 | >12.78 |
| Alst W | >100 | >100 | - | - | - | - | |
| Alst D | >100 | >100 | - | - | - | - | |
| Altw W | 38.42 ± 0.46 | 40.20 ± 1.61 | 1.04 | - | - | - | |
| Altw E | 16.64 ± 0.63 | 12.44 ± 0.33 | 0.74 | >200 | >12.01 | >16.33 | |
| Altw D | 48.42 ± 0.60 | 54.20 ± 0.61 | 1.11 | - | - | - | |
| Altr W | >100 | >100 | - | - | - | - | |
| Altr D | >100 | >100 | - | - | - | - | |
| All E | 41.38 ± 0.36 | 50.83 ± 1.60 | 1.22 | - | - | - | |
| All D | 49.80 ± 0.45 | 56.83 ± 1.01 | 1.14 | - | - | - | |
| Annona Senegalensis (Annonaceae) 32071/HNC | Asl Wet | 25.08 ± 0.30 | 14.09 ± 0.88 | 0.56 | 81.61 ± 0.48 | 3.25 | 5.79 |
| Asl W | >100 | >100 | − | - | - | - | |
| Asl Et | 39.40 ± 0.80 | 28.72 ± 2.32 | 0.72 | - | - | - | |
| Asl D | 42.10 ± 0.90 | 29.20 ± 1.30 | 0.69 | - | - | - | |
| Asb W | 14.47 ± 0.30 | >100 | >6.91 | - | - | - | |
| Asb Et | 19.82 ± 1.82 | 16.80 ± 0.17 | 0.84 | 97.95 ± 0.25 | 4.94 | 5.82 | |
| Asb Wet | 25.07 ± 1.36 | 13.16 ± 0.00 | 0.52 | >200 | >7.97 | >15.19 | |
| Asb D | 29.07 ± 1.60 | 30.60 ± 1.09 | 1.05 | - | - | - | |
| Asst W | >100 | >100 | - | - | - | - | |
| Asst Wet | 18.89 ± 0.46 | 20.20 ± 0.98 | 1.06 | >200 | >10.58 | >9.90 | |
| Asst Et | >100 | >100 | - | - | - | - | |
| Asst D | >100 | >100 | - | - | - | - | |
| Astw Wet | 30.41 ± 0.52 | 13.17 ± 0.00 | 0.43 | - | - | - | |
| Astw Et | >100 | >100 | - | - | - | - | |
| Astw W | >100 | >100 | - | - | - | - | |
| Astw D | >100 | >100 | - | - | - | - | |
| Cananga Odorata (Annonaceae) 42250/HNC | Cofl D | >100 | >100 | - | - | - | - |
| Drypetes Principum (Euphorbiaceae) 52007/HNC | Dptw W | 31.52 ± 0.39 | 35.94 ± 2.75 | 1.14 | - | - | - |
| Dptw E | 12.68 ± 0.00 | 12.74 ± 0.00 | 1.00 | 98.14 ± 0.48 | 7.73 | 7.70 | |
| Dptw D | 30.21 ± 0.91 | 26.40 ± 1.75 | 0.87 | - | - | - | |
| Dpl W | 4.91 ± 0.29 | 6.64 ± 0.00 | 1.35 | >200 | >40.73 | >30.12 | |
| Dpl E | 5.49 ± 0.63 | 5.98 ± 0.40 | 1.08 | >200 | >36.43 | >33.44 | |
| Dpl D | 6.49 ± 0.58 | 7.10 ± 0.82 | 1.09 | >200 | >30.81 | >28.16 | |
| Dpst W | >100 | >100 | - | - | - | - | |
| Dpst E | 27.78 ± 0.32 | 16.71 ± 0.25 | 0.60 | - | - | - | |
| Dpst D | >100 | >100 | - | - | - | - | |
| Ficus Benjamina (Moraceae) 65599/HNC | Fbfr Wet | >100 | >100 | - | - | - | - |
| Fbfr W | >100 | >100 | - | - | - | - | |
| Fbfr D | >100 | >100 | - | - | - | - | |
| Fbl W | 12.41 ± 0.36 | 26.35 ± 1.58 | 2.12 | >200 | >16.11 | >7.59 | |
| Fbl Wet | >100 | >100 | - | - | - | - | |
| Fbl D | >100 | >100 | - | - | - | - | |
| Fbst W | >100 | >100 | - | - | - | - | |
| Fbst Wet | >100 | 52.91 ± 2.29 | NA | - | - | - | |
| Fbst D | >100 | >100 | - | - | - | - | |
| Ficus Exasperate (Moraceae) 19095/HNC (YA) | Fest W | >100 | >100 | - | - | - | - |
| Fest Wet | 55.70 ± 0.50 | 27.22 ± 1.29 | 0.48 | - | - | - | |
| Fest D | 57.60 ± 0.40 | 25.12 ± 1.90 | 0.43 | - | - | - | |
| Fel W | 23.84 ± 0.48 | 28.00 ± 1.67 | 1.17 | - | - | - | |
| Fel Wet | 26.99 ± 0.60 | 35.41 ± 3.23 | 1.31 | - | - | - | |
| Fel D | 27.29 ± 0.60 | 39.41 ± 1.30 | 1.44 | - | - | - | |
| Occimum Gratissimum(Lamiaceae) 5817/SRF/Cam | Ogst Et | >100 | >100 | - | - | - | - |
| Ogst M | >100 | 46.36 ± 3.38 | NA | - | - | - | |
| Ogl Et | 54.41 ± 0.03 | 27.50 ± 2.56 | 0.50 | - | - | - | |
| Ogl M | 48.11 ± 0.68 | 21.79 ± 2.49 | 0.45 | - | - | - | |
| Ogr Et | 52.41 ± 1.20 | 29.01 ± 2.90 | 0.55 | - | - | - | |
| Ogr M | 54.22 ± 0.75 | 24.33 ± 1.13 | 0.44 | - | - | - | |
| Senna Alata(Fabaceae) 1871/HNC (YA) | Catw Et | >100 | 37.06 ± 1.80 | NA | - | - | - |
| Catw W | >100 | >100 | - | - | - | - | |
| Catw D | >100 | >100 | - | - | - | - | |
| Cal Et | 31.36 ± 0.73 | 32.38 ± 2.84 | 1.03 | - | - | - | |
| Cal D | 41.60 ± 0.34 | 52.80 ± 1.40 | 1.26 | - | - | - | |
| Cast W | >100 | >100 | - | - | - | - | |
| Cast D | >100 | >100 | - | - | - | - | |
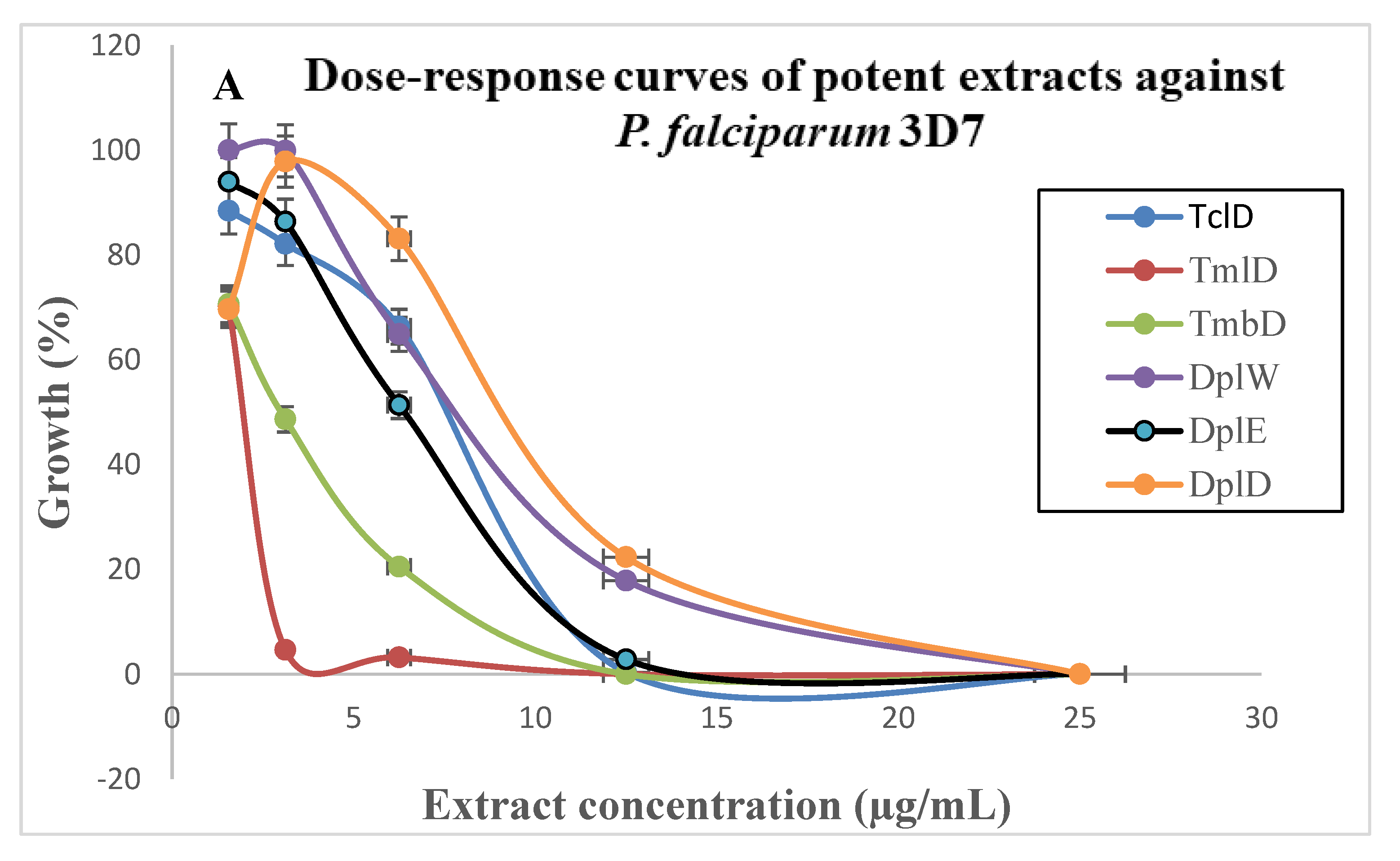
| Terminalia Catappa (Combretaceae) 51244/HNC | Tcl D | 6.41 ± 0.43 | 8.10 ± 0.30 | 1.26 | >200 | >31.20 | >24.69 |
| Terminalia Mantaly (Combretaceae) 64212/HNC | Tml D | 2.49 ± 0.09 | 1.90 ± 0.10 | 0.76 | >200 | >80.32 | >105.26 |
| Tmb D | 3.70 ± 0.16 | 2.80 ± 0.60 | 0.75 | >200 | >54.05 | >71.42 | |
| Chloroquine (µM) | 40 | 400 | 10 | - | - | - | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufin Marie, T.K.; Mbetyoumoun Mfouapon, H.; Madiesse Kemgne, E.A.; Jiatsa Mbouna, C.D.; Tsouh Fokou, P.V.; Sahal, D.; Fekam Boyom, F. Anti-Plasmodium falciparum Activity of Extracts from 10 Cameroonian Medicinal Plants. Medicines 2018, 5, 115. https://doi.org/10.3390/medicines5040115
Rufin Marie TK, Mbetyoumoun Mfouapon H, Madiesse Kemgne EA, Jiatsa Mbouna CD, Tsouh Fokou PV, Sahal D, Fekam Boyom F. Anti-Plasmodium falciparum Activity of Extracts from 10 Cameroonian Medicinal Plants. Medicines. 2018; 5(4):115. https://doi.org/10.3390/medicines5040115
Chicago/Turabian StyleRufin Marie, Toghueo Kouipou, Heroine Mbetyoumoun Mfouapon, Eugenie Aimée Madiesse Kemgne, Cedric Derick Jiatsa Mbouna, Patrick Valere Tsouh Fokou, Dinkar Sahal, and Fabrice Fekam Boyom. 2018. "Anti-Plasmodium falciparum Activity of Extracts from 10 Cameroonian Medicinal Plants" Medicines 5, no. 4: 115. https://doi.org/10.3390/medicines5040115
APA StyleRufin Marie, T. K., Mbetyoumoun Mfouapon, H., Madiesse Kemgne, E. A., Jiatsa Mbouna, C. D., Tsouh Fokou, P. V., Sahal, D., & Fekam Boyom, F. (2018). Anti-Plasmodium falciparum Activity of Extracts from 10 Cameroonian Medicinal Plants. Medicines, 5(4), 115. https://doi.org/10.3390/medicines5040115