The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant
2.2. Extract Preparation
2.3. Phenols and Flavonoids Assay
2.4. Animal Studies
2.4.1. Animal Conditions
2.4.2. Preparation of Diabetic Mice
2.4.3. Drug Administration
2.4.4. Experimental Design
2.4.5. Serum Glucose Assay
2.4.6. Glutathione and Lipid Peroxidation Assay
Tissue Preparation and Subcellular Fractionation
Lipid Peroxidation Assay
Glutathione Assay
2.4.7. Statistical Analysis
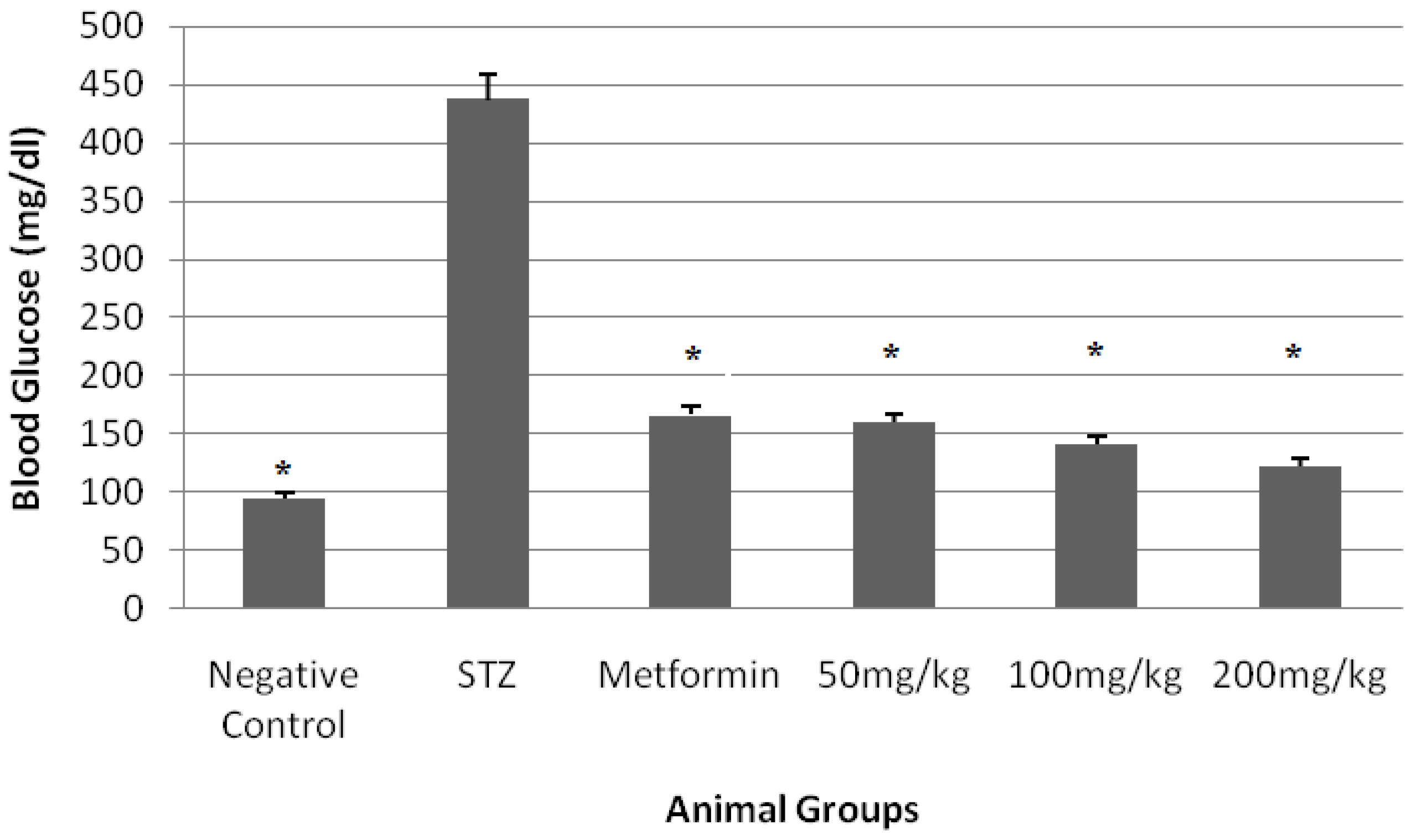
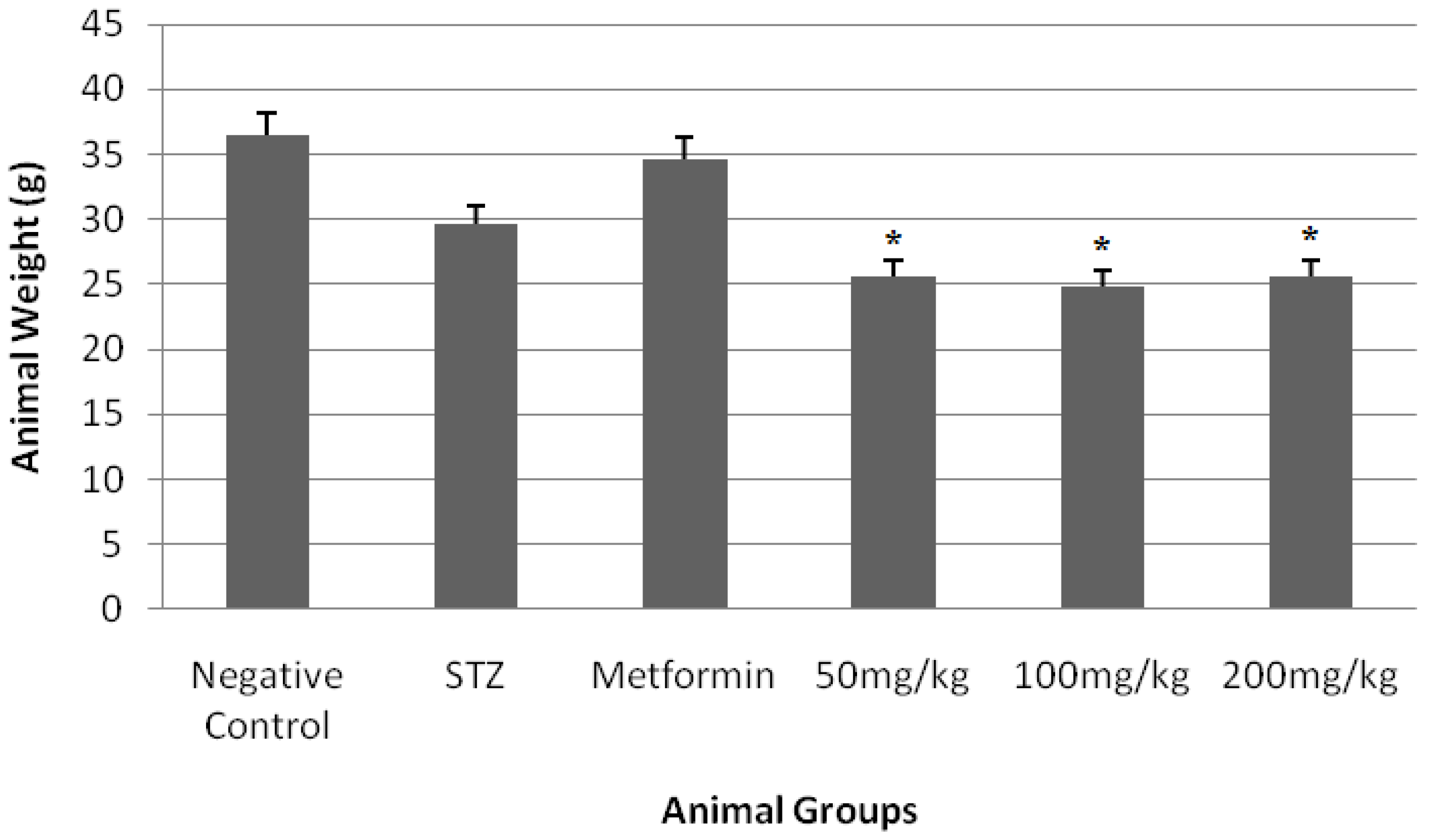
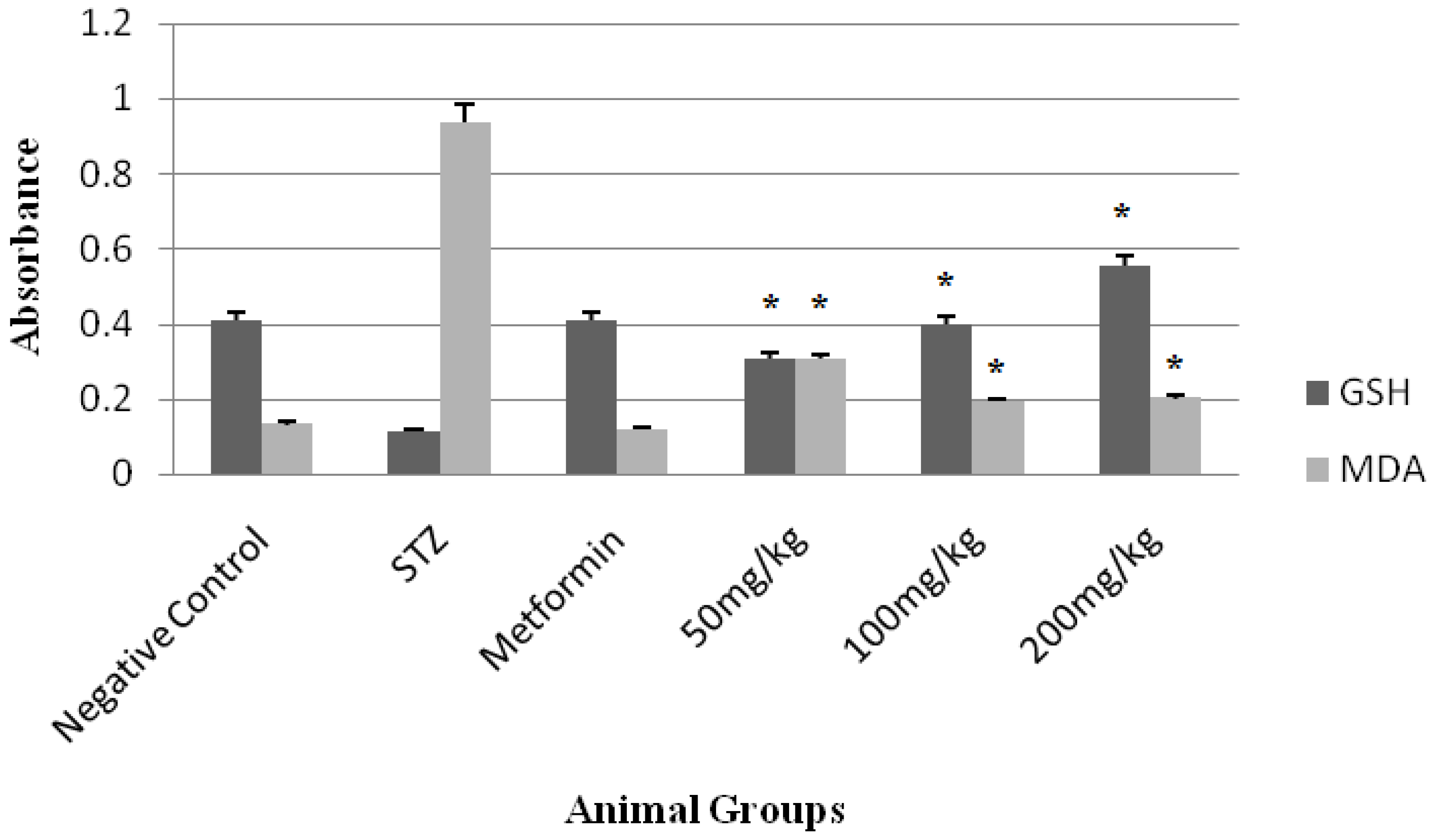
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Unnikrishnan, A. Diabetes secondary to endocrine and pancreatic disorders. Ind. J. Med. Res. 2016, 143, 670. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Müller-Wieland, D. Type 2 Diabetes: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2016; Available online: https://scholar.google.com/scholar?q=Goldstein+BJ (accessed on 31 December 2017).
- Guariguata, L.; Whiting, D.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.; Mazumder, P. Methanolic Extract of Curcuma caesia Roxb. Prevents the Toxicity Caused by Cyclophosphamide to Bone Marrow Cells, Liver and Kidney of Mice. Pharmacogn. Res. 2016, 8, 43–49. [Google Scholar] [CrossRef]
- Panjeshahin, M.R.; Azadbakht, M.; Akbari, N. Antidiabetic Activity of Different Extracts of Myrtus Communis in Streptozotocin Induced Diabetic Rats. Rom. J. Diabetes Nutr. Metab. Diseases 2016, 23, 183–190. [Google Scholar] [CrossRef]
- Mirzaee, F.; Hosseini, A.; Jouybari, H.B.; Davoodi, A.; Azadbakht, M. Medicinal, biological and phytochemical properties of Gentiana species. J. Tradit. Complement. Med. 2017, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Gharaghani, A.; Solhjoo, S.; Oraguzie, N. A review of genetic resources of pome fruits in Iran. Genet. Resour. Crop Evol. 2016, 63, 151–172. [Google Scholar] [CrossRef]
- Rop, O.; Sochor, J.; Jurikova, T.; Zitka, O.; Skutkova, H.; Mlcek, J.; Salas, P.; Krska, B.; Babula, P.; Adam, V.; et al. Effect of five different stages of ripening on chemical compounds in medlar (Mespilus germanica, L.). Molecules 2011, 16, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Naghdi, N.; Rafieian-Kopaei, M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Yazdi, F.; Alizadeh-Behbahani, B.; Zanganeh, H. The Comparison among Antibacterial Activity of Mespilus germanica Extracts and Number of Common Therapeutic Antibiotics “In Vitro”. Zahedan J. Res. Med. Sci. 2015, 17, 29–34. [Google Scholar] [CrossRef]
- Avram, M.; Stoica, A.; Dobre, T.; Stroescu, M. Extraction of vegetable oil from ground seeds by percolation techniques. UPB Sci. Bull. B 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Do, Q.; Angkawijaya, A.; Tran-Nguyen, P.; Huynh, L.; Soetaredjo, F.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Shokri, G.; Fathi, H.; Jafari Sabet, M.; Nasri Nasrabadi, N.; Ataee, R. Evaluation of anti-diabetic effects of hydroalcoholic extract of green tea and cinnamon on streptozotocin-induced diabetic rats. Pharm. Biomed. Res. 2015, 1, 20–29. [Google Scholar] [CrossRef]
- Kabir, M.; Al Noman, M.; Rahman, M.; Ara, J.; Hossain, M.; Hasanat, A.; Zaheed, F. Antibacterial activity of organic and aqueous extracts of Hopea odorata Roxb. leaves and their total flavonoid content. Br. Microbiol. Res. J. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Khuwaja, G.; Khan, M.; Ishrat, T.; Ahmad, A.; Raza, S.; Ashafaq, M.; Javed, H.; Khan, M.B.; Khan, A.; Vaibhav, K.; et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: Behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011, 1368, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Hosseini, M.-J.; Ghazi-Khansari, M.; Pourahmad, J. Depleted uranium induces disruption of energy homeostasis and oxidative stress in isolated rat brain mitochondria. Metallomics 2013, 5, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Kharouta, M.; Miller, K.; Kim, A.; Wojcik, P.; Kilimnik, G.; Dey, A.; Steiner, D.F.; Hara, M. No mantle formation in rodent islets—The prototype of islet revisited. Diabtes Res. Clin. Pract. 2009, 85, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hatice, B.; Mine, G.; LaIe, G.; Galip, M.D.; Dogukan, C.; Halis, S.; Zekai, H.; Nurcan, K.B. Effects of Aqueous Extract of Myrtus Communis, L. Leaves on Streptozotocin-Induced Diabetic Rats. J. Res. Med. Dent. Sci. 2016, 4. [Google Scholar] [CrossRef]
- Li, S.; Brault, A.; Sanchez Villavicencio, M.; Haddad, P.S. Rhododendron groenlandicum (Labrador tea), an antidiabetic plant from the traditional pharmacopoeia of the Canadian Eastern James Bay Cree, improves renal integrity in the diet-induced obese mouse model. Pharm. Biol. 2016, 54, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Shantha, T.; Patchaimal, P.; Reddy, M.P.; Kumar, R.K.; Tewari, D.; Bharti, V.; Venkateshwarlu, G.; Mangal, A.K.; Padhi, M.M.; Dhiman, K.S. Pharmacognostical Standardization of Upodika-Basella alba, L.: An Important Ayurvedic Antidiabetic Plant. Anc. Sci. Life 2016, 36, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Bazaz, R.D.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. DARU J. Pharm. Sci. 2012, 20, 37. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiee, F.; Khoshvishkaie, E.; Davoodi, A.; Dashti Kalantar, A.; Bakhshi Jouybari, H.; Ataee, R. The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice. Medicines 2018, 5, 1. https://doi.org/10.3390/medicines5010001
Shafiee F, Khoshvishkaie E, Davoodi A, Dashti Kalantar A, Bakhshi Jouybari H, Ataee R. The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice. Medicines. 2018; 5(1):1. https://doi.org/10.3390/medicines5010001
Chicago/Turabian StyleShafiee, Fatemeh, Elnaz Khoshvishkaie, Ali Davoodi, Ayat Dashti Kalantar, Hossein Bakhshi Jouybari, and Ramin Ataee. 2018. "The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice" Medicines 5, no. 1: 1. https://doi.org/10.3390/medicines5010001
APA StyleShafiee, F., Khoshvishkaie, E., Davoodi, A., Dashti Kalantar, A., Bakhshi Jouybari, H., & Ataee, R. (2018). The Determination of Blood Glucose Lowering and Metabolic Effects of Mespilus germanica L. Hydroacetonic Extract on Streptozocin-Induced Diabetic Balb/c Mice. Medicines, 5(1), 1. https://doi.org/10.3390/medicines5010001




