Mini-Review: Assessing the Potential Impact of Microneedle Technologies on Home Healthcare Applications
Abstract
1. Introduction
2. Home Healthcare
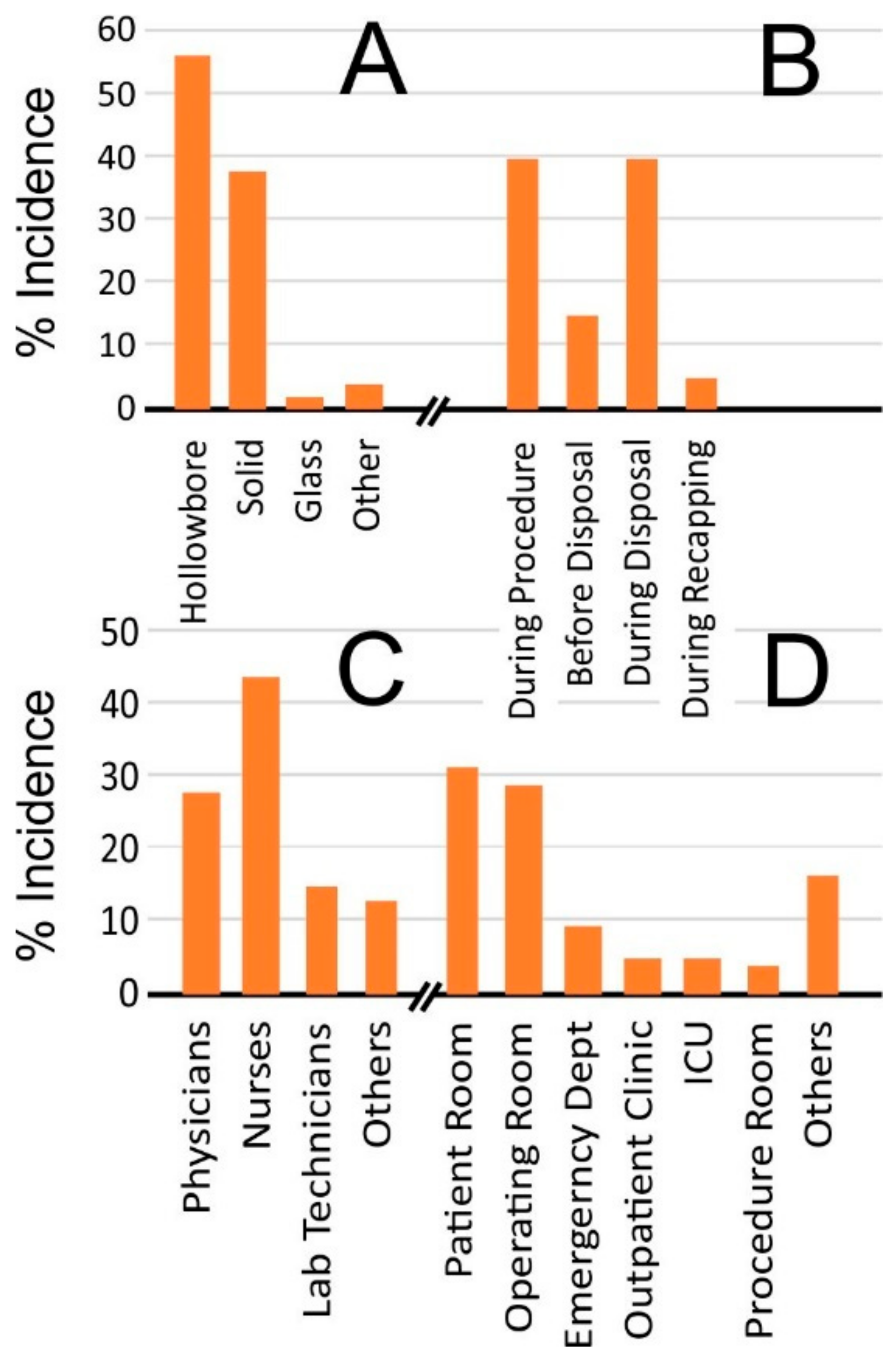
3. Engineering a Solution to Needlestick Injuries
4. The Quest for Needle-Free Delivery
4.1. Microneedle Designs and Implications
4.2. Solid Microneedles/Coated Microneedles
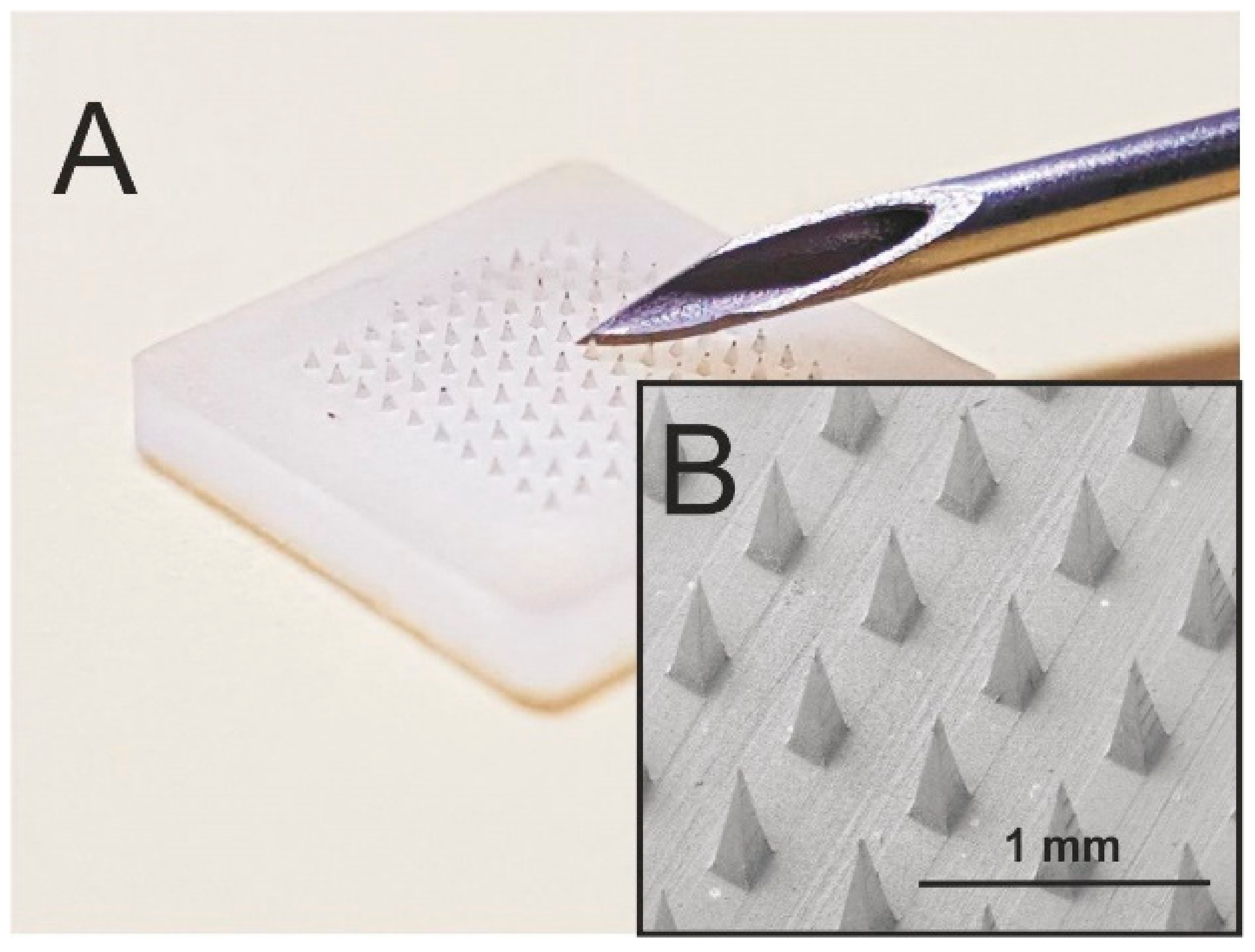
4.3. Hollow Microneedles
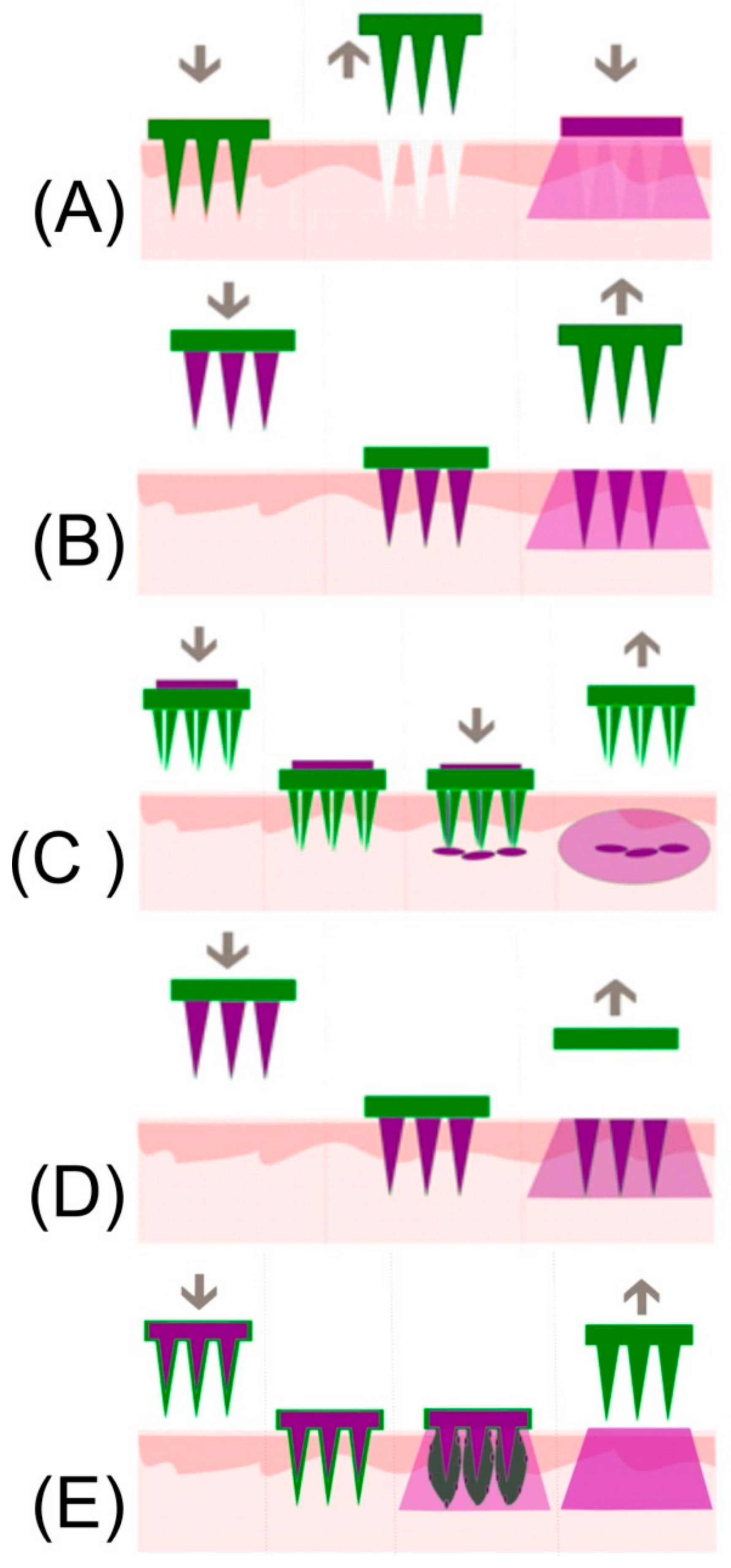
4.4. Dissolvable and Swellable Microneedles
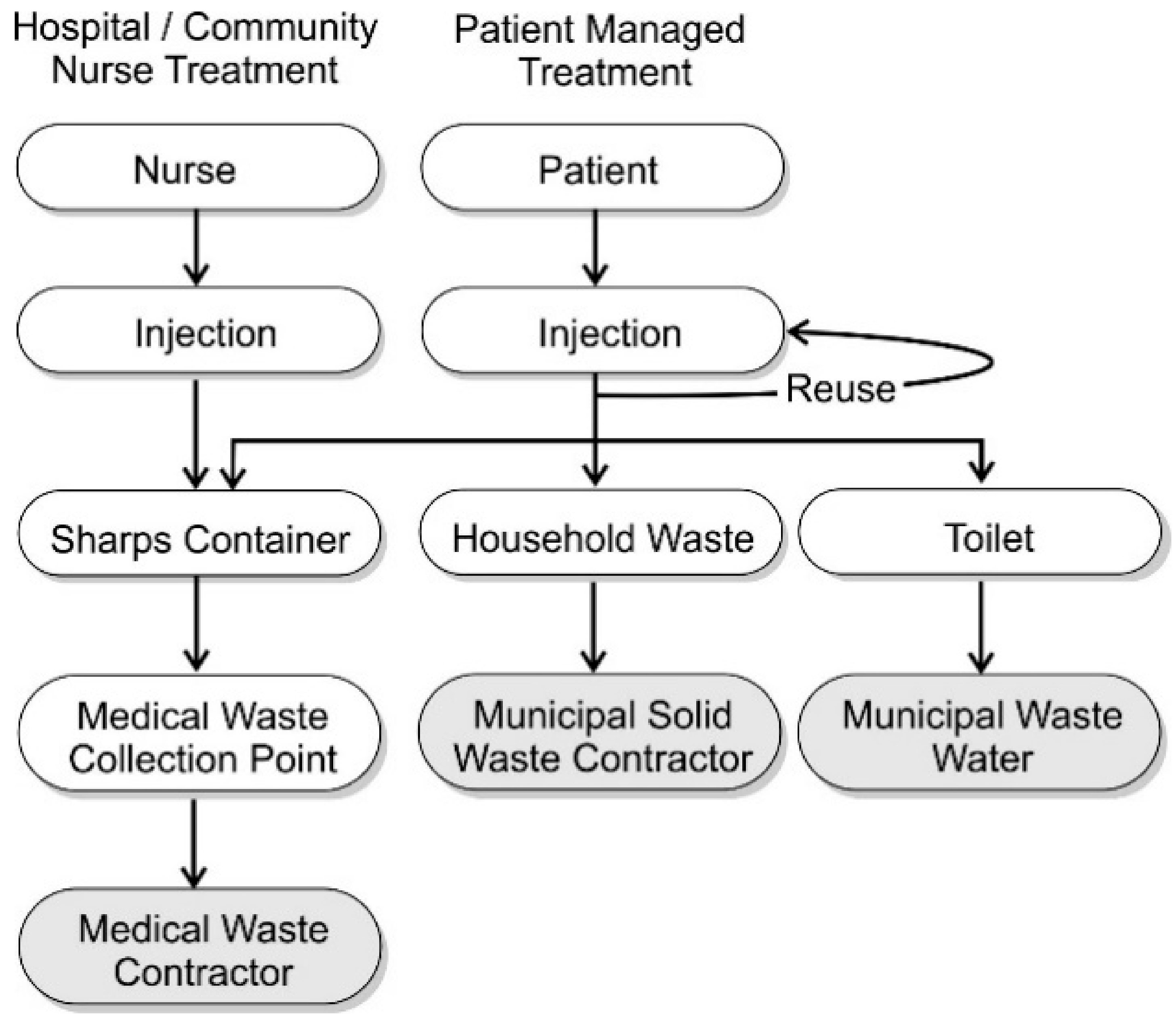
5. Needle Handling and Disposal
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jagger, J.; Berguer, R.; Phillips, E.K.; Parker, G.; Gomaa, A.E. Increase in Sharps Injuries in Surgical Settings versus Nonsurgical Settings after Passage of National Needlestick Legislation. AORN J. 2011, 93, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Jagger, J.; Parker, G.; Phillips, E.K.; Gomaa, A. Disposal of sharps medical waste in the United States: Impact of recommendations and regulations, 1987–2007. Am. J. Infect. Control 2012, 40, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Massachusetts Department of Public Health Occupational Health Surveillance Program. Sharps Injuries among Hospital Workers in Massachusetts. 2007. Available online: http://www.mass.gov/Eeohhs2/docs/dph/occupational_health/injuries_hospital_2007.pdf (accessed on 12 February 2018).
- CDC. Workbook for Designing, Implementing and Evaluating a Sharps Injury Prevention Program; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2008; pp. 1–168. [CrossRef]
- Tatelbaum, M.F. Needlestick safety and prevention act. Pain Physician 2011, 4, 193–195. [Google Scholar] [CrossRef]
- Jagger, J.; Perry, J.; Gomaa, A.; Phillips, E.K. The impact of U.S. policies to protect healthcare workers from bloodborne pathogens: The critical role of safety-engineered devices. J. Infect. Public Health 2008, 1, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, A.L.; Orelien, J.G.; Srivastava, P.U.; Jagger, J.; Cohn, R.D.; Cardo, D.M.; Group, N.S.; Network, E.D.S. Estimate of the Annual Number of Percutaneous Injuries among Hospital-Based Healthcare Workers in the United States, 1997–1998. Infect. Control Hosp. Epidemiol. 2004, 25, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Grimmond, T.; Good, L. Exposure Survey of Trends in Occupational Practice (EXPO-S.T.O.P.) 2015: A national survey of sharps injuries and mucocutaneous blood exposures among health care workers in US hospitals. Am. J. Infect. Control 2017, 45, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Quinn, H.L.; Kearney, M.-C.; Courtenay, A.J.; McCrudden, M.T.C.; Donnelly, R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014, 11, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and progress. Drug Deliv. 2016, 23, 2338–2354. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Shin, J.-H.; Seok, H.Y.; Kim, Y.-C. Biomedical applications of microneedles in therapeutics: Recent advancements and implications in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Health Protection Report; Public Health England: London, UK, 2017; Volume 11. [Google Scholar]
- Tarantola, A.; Abiteboul, D.; Rachline, A. Infection risks following accidental exposure to blood or body fluids in health care workers: A review of pathogens transmitted in published cases. Am. J. Infect. Control 2006, 34, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Elseviers, M.M.; Arias-Guillén, M.; Gorke, A.; Arens, H.-J. Sharps Injuries amongst Healthcare Workers: Review of Incidence, Transmissions and Costs. J. Ren. Care 2014, 40, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Ogden, S.; Lavery, D.; Griffiths, C.E.M. Making a ‘point’ about the safe disposal of sharps in patients on biological therapies. Br. J. Dermatol. 2008, 159, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Markkanen, P.K.; Galligan, C.J.; Kriebel, D.; Chalupka, S.M.; Kim, H.; Gore, R.J.; Sama, S.R.; Laramie, A.K.; Davis, L. Sharps injuries and other blood and body fluid exposures among home health care nurses and aides. Am. J. Public Health 2009, 99, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Labor Statistics. Occupational Outlook Handbook, 2016–17 ed.; Home Health Aides and Personal Care Aides, 2015. Available online: http://www.bls.gov/ooh/personal-care-and-service/personal-care-aides.htm (accessed on 13 February 2018).
- Bureau of Labor Statistics. Occupational Outlook Handbook: Fastest Growing Occupations. 2015. Available online: http://www.bls.gov/ooh/fastest-growing.htm (accessed on 14 February 2018).
- National Association for Home Care & Hospice. Basic Statistics about the Home Care. 2010. Available online: http://www.nahc.org/assets/1/7/10HC_Stats.pdf (accessed on 14 February 2018).
- National Research Council. Health Care Comes Home: The Human Factors. Available online: http://www.nap.edu/catalog/13149/health-care-comes-home%0A-the-human-factors%0A (accessed on 14 February 2018).
- Lipscomb, J.; Sokas, R.; McPhaul, K.; Scharf, B.; Barker, P.; Trinkoff, A.; Storr, C. Occupational blood exposure among unlicensed home care workers and home care registered nurses: Are they protected? Am. J. Ind. Med. 2009, 52, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, P.; Quinn, M.; Galligan, C.; Sama, S.; Brouillette, N.; Okyere, D. Characterizing the nature of home care work and occupational hazards: A developmental intervention study. Am. J. Ind. Med. 2014, 57, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Haiduven, D.; Ferrol, S. Sharps injuries in the home health care setting: Risks for home health care workers. AAOHN J. 2004, 52, 102–108. [Google Scholar] [PubMed]
- Gold, K.; Schumann, J. Dangers of used sharps in household trash: Implications for home care. Home Healthc. Now 2007, 25, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Becton-Dickinson. A Look at the Reuse of Insulin Needles. 2006. Available online: https://www.bd.com/.../DC_A-Look-at-the-Reuse-of-Insulin-Needles_WP_EN.pdf (accessed on 1 March 2018).
- Costello, J.; Parikh, A. The sticking point: Diabetic sharps disposal practices in the community. J. Gen. Intern. Med. 2013, 28, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Chege, M.; McConville, A.; Davis, J. Microneedle drug delivery systems: Appraising opportunities for improving safety and assessing areas of concern. J. Chem. Health Safety 2017, 24, 6–14. [Google Scholar] [CrossRef]
- Gomaa, Y.A.; Morrow, D.I.J.; Garland, M.J.; Donnelly, R.F.; EI-Khordagui, L.K.; Meidan, V.M. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: Assessments by transepidermal water loss. Toxicol. In Vitro 2010, 24, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Birchall, J.C.; Clemo, R.; Anstey, A.; John, D.N. Microneedles in clinical practice-an exploratory study into the opinions of healthcare professionals and the public. Pharm. Res. 2011, 28, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.I.; Smith, E.; John, D.N.; Kalavala, M.; Edwards, C.; Anstey, A.; Morrissey, A.; Birchall, J.C. Clinical administration of microneedles: Skin puncture, pain and sensation. Biomed. Microdevices 2008, 11, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Brogden, N.K.; Stinchcomb, A.L. Fluvastatin as a Micropore Lifetime Enhancer for Sustained Delivery across Microneedle-Treated Skin. J. Pharm. Sci. 2014, 103, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Brogden, N.K.; Banks, S.L.; Crofford, L.J.; Stinchcomb, A.L. Diclofenac enables unprecedented week-long microneedle-enhanced delivery of a skin impermeable medication in humans. Pharm. Res. 2013, 30, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Huo, M.R.; Zhou, J.P.; Zhou, Y.Q.; Hao, B.H.; Liu, T.; Zhang, Y. Super-short solid silicon microneedles for transdermal drug delivery applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Alkilani, A.Z.; McCrudden, M.T.C.; O’Neill, S.; O’Mahoy, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013, 451, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Kolli, C.S.; Banga, A.K. Characterization of Microchannels Created by Metal Microneedles: Formation and Closure. AAPS J. 2011, 13, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, Y.; Zhang, S.; Yang, G.; Gao, Y. Controllable coating of microneedles for transdermal drug delivery. Drug Dev. Ind. Pharm. 2015, 41, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.; Mikszta, J.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [PubMed]
- Al-Zahrani, S.; Zaric, M.; McCrudden, C.; Scott, C.; Kissenpfennig, A.; Donnelly, R.F. Microneedle-mediated vaccine delivery: Harnessing cutaneous immunobiology to improve efficacy. Expert Opin. Drug Deliv. 2012, 9, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Haj-Ahmad, R.; Khan, H.; Arshad, M.S.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.-W.; Ahmad, Z. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics 2015, 7, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Bonificio, A.; Gallorini, S.; Palladino, G.; Determan, A.S.; Dohmeier, D.M.; Kroells, K.D.; Sternijohn, J.R.; Singh, M.; et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 2013, 31, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Gill, H.S.; He, C.; Ou, C.; Wang, L.; Wang, Y.-C.; Feng, H.; Zhang, H.; Prausnitz, M.R.; Compans, R.W. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J. Control. Release 2014, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Burton, S.A.; Ng, C.-Y.; Simmers, R.; Moeckly, C.; Brandwein, D.; Gilbert, T.; Johnson, N.; Brown, K.; Alston, T.; Prochnow, G.; et al. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm. Res. 2011, 28, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gardeniers, H.J.G.E.; Luttge, R.; Berenschot, E.J.W.; de Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Paik, S.J.; Kim, S.H.; Allen, M.G. Hypodermic-needle-like hollow polymer microneedle array: Fabrication and characterization. J. Microelectromech. Syst. 2014, 23, 991–998. [Google Scholar] [CrossRef]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Axial and shear fracture strength evaluation of silicon microneedles. Microsyst. Technol. 2010, 16, 973–978. [Google Scholar] [CrossRef]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J. Micromech. Microeng. 2010, 20, 045011. [Google Scholar] [CrossRef]
- Zahn, J.D.; Talbot, N.H.; Liepmann, D.; Pisano, A.P. Microfabricated polysilicon microneedles for minimally invasive biomedical devices. Biomed. Microdevices 2000, 2, 295–303. [Google Scholar] [CrossRef]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. microRNA-10A* and microRNA-21 Modulate Endothelial Progenitor Cell Senescencevia Suppressing Hmga2. NIH Public Access 2014, 112, 152–164. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Quek, T.C.; Soon, W.J.; Choi, J.; Zou, S.; Kang, L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J. Pharm. Sci. 2013, 102, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The Effects of Geometry on Skin Penetration and Failure of Polymer Microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Migalska, K.; Tuan-Mahmood, T.M.; Singh, T.R.R.; Majithija, R.; Caffarel-Salvador, E.; McCrudden, C.M.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Influence of skin model on in vitro performance of drug-loaded soluble microneedle arrays. Int. J. Pharm. 2012, 434, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Migalska, K.; Morrow, D.I.J.; Garland, M.J.; Thakur, R.; Woolfson, A.D.; Donnelly, R.F. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm. Res. 2011, 28, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; Garland, M.J.; McInnes, F.J.; Donnelly, R.F.; EI-Khordagui, L.K.; Wilson, C.G. Flux of ionic dyes across microneedle treated skin: Effect of dye molecular characteristics. Int. J. Pharm. 2012, 438, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; Garland, M.J.; McInnes, F.J.; Donnelly, R.F.; EI-Khordagui, L.K.; Wilson, C.G. Microneedle/nanoencapsulation-mediated transdermal delivery: Mechanistic insights. Eur. J. Pharm. Biopharm. 2014, 86, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Murano, H.; Hamasaki, N.; Fukushima, K.; Takada, K. Incidence of low bioavailability of leuprolide acetate after percutaneous administration to rats by dissolving microneedles. Int. J. Pharm. 2011, 407, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jung, B.; Park, J.H. Hydrogel swelling as a trigger to release biodegradable polymer microneedles in skin. Biomaterials 2012, 33, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castañeda, P.; Escobar-Chávez, J.J.; Rodríguez-Cruz, I.M.; Melgoza-Contreras, L.M.; Martínez-Hernández, J. Microneedles as Enhancer of Drug Absorption Through the Skin and Applications in Medicine and Cosmetology. J. Pharm. Pharm. Sci. 2018, 21, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Joyce, J.C.; Nguyen, H.X.; Eangoor, P.; Knaack, J.S.; Banga, A.K.; Prausnitz, M.R. Dihydroergotamine mesylate-loaded dissolving microneedle patch made of polyvinylpyrrolidone for management of acute migraine therapy. J. Control. Release 2017, 268, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cai, B.; Quan, G.; Peng, T.; Yao, G.; Zhu, C.; Wu, A.; Ran, H.; Pan, X.; Wu, C. Novel strategy for immunomodulation: Dissolving microneedle array encapsulating thymopentin fabricated by modified two-step molding technology. Eur. J. Pharm. Biopharm. 2018, 122, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Jang, Y.; Huh, I.; Yang, H.; Jang, M.; Jung, H. Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018, 8, 1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Zhang, X.P.; Chen, B.Z.; Guo, X.D. Dissolvable layered microneedles with core-shell structures for transdermal drug delivery. Mater. Sci. Eng. C 2018, 83, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ronnander, P.; Simon, L.; Spilgies, H.; Koch, A.; Scherr, S. Dissolving polyvinylpyrrolidone-based microneedle systems for in-vitro delivery of sumatriptan succinate. Eur. J. Pharm. Sci. 2018, 114, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Tu, T.N.T.; Kim, S.; Yang, H.; Jang, M.; Jo, D.; Ryu, J.; Baek, J.; Jung, H. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int. J. Cosmet. Sci. 2018, 40, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hutton, A.R.J.; Quinn, H.L.; McCague, P.J.; Jarrahian, C.; Rein-Weston, A.; Coffey, P.S.; Gerth-Guyette, E.; Zehrung, D.; Larrañeta, E.; Donnelly, R.F. Transdermal delivery of vitamin K using dissolving microneedles for the prevention of vitamin K deficiency bleeding. Int. J. Pharm. 2018, 541, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Jang, Y.; Ma, Y.; Dangol, M.; Yang, H.; Jang, M.; Jung, H. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme. Eur. J. Pharm. Sci. 2018, 117, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Seo, S.H.; Kim, S.; Dangol, M.; Shim, J.; Li, C.G.; Ma, Y.; Lee, C.; Kang, G.; Yang, H.; et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials 2018, 167, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Saju, A.; Cheerla, K.; Gade, S.K.; Garg, P.; Venuganti, V.V.K. Corneal delivery of besifloxacin using rapidly dissolving polymeric microneedles. Drug Deliv. Transl. Res. 2018, 8, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Metin, A.U.; Satıroglu, B.; Solmaz, M.E.; Kayser, V.; Mäder, K. Poly (methyl vinyl ether-co-maleic acid)—Pectin based hydrogel forming systems: Gel, film, and microneedles. Eur. J. Pharm. Biopharm. 2017, 117, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Kuang, D.; Wang, S.; Zheng, Z.; Yadavalli, V.K.; Lua, S. Swellable silk fibroin microneedles for transdermal drug delivery. Int. J. Biol. Macromol. 2018, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Barturen, L.; Ervine, M.; Donnelly, R.F. Hydrogels based on poly(methyl vinyl ether-co-maleic acid) and Tween 85 for sustained delivery of hydrophobic drugs. Int. J. Pharm. 2018, 538, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Blenkharn, J.I. Healthcare waste and sharps management. Br. J. Healthc. Manag. 2014, 20, 424–427. [Google Scholar] [CrossRef]
- Grimmond, T.; Bylund, S.; Anglea, C.; Beeke, L.; Callahan, A.; Christiansen, E.; Flewelling, K.; Mclntosh, K.; Richter, K.; Vitale, M. Sharps injury reduction using a sharps container with enhanced engineering: A 28 hospital nonrandomized intervention and cohort study. Am. J. Infect. Control 2010, 38, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Blenkharn, J.I.; Odd, C. Sharps Injuries in Healthcare Waste Handlers. Ann. Occup. Hyg. 2008, 52, 281–286. [Google Scholar]
- Turnberg, W.L. Biohazardous Waste: Risk Assessment, Policy and Management; John Wiley & Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Blenkharn, J.I. Safe disposal and effective destruction of clinical wastes. J. Hosp. Infect. 2005, 60, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.F.; Eggerth, L.L.; Enkhtsetseg, S.H.; Savage, G.M. Characteristics of healthcare waste. Waste Manag. 2008, 28, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Ellenbecker, M.J.; Moure-Eraso, R. Alternatives for treatment and disposal cost reduction of regulated medical waste. Waste Manag. 2004, 24, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.; Sangodoyin, A.; Sridhar, M.; Booth, C.; Olomolaiye, P.; Hammond, F. Medical waste management in Ibadan, Nigeria: Obstacles and prospects. Waste Manag. 2009, 29, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Marincovic, N.; Pavic, T.; Vitale, K.; Holcer, N.J.; Dzakula, A. Management of hazardous medical waste in Croatia. Waste Manag. 2008, 28, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, J.; Ferrer, M.; Ferrer, F. Waste Management and Disposal during the Philippine Follow-Up Measles Campaign; Health Care without Harm: Quezon City, Philippines; Washington, DC, USA; Health Department of the Philippines: Manila, Philippines, 2004; Available online: https://saudesemdano.org/sites/default/files/documents-files/2513/Waste_Mgmt_PMEC_2004.pdf (accessed on 7 June 2018).
- Hossain, M.S.; Santhanam, A.; Norulaini, N.A.N.; Omar, A.K.M. Clinical solid waste management practices and its impact on human health and environment—A review. Waste Manag. 2011, 31, 754–766. [Google Scholar] [CrossRef] [PubMed]
- McConville, A.; Davis, J. Transdermal microneedle sensor arrays based on palladium: Polymer composites. Electrochem. Commun. 2016, 72, 162–165. [Google Scholar] [CrossRef]
- Bennett, N.T.; Howard, R.J. Quantity of Blood Inoculated in a Needlestick Injury from Suture Needles. Obstet. Gynecol. Surv. 1994, 49, 398. [Google Scholar] [CrossRef]
- Gerberding, J.L.; Mast, S.T.; Woolwine, J.D. Efficacy of Gloves in Reducing Blood Volumes Transferred during Simulated Needlestick Injury. J. Infect. Dis. 1993, 168, 1589–1592. [Google Scholar]
| Drug | Polymer | Type | Ref. |
|---|---|---|---|
| Dihydroergotamine mesylate | Polyvinylpyrrolidone | D | [70] |
| Thymopentin | Polyvinylpyrrolidone | D | [71] |
| Exendin-4 | Carboxymethylcellulose | D | [72] |
| Fluorescent Model | Hyaluronic acid/PVA | D | [73] |
| Sumatriptan succinate | Polyvinylpyrrolidone | D | [74] |
| Adenosine | Hyaluronic acid | D | [75] |
| Vitamin K | Gantrez® S-97, a copolymer of methyl vinyl ether and maleic acid | D | [76] |
| Lysozyme | Polyvinylpyrrolidone | D | [77] |
| Valproic acid | Carboxymethylcellulose | D | [78] |
| Besifloxacin | Polyvinylpyrrolidone | D | [79] |
| Caffeine/Theophylline | Hydrolysed poly(methyl-vinyl ether-co-maleic anhydride) and poly(ethyleneglycol) | S-E | [67] |
| None Specified | Poly(methyl vinyl ether-co-maleic acid) and pectin | S | [80] |
| Glucose/Cholesterol | Methacrylated hyaluronic acid | S-E | [68] |
| FITC-dextrans | Silk fibroin | S | [81] |
| Curcumin | Gantrez® S-97 poly(methyl vinyl ether-co-maleic acid) and Tween 85 | S | [82] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McConville, A.; Hegarty, C.; Davis, J. Mini-Review: Assessing the Potential Impact of Microneedle Technologies on Home Healthcare Applications. Medicines 2018, 5, 50. https://doi.org/10.3390/medicines5020050
McConville A, Hegarty C, Davis J. Mini-Review: Assessing the Potential Impact of Microneedle Technologies on Home Healthcare Applications. Medicines. 2018; 5(2):50. https://doi.org/10.3390/medicines5020050
Chicago/Turabian StyleMcConville, Aaron, Catherine Hegarty, and James Davis. 2018. "Mini-Review: Assessing the Potential Impact of Microneedle Technologies on Home Healthcare Applications" Medicines 5, no. 2: 50. https://doi.org/10.3390/medicines5020050
APA StyleMcConville, A., Hegarty, C., & Davis, J. (2018). Mini-Review: Assessing the Potential Impact of Microneedle Technologies on Home Healthcare Applications. Medicines, 5(2), 50. https://doi.org/10.3390/medicines5020050






