Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Determination of Total Polyphenols
2.4. Determination of Antioxidant Capacity
2.5. Statistical Analysis
3. Results
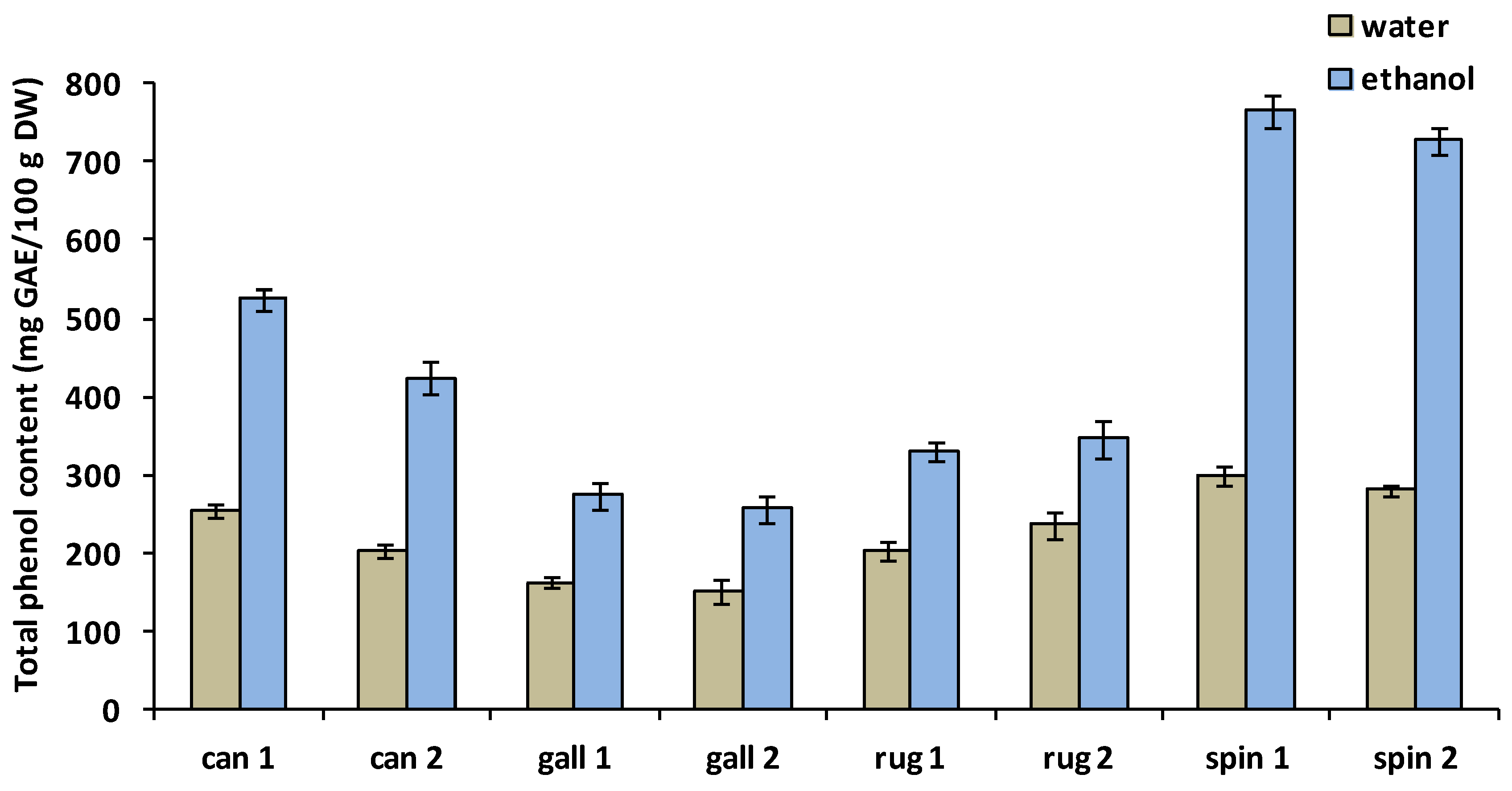
3.1. Total Polyphenol Content
3.2. Antioxidant Capacity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bruneau, A.; Starr, J.R.; Joly, S. Phylogenetic relationships in the genus Rosa: New evidence from chloroplast DNA sequences and an appraisal of current knowledge. Syst. Bot. 2007, 32, 366–378. [Google Scholar] [CrossRef]
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/search?q=rosa (accessed on 28 June 2018).
- Brown, D. New Encyclopedia of Herbs & Their Uses, 1st ed.; Dorling Kindersley: London, UK, 2002; pp. 346–347. ISBN 0-7513-3386-7. [Google Scholar]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Am. Soc. Hort. Sci. 2014, 139, 157–166. [Google Scholar]
- Orhan, D.D.; Hartevioğlu, A.; Küpeli, E.; Yesilada, E.J. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Willich, S.N.; Rossnagel, K.; Roll, S.; Wagner, A.; Mune, O.; Erlendson, J.; Kharazmi, A.; Sörensen, H.; Winther, K. Rose hip herbal remedy in patients with rheumatoid arthritis—A randomized controlled trial. Phytomedicine 2010, 17, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yold, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Tumbas, V.T.; Canadanovic-Brunet, J.M.; Cetojevic-Simin, D.D.; Cetkovic, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Rose hips as complementary and alternative medicine: Overview of the present status and prospects. Mediterr. J. Nutr. Metab. 2013, 6, 89. [Google Scholar] [CrossRef]
- Fan, C.; Pacier, C.; Martirosyan, D.M. Rose hip (Rosa canina L.): A functional food perspective. Funct. Foods Health Dis. 2014, 4, 493–509. [Google Scholar]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Adamczak, A.; Buchwald, W.; Zielinski, J.; Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., sect. Caninae dc. Em. Christ.). Acta Biol. Cracov. Bot. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Nowak, R. Chemical composition of hips essential oils of some Rosa L. species. Z. Naturforsch. 2005, 60c, 369–378. [Google Scholar] [CrossRef]
- Adamczak, A.; Grys, A.; Buchwald, W.; Zieliński, J. Content of oil and main fatty acids in hips of rose species native in Poland. Dendrobiology 2011, 66, 55–62. [Google Scholar]
- Sharma, B.; Singh, B.; Dhyani, D.; Verma, P.K.; Karthigeyan, S. Fatty acid composition of wild growing rose species. J. Med. Plants Res. 2012, 6, 1046–1049. [Google Scholar] [CrossRef]
- Wenzig, E.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Ogah, O.; Watkins, C.S.; Ubi, B.E.; Oraguzie, N.C. Phenolic compounds in Rosaceae fruit and nut crops. J. Agric. Food Chem. 2014, 62, 9369–9386. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Chrif, R.; Zahed, N.; Abid, I.; Medfei, W.; Sebei, H.; Brahim, N.B. Fatty acid and phenolic constituents of leaves, flowers and fruits of tunisian dog rose (Rosa canina L.). Riv. Ital. Sostanze Gr. 2010, 87, 117–123. [Google Scholar]
- Türkben, C.; Uylaşer, V.; İncedayı, B.; Çelikkol, I. Effects of different maturity periods and processes on nutritional components of rose hip (Rosa canina L.). J. Food Agric. Environ. 2010, 8, 26–30. [Google Scholar]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Elmastas, M.; Demir, A.; Genc, N.; Dölek, Ü.; Günes, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Nadpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anackov, G.T.; Cetojevic´-Simin, D.D.; Mimica-Dukic, N.M.; Beara, I.N. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Najda, A.; Buczkowska, H. Morphological and chemical characteristics of fruits of selected Rosa sp. Mod. Phytomorph. 2013, 3, 99–103. [Google Scholar] [CrossRef]
- Jiménez, S.; Jiménez-Moreno, N.; Luquin, A.; Laguna, M.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Chemical composition of rosehips from different Rosa species: An alternative source of antioxidants for the food industry. Food Add. Cont. A 2017, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, S. Heilpflanzen. In Praxis heute, 2nd ed.; Urban & Fischer Verlag: München, Germany, 2012; pp. 261–262. ISBN 978-3-437-57272-2. (In German) [Google Scholar]
- USDA (United States Department of Agriculture), Natural Resources Conservation Service. Rosa canina L. Dog Rose. In The PLANTS Database; National Plant Data Team: Greensboro, NC, USA. Available online: https://plants.usda.gov/plantguide/pdf/pg_roca3.pdf (accessed on 20 June 2018).
- European Medicines Agency. Rosa Flower. EMA/237220/2017. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Summary_of_assessment_report_for_the_public/2017/07/WC500230910.pdf (accessed on 20 June 2018).
- USDA (United States Department of Agriculture), Natural Resources Conservation Service. Rosa rugosa Thunb. Rugosa Rose. In The PLANTS Database; National Plant Data Team: Greensboro, NC, USA. Available online: https://plants.usda.gov/factsheet/pdf/fs_roru.pdf (accessed on 20 June 2018).
- Boyd, P.D.A. Scots Roses and Related Cultivars of Rosa spinosissima—A Review. In Proceedings of the ISHS Acta Horticulturae 1064 VI International Symposium on Rose Research and Cultivation, Hannover, Germany, 25 January 2015. [Google Scholar]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, K.; Özdemir, E. The effects of genotype and growing conditions on antioxidant capacity, phenolic compounds, organic acid and individual sugars of strawberry. Food Chem. 2014, 155, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.L.A.G.; Mélo, E.A.; Maciel, M.I.S.; Prazeres, F.G.; Musser, R.S.; Lima, D.A.E.S. Total phenolic and carotenoid contents in acerola genotypes harvested at three ripening stages. Food Chem. 2005, 90, 565–568. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). J. Sci. Food Agric. 2016, 96, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Béla, L. Pharmacopoea Hungarica, 8th ed.; Medicina Kiadó: Budapest, Hungary, 2004; p. 1432. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 1, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Fattahi, S.; Jamei, R.; Sarghein, S.H. Antioxidant and antiradicalic activity of Rosa canina and Rosa pimpinellifolia fruits from West Azerbaijan. Iran. J. Plant Physiol. 2012, 2, 523–529. [Google Scholar]
- Yilmaz, S.O.; Ercisli, S. Antibacterial and antioxidant activity of fruits of some rose species from Turkey. Rom. Biotechnol. Lett. 2011, 16, 6407–6411. [Google Scholar]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic fruits as a source of important phytochemicals: Improving the traditional use of Rosa canina fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Gao, X.; Björk, L.; Trajkovski, V.; Uggla, M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000, 80, 2021–2027. [Google Scholar] [CrossRef]
- Taneva, I.; Petkova, N.; Dimov, I.; Ivanov, I.; Denev, P. Characterization of rose hip (Rosa canina L.) fruits extracts and evaluation of their in vitro antioxidant activity. J. Pharmacogn. Phytochem. 2016, 5, 35–38. [Google Scholar]
- Koca, I.; Ustun, N.S.; Koyuncu, T. Effect of drying conditions on antioxidant properties of rosehip fruits (Rosa canina sp.). Asian J. Chem. 2009, 21, 1061–1068. [Google Scholar]
- Montazeri, N.; Baher, E.; Mirzajani, F.; Barami, Z.; Yousefian, S. Phytochemical contents and biological activities of Rosa canina fruit from Iran. J. Med. Plants Res. 2011, 5, 4584–4589. [Google Scholar]
- Franco, D.; Pinelo, M.; Sineiro, J.; Núñez, M.J. Processing of Rosa rubiginosa: Extraction of oil and antioxidant substances. Bioresour. Technol. 2007, 98, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Cent. Eur. J. Biol. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- İlbay, Z.; Şahin, S.; Kırbaşlar, S.I. Investigation of polyhenolic content of rose hip (Rosa canina L.) tea extracts: A comparative study. Foods 2013, 2, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Koczka, N.; Móczár, Z.; Stefanovits-Bányai, É.; Ombódi, A. Differences in antioxidant properties of ginkgo leaves collected from male and female trees. Acta Pharm. 2015, 65, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Butsat, S.; Siriamornpun, S. Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C. Int. Food Res. J. 2016, 23, 180–187. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of Hashemi rice bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, L.H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin characterization and bioactivity of extracts from different parts of Uncaria tomentosa L. (cat’s claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Lee, K.S.; Hyacinth, H.I.; Hibbert, J.M.; Reid, M.E.; Wheatley, A.O.; Asemota, H.N. An investigation of the antioxidant capacity in extracts from Moringa oleifera plants grown in Jamaica. Plants 2017, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
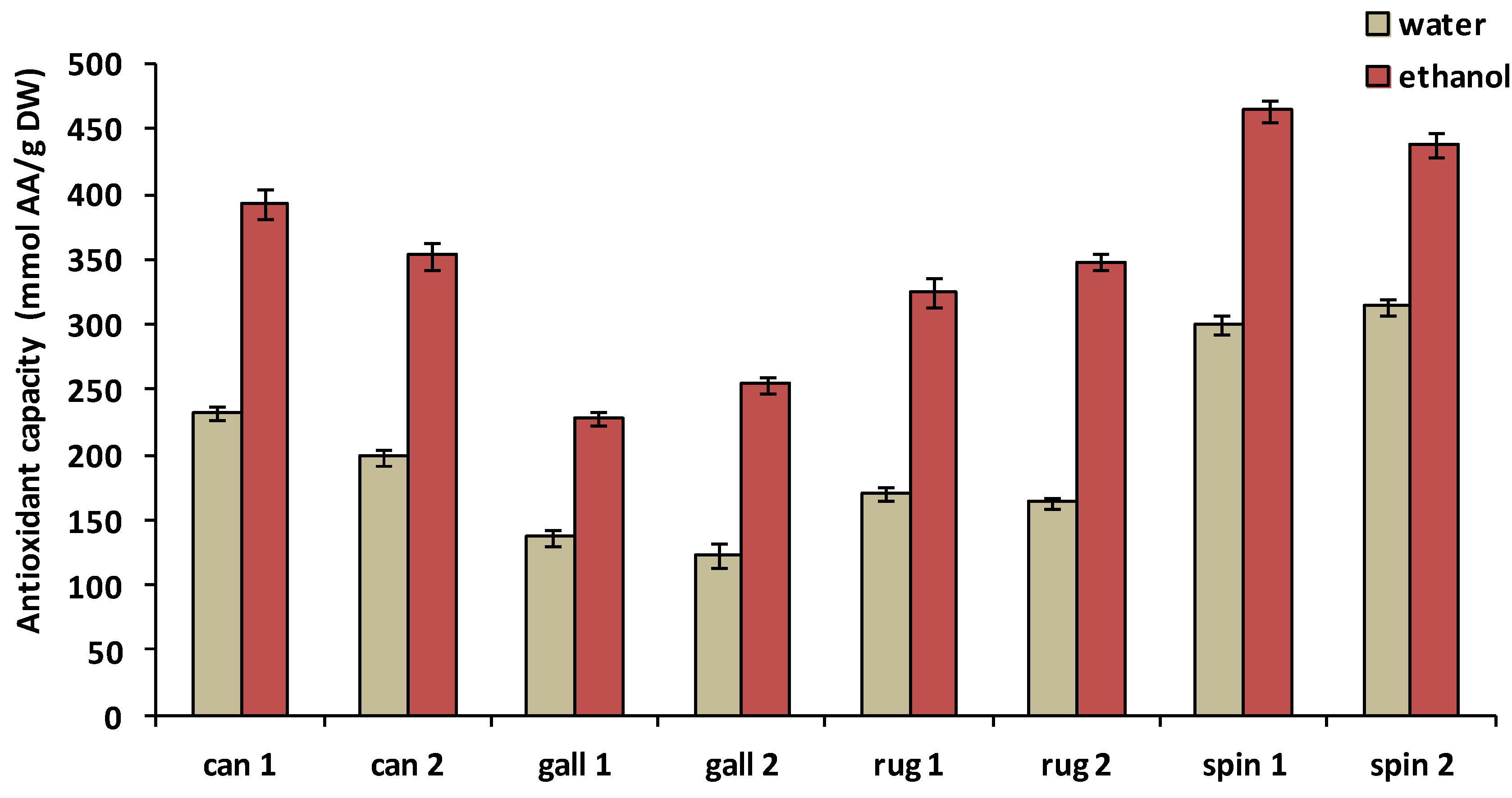
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. https://doi.org/10.3390/medicines5030084
Koczka N, Stefanovits-Bányai É, Ombódi A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines. 2018; 5(3):84. https://doi.org/10.3390/medicines5030084
Chicago/Turabian StyleKoczka, Noémi, Éva Stefanovits-Bányai, and Attila Ombódi. 2018. "Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species" Medicines 5, no. 3: 84. https://doi.org/10.3390/medicines5030084
APA StyleKoczka, N., Stefanovits-Bányai, É., & Ombódi, A. (2018). Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines, 5(3), 84. https://doi.org/10.3390/medicines5030084



