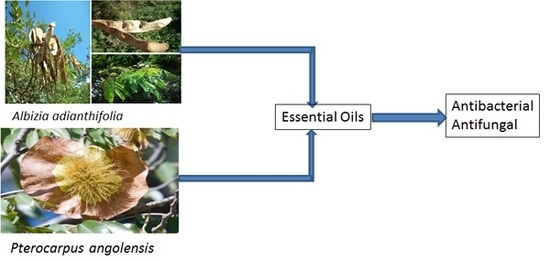
GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Materials
2.2. Extraction Method
2.3. Procedure for GC-MS Analysis
2.4. Procedure for Antimicrobial Activity
3. Results and Discussions
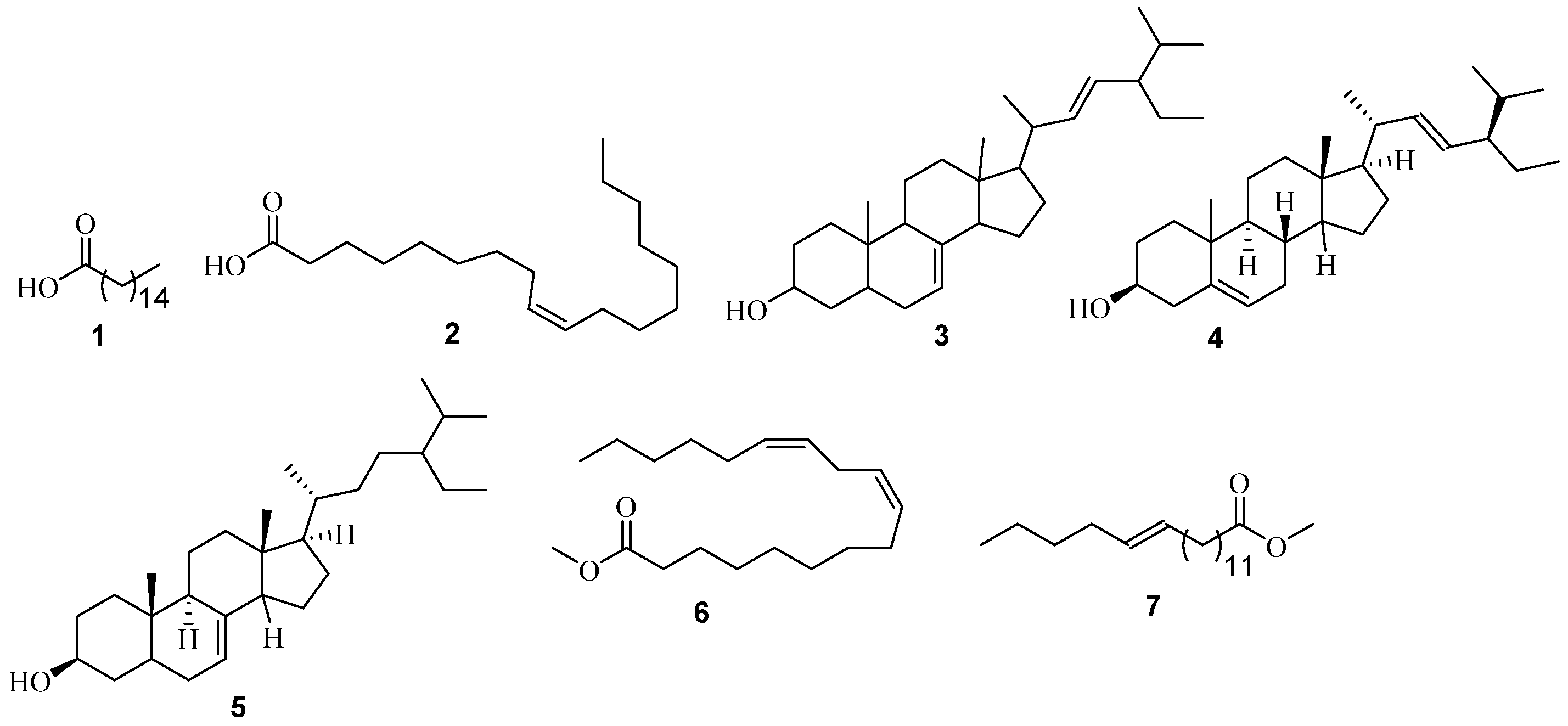
3.1. GC-MS Analysis
3.1.1. GC-MS Analysis of A. adianthifolia
| Species | Compd. Name/No | Mol. Formula | Mol. Wt. | NIST Match Factor: Forward, Reverse | RT (min) | Peak Area (%) | Reported Bioactivity |
|---|---|---|---|---|---|---|---|
| A. adianthifolia (n-hexane extract) | n-hexadecanoic acid 1 | C16H32O2 | 256 | 858, 880 | 16.65 | 34.85 | Anti-inflammatory [15], Antioxidant, hypocholesterolemic nematicide, pesticide, anti androgenic flavor, hemolytic, 5-Alpha reductase inhibitor [16], potent mosquito larvicide [17]. |
| oleic acid 2 | C18H34O2 | 282 | 754, 892 | 18.27 | 6.28 | Antibacterial [18]. | |
| chondrillasterol 3 | C29H48O | 412 | 805, 935 | 29.29 | 18.23 | Cytotoxicity [19]. | |
| stigmasterol 4 | C29H48O | 412 | 818, 830 | 29.30 | 28.64 | Thyroid inhibitory, antiperoxidative and hypoglycemic effects [20]. | |
| 24S, 5α stigmast-7-en-3β-ol 5 | C29H50O | 414 | 809, 930 | 30.02 | 4.37 | Antimutagenic [21]. | |
| Total = 92.37 | |||||||
| A. adianthifolia (chloroform extract) | 9,12-octadecadienoic acid (Z,Z)-, methyl ester 6 | C19H34O2 | 294 | 750, 895 | 17.76 | 17.58 | Anti-cancer [22]. |
| trans-13-octadecanoic acid, methyl ester 7 | C19H36O2 | 296 | 857, 907 | 17.81 | 37.23 | Anti-inflammatory, antiandrogenic, cancer preventive, dermatitigenic, irritant, antileukotriene—D4, hypocholesterolemic, 5-alpha reductase inhibitor, anemiagenic, insectifuge, flavor [23]. | |
| Total = 54.81 | |||||||
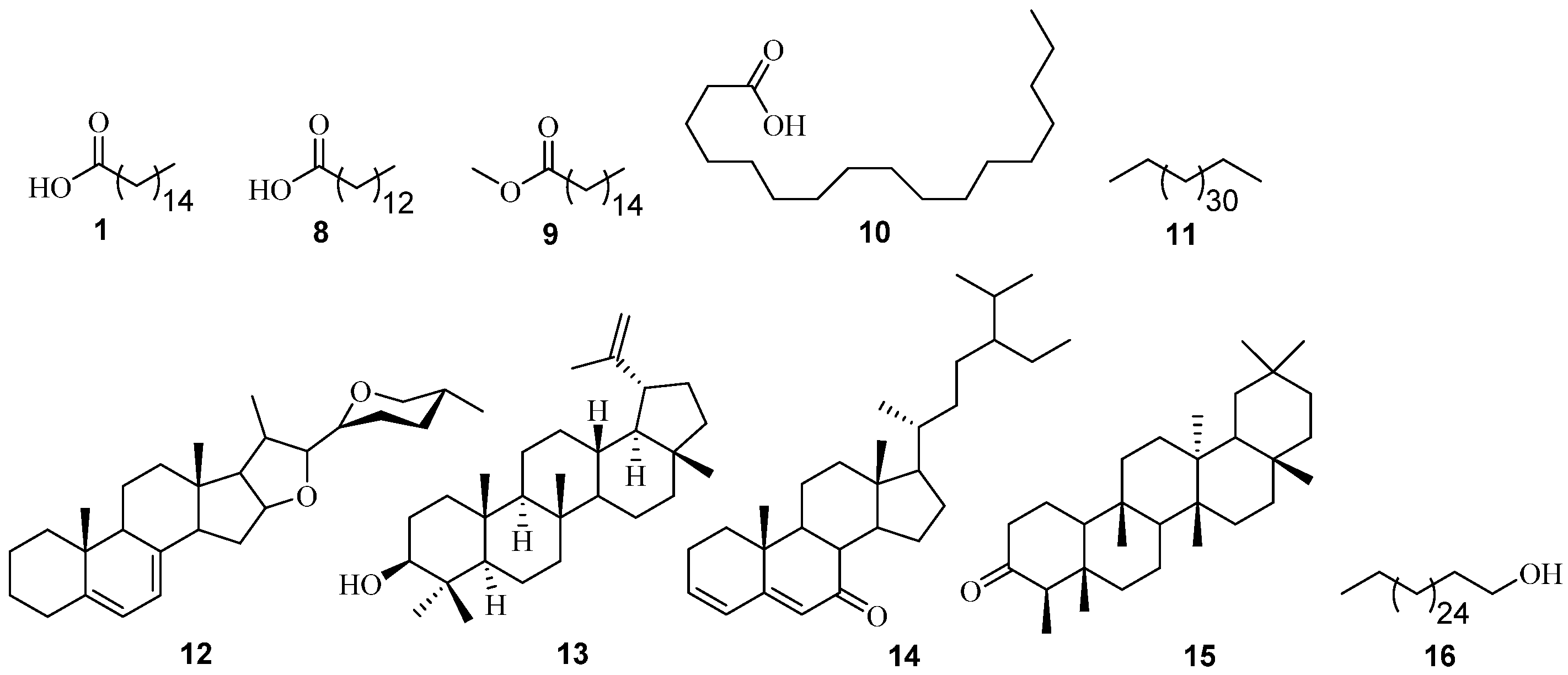
3.1.2. GC-MS Analysis of P. angolensis
| Species | Compd. Name/No. | Mol. Formula | Mol. Wt. | NIST Match Factor: Forward, Reverse | RT (min) | Peak Area (%) | Reported Bioactivity |
|---|---|---|---|---|---|---|---|
| P. angolensis (n-hexane extract) | tetradecanoic acid 8 | C14H28O2 | 228 | 835, 902 | 14.52 | 1.84 | Larvicidal and repellent activity [24]. |
| hexadecanoic acid, methyl ester 9 | C17H34O2 | 270 | 879, 903 | 16.15 | 1.84 | Antibacterial and antifungal [25] | |
| n-hexadecanoic acid 1 | C16H32O2 | 256 | 864, 879 | 16.70 | 10.29 | See above | |
| octadecanoic acid 10 | C18H36O2 | 284 | 780, 825 | 18.48 | 5.89 | Antimicrobial activity [17]. | |
| tetratriacontane 11 | C34H70 | 478 | 759, 874 | 24.79 | 31.67 | Antibacterial and antifungal [26] | |
| 7-dehydrodiosgenin 12 | C27H40O3 | 412 | 721, 736 | 26.59 | 9.58 | Antibacterial, antifungal,, antioxidant, cytotoxic [27,28] | |
| lupeol 13 | C30H50O | 426 | 832, 897 | 30.48 | 6.54 | Anti-inflammatory activity [29], Anti-cancer [30]. | |
| stigmasta-3,5-dien-7-one 14 | C29H46O | 410 | 817, 864 | 30.66 | 7.13 | Free radical scavenging? Anti-diabetic, anticancer? [31,32] | |
| friedelan-3-one 15 | C30H50O | 426 | 882, 903 | 32.91 | 2.56 | Antibacterial, antifungal, anti-inflammatory, analgesic, antipyretic, antihypertensive [33,34] | |
| Total = 77.34 | |||||||
| P. angolensis (chloroform extract) | * 1-octacosanol 16 | C28H58O | 410 | 837, 918 813, 891 | 26.44 28.61 | 37.73 62.27 | Antioxidant [35]. |
| Total = 100 | |||||||
3.2. Structure Elucidation of Compound 16
| Position | δH(ppm) | δC (ppm) |
|---|---|---|
| 1 | 3.69, 2H, br s * | 63.1 |
| 2 | 2.22, 2H, s * | 32.9 |
| 3 | 1.63, 2H, s * | 32.0 |
| 4–27 | 1.35–1.30, 49H, s | 29.7–22.7 |
| 28 | 0.93, 3H, t, (6.6) | 14.1 |
3.3. Result for the Preliminary Antimicrobial Assay
| Species | Part | Extract | Microbial Strains and MIQ (μg) | ||||
|---|---|---|---|---|---|---|---|
| Gram+ve Bacteria | Gram−ve Bacteria | Fungus | |||||
| E. coli | P. aeruginosa | B. subtilis | S. aureus | C. albicans | |||
| A. adianthifolia | heartwood | n-hexane | 1 | 50 | 50 | 50 | >100 |
| A. adianthifolia | heartwood | Chloroform | 1 | 50 | 50 | 50 | >100 |
| P. angolensis | stem bark | n-hexane | >100 | >100 | 100 | >100 | 100 |
| P. angolensis | stem bark | Chloroform | >100 | >100 | 50 | >100 | 100 |
| Chloramphenicol | 0.50 | 10 | 0.25 | 0.50 | N.A | ||
| Miconazole | N.A | N.A | N.A | N.A | 0.25 | ||
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Abdel-Kader, M.; Hoch, J.; Berger, J.M.; Evans, R.; Miller, J.S.; Wisse, J.H.; Mamber, S.W.; Dalton, J.M.; Kingston, D.G.I. Two bioactive saponins from Albizia subdimidiata from the Suriname rainforest. J. Nat. Prod. 2001, 64, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Uzokwe, N.E.; Igboanugo, A.B.I.; Adio, A.F.; Awosan, E.A.; Nwogwugwu, J.O.; Faloye, B.; Olatunji, B.P.; Adesoga, A.A. Ethno-medicinal information on collation and identification of some medicinal plants in research institutes of South-west Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 1–7. [Google Scholar]
- Tamokou, J.; Mpetga, D.J.S.; Lunga, P.K.; Tene, M.; Tane, P.; Kuiate, J.R. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae). BMC Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.P. Pterocarpus (Leguminoseae-Papillinaceae) Revised for the World; Verlag Von J Cramer: Lehre, Germany, 1972. [Google Scholar]
- King, F.E.; King, T.J.; Warwick, A.J. The chemistry of extractives from hardwoods. Part VI. Constituents of muninga, the heartwood of Pterocarpus Angolensis, A. : 6: 4′-dihydroxy-5 : 7-dimethoxyisojavone (muningin). J. Chem. Soc. 1952. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Klitgaard, B.B.; Forest, F.; Francis, L.; Savolainen, V.; Williamson, E.M.; Hawkins, J.A. The use of phylogeny to interpret cross-cultural patterns in plant use and guide medicinal plant discovery: An example from Pterocarpus (Leguminosae). PloS One 2011. [Google Scholar] [CrossRef] [PubMed]
- Ama, I.U.; David, C.O.; UcheOrji, O.; Maduabuchi, A.P.; Chukwu, C.; Obasi, J.N. GC-MS analysis, acute toxicity and oxidative stress potentials (effects) of Albizia chevalieri extract on juvenile African catfish (Clarias gariepinus). Middle East J. Sci. Res. 2015, 23, 192–199. [Google Scholar]
- Maruthupandian, A.; Mohan, V.R. GC-MS analysis of some bioactive constituents of Pterocarpus marsupium Roxb. Int. J. Chem. Tech. Res. 2011, 3, 1652–1657. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.T.; Sparkman, O.D. Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation, 4th ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 433–441. [Google Scholar]
- Saxena, G.; Farmer, S.; Towers, G.H.N.; Hancock, R.E.W. Use of specific dyes in the detection of antimicrobial compounds from crude plant extracts using a thin layer chromatography agar overlay technique. Phytochem. Anal. 1995, 6, 125–129. [Google Scholar] [CrossRef]
- Rahalison, M.; Hamburger, M.; Hostettmann, K. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem. Anal. 1991, 2, 199–203. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Awa, E.P.; Ibrahim, S.; Ameh, D.A. GC/MS analysis and antimicrobial activity of diethyl ether fraction of methanolic extract from the stem bark of Annona senegalensis Pers. Int. J. Pharm. Sci. Res. 2012, 3, 4213–4218. [Google Scholar]
- Chen, J.J.; Duh, C.Y.; Chen, I.S. Cytotoxic chromenes from Myriactis humilis. Planta Med. 2005, 71, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B.K. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Guevara, A.P.; Amor, E.; Russell, G. Antimutagens from Plumeria acuminata ait. Mutat. Res. Environ. Mutagen. Relat. Subj. 1996, 361, 67–72. [Google Scholar] [CrossRef]
- Yu, F.R.; Lian, X.Z.; Guo, H.Y.; McGuire, P.M.; Li, R.D.; Wang, R.; Yu, F.H. Isolation and characterization of methyl esters and derivatives from Euphorbia kansui (Euphorbiaceae) and their inhibitory effects on the human SGC-7901 cells. J. Pharm. Pharm. Sci. 2005, 8, 528–535. [Google Scholar] [PubMed]
- Krishnamoorthy, K.; Subramaniam, P. Phytochemical profiling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) Gandhi Using GC-MS. Int. Sch. Res. Not. 2014. [Google Scholar] [CrossRef]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmcol. Sci. 2011, 15, 775–780. [Google Scholar]
- Mahmood, A.; Ahmed, R.; Kosar, S. Phytochemical screening and biological activities of the oil components of Prunus domestica Linn. J. Saudi Chem. Soc. 2009, 13, 273–277. [Google Scholar] [CrossRef]
- Reddy, K.S.; Shekhami, M.S.; Berry, D.E.; Lynn, D.G.; Hecht, S.M. Afrontoside a new cytotoxic principle from Dracaena afromontana. J. Chem. Soc. Perkin Trans. 1984, 5, 987–992. [Google Scholar] [CrossRef]
- Karabay-Yavasoglu, N.U.; Sukatar, A.; Ozdemir, G.; Horzum, Z. Antimicrobial activity of volatile components and various extracts of the red algae Jania rubens. Phytother. Res. 2007, 21, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001, 76, 77–80. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Balogun, O.S.; Oladosu, I.A.; Akiinnusi, A.; Zhiqiang, L. Fatty acid composition α-glucosidae inhibitory potential and cytotoxicity activity of Oncoba spinosa Forssk. Elix. Appl. Chem. 2013, 59, 15637–15641. [Google Scholar]
- Delazar, A.; Nazifi, E.; Movafeghi, A.; Nazemiyey, H.; Hemmati, S.; Nahar, L.; Sarker, S.D. Analyses of phytosterols and free radical scavengers in the bulbs of Ornithogalum cuspidatum Bertol. Bol. Latinoam. Caribe Plant. Med. Aromat. 2010, 9, 87–92. [Google Scholar]
- Antonisamy, P.; Duraipandiyan, V.; Ignacimuthu, S. Anti-inflammatory, analgesic and antipyretic effects of friedelin isolated from Azima tetracantha Lam. in mouse and rat models. J. Pharm. Pharmacol. 2011, 63, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Chakraborty, P.; Basak, G. Antibacterial, antifungal and phytotoxic screening of some prepared pyrazine derivatives in comparison to their respective α-diketo precursors. Int. J. Pharm. Sci. Res. 2011, 2, 1687–1692. [Google Scholar]
- Firdous, S.; Khan, K.; Zikr-Ur-Rehman, S.; Ali, Z.; Soomro, S.; Ahmad, V.U.; Rasheed, M.; Mesaik, M.A.; Faizi, S. Isolation of phytochemicals from Cordia rothii (Boraginaceae) and evaluation of their immunomodulatory properties. Rec. Nat. Prod. 2014, 8, 51–55. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, M.N.; Majinda, R.R.T. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 2016, 3, 3. https://doi.org/10.3390/medicines3010003
Abubakar MN, Majinda RRT. GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines. 2016; 3(1):3. https://doi.org/10.3390/medicines3010003
Chicago/Turabian StyleAbubakar, Mustapha N., and Runner R. T. Majinda. 2016. "GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC)" Medicines 3, no. 1: 3. https://doi.org/10.3390/medicines3010003
APA StyleAbubakar, M. N., & Majinda, R. R. T. (2016). GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines, 3(1), 3. https://doi.org/10.3390/medicines3010003