1. Introduction
1.1. Background
Febrile neutropenia (FN) is a life-threatening complication of patients with cancer [
1,
2]. In the U.S., in 2012 there were 91,560 adult cancer-related neutropenia hospitalizations, with a total cost of USD 2.3 billion and an average of 9.6 hospital days [
1]. Since then, for many reasons (increased cancer diagnosis, etc.), these numbers have likely only increased. Additionally, the risk of infection increases with the degree and duration of neutropenia [
2,
3,
4]. Patients with FN have a 30% risk of developing end-organ dysfunction and an 11% risk of mortality [
2,
3,
4]. Furthermore, FN patients with sepsis have a 50% mortality risk [
2,
3,
4]. Clinical guidelines developed by the American Society of Clinical Oncology (ASCO) and the Infectious Diseases Society of America (IDSA) recommend that the first empiric antibiotic dose be administered within one hour of triage in an effort to improve outcomes [
2,
3]. However, with average emergency department (ED) wait times to see a provider exceeding 15 min, a 60 min time to antibiotic administration (TTA) is very difficult to achieve [
5].
1.2. Importance
At the time of this study, during our search, only five articles published since 2009 were identified when searching OVID and PubMed for the specific words “febrile neutropenia time to administration”, and all except one were pediatric studies that investigated TTA for patients with FN [
6,
7,
8,
9,
10,
11,
12]. Only one adult publication provided a suggested process towards achieving guideline-recommended goals [
9]. Therefore, improving time to administration for patients with febrile neutropenia is likely an opportunity for many emergency departments.
1.3. Goals of This Investigation
This study was designed to evaluate whether a nursing and pharmacy ED triage screening tool and protocol improves TTA and patient outcomes.
2. Methods
2.1. Study Design and Setting
This was a retrospective, quasi-experimental study of FN patients who presented to the ED at an 808-bed not-for-profit, community-teaching level II trauma hospital from April 2014 to April 2017. Renown Health receives over 100,000 annual emergency department visits, with over 31,000 annual admissions, and Renown’s Institute for Cancer offers services in surgical, medical, and radiation oncology.
2.2. Selection of Participants
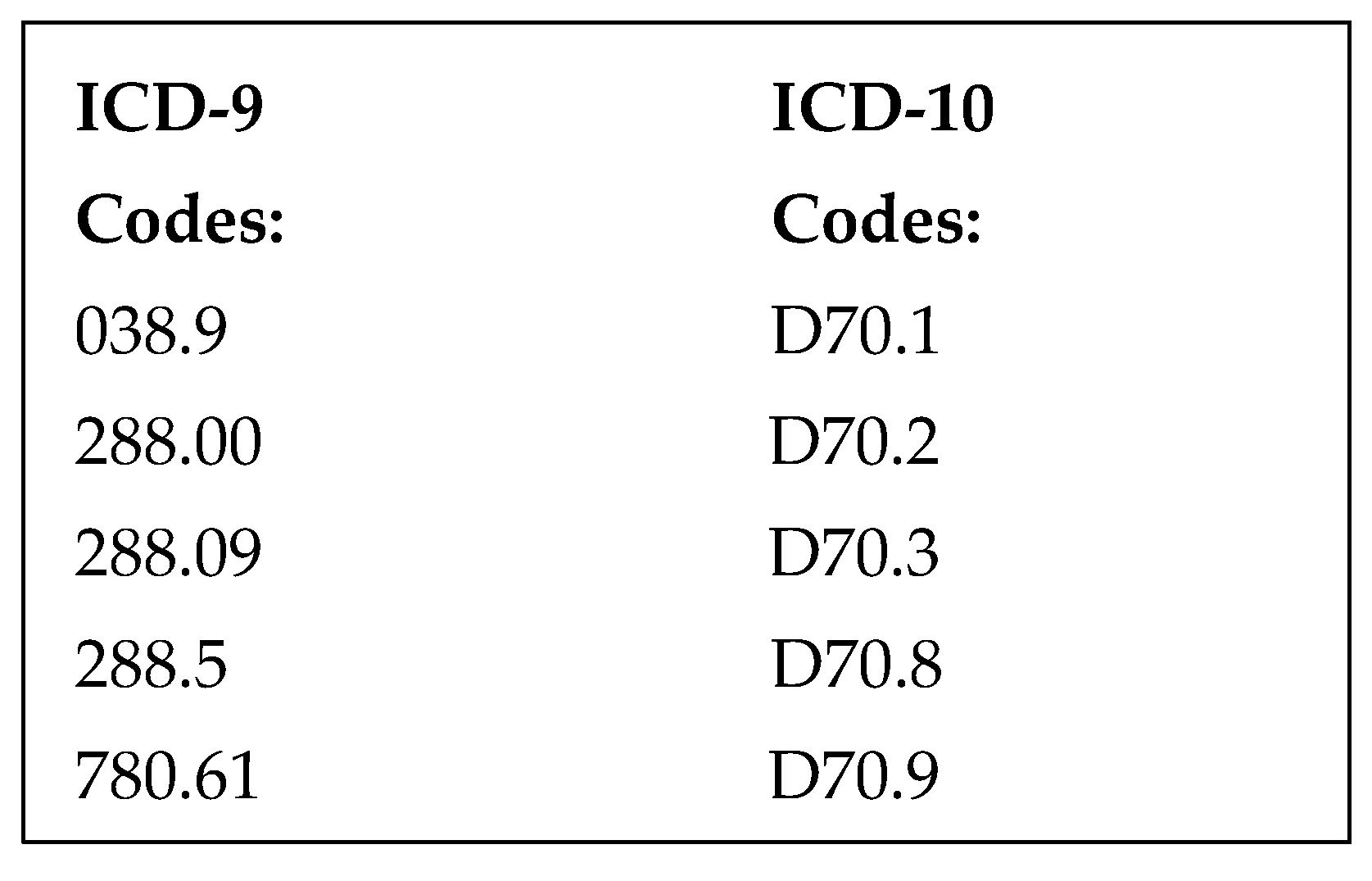
Patients were included in the study if they had an ICD-9 or ICD-10 code for neutropenia, cancer, or chemotherapy (
Figure 1) and were admitted to the hospital through the ED with fever and neutropenia. Fever was defined as either ≥100.4 °F (38 °C) or <96.5 °F (35.8 °C) within 24 h of presentation or fever prior to arrival with the use of acetaminophen or NSAIDs. Neutropenia was defined as an absolute neutrophil count (ANC) < 1000 per microliter within 24 h of admission. Patients were excluded if they were <18 years of age, pregnant, or prisoners.
2.3. Interventions
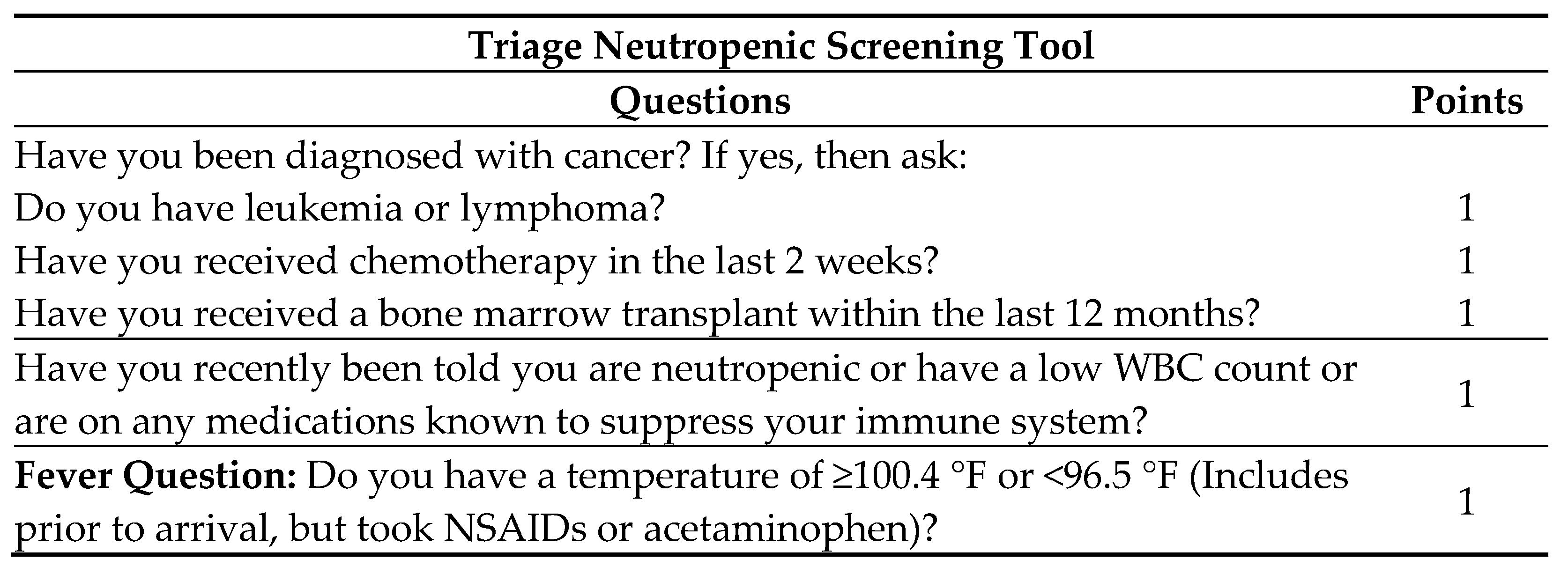
During patient triage in the ED, the neutropenic triage screening tool is completed by nursing (
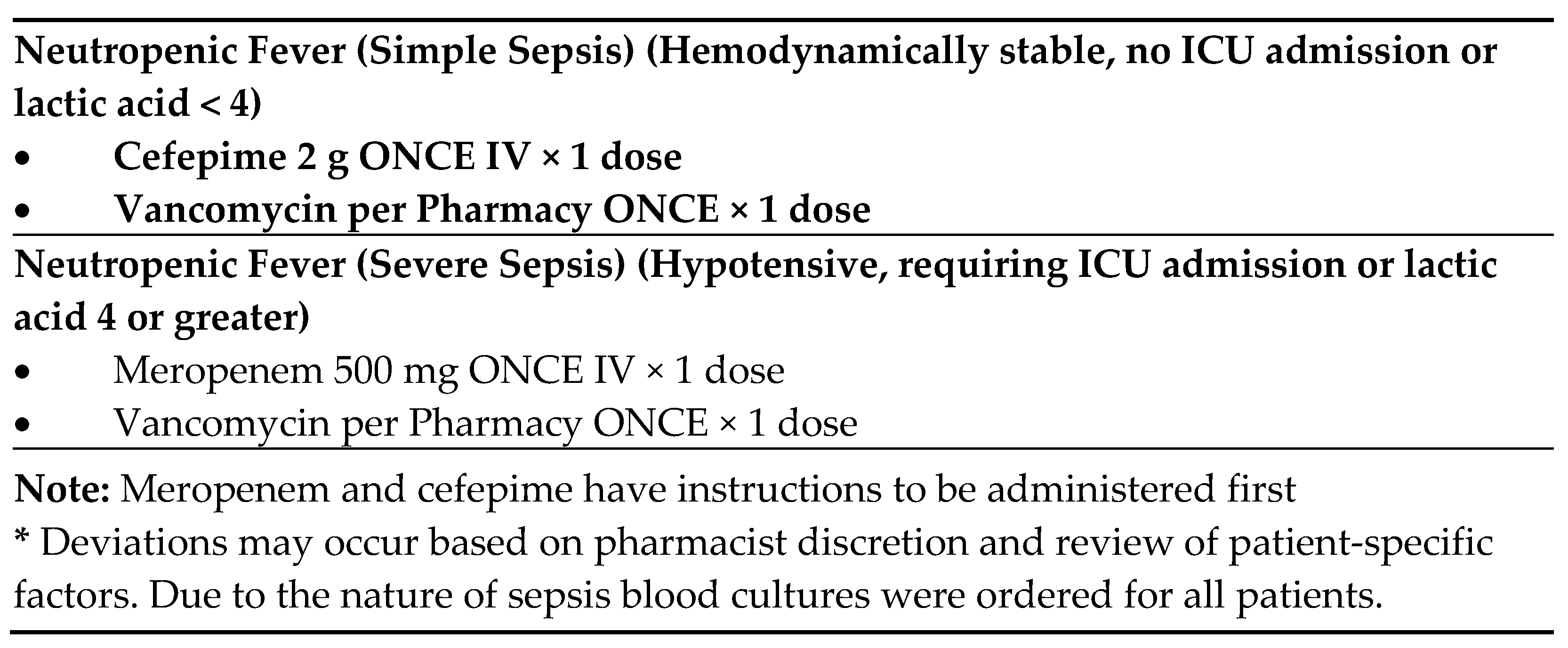
Figure 2). If positive on screening, the protocol order set is initiated. A positive triage screen is a score of 2 or more, with 1 point coming from the question pertaining to fever. The protocol includes orders for cultures from potential infectious sites, diagnostic laboratory tests, and a “nursing communication alert” for nursing to notify the physician and pharmacy of the patient, and for a pharmacy consult to initiate broad-spectrum antibiotics. If no antibiotic orders from the physician are received within 15 min from receipt of the consult, the pharmacist will initiate recommended antibiotics (
Figure 3) independently of the physician per protocol. Deviations from recommendations may occur based on pharmacist discretion and review of patient-specific factors. This process occurs roughly 30 min after presentation to the ED. All of this is an effort to reach a TTA of under 60 min.
2.4. Data Collection
Data was collected from the electronic medical record. State death certificate data was collected in conjunction with Desert Research Institute, as contracted with Renown Health. Patient baseline characteristics gathered from the EMR included age, weight, height, gender, ethnicity, allergy information, admission diagnosis, ICD-10 and ICD-9 diagnosis, first temperature recorded, maximum temperature recorded during hospital stay, minimum absolute neutrophil count (ANC) recorded, maximum ANC recorded, febrile neutropenia screening score and flowsheet information, source of infection, time to first antibiotic, first antibiotic administered, microbiology results, ED length of stay, ED door-to-doctor time, hospital length of stay, and 30-day mortality.
2.5. Outcomes
The primary outcome was time to first antibiotic. This was defined as the time from arrival at the ED until the first antibiotic was administered. Secondary outcomes included: 30-day mortality, ED length of stay, and hospital length of stay.
2.6. Statistical Analysis
Statistical analyses were performed with the SAS statistical program. Chi-squared tests and Fisher’s Exact Tests were used for categorical data and Wilcoxon Rank-Sum Tests for continuous variables. A p value of <0.05 was considered statistically significant. Power analysis estimated that 139 total patients were required to demonstrate a 30% reduction in time to administration of antibiotics with a 95% confidence interval.
This study was approved by the institutional review board: 1295724-1.
3. Results
3.1. Characteristics of Study Subjects
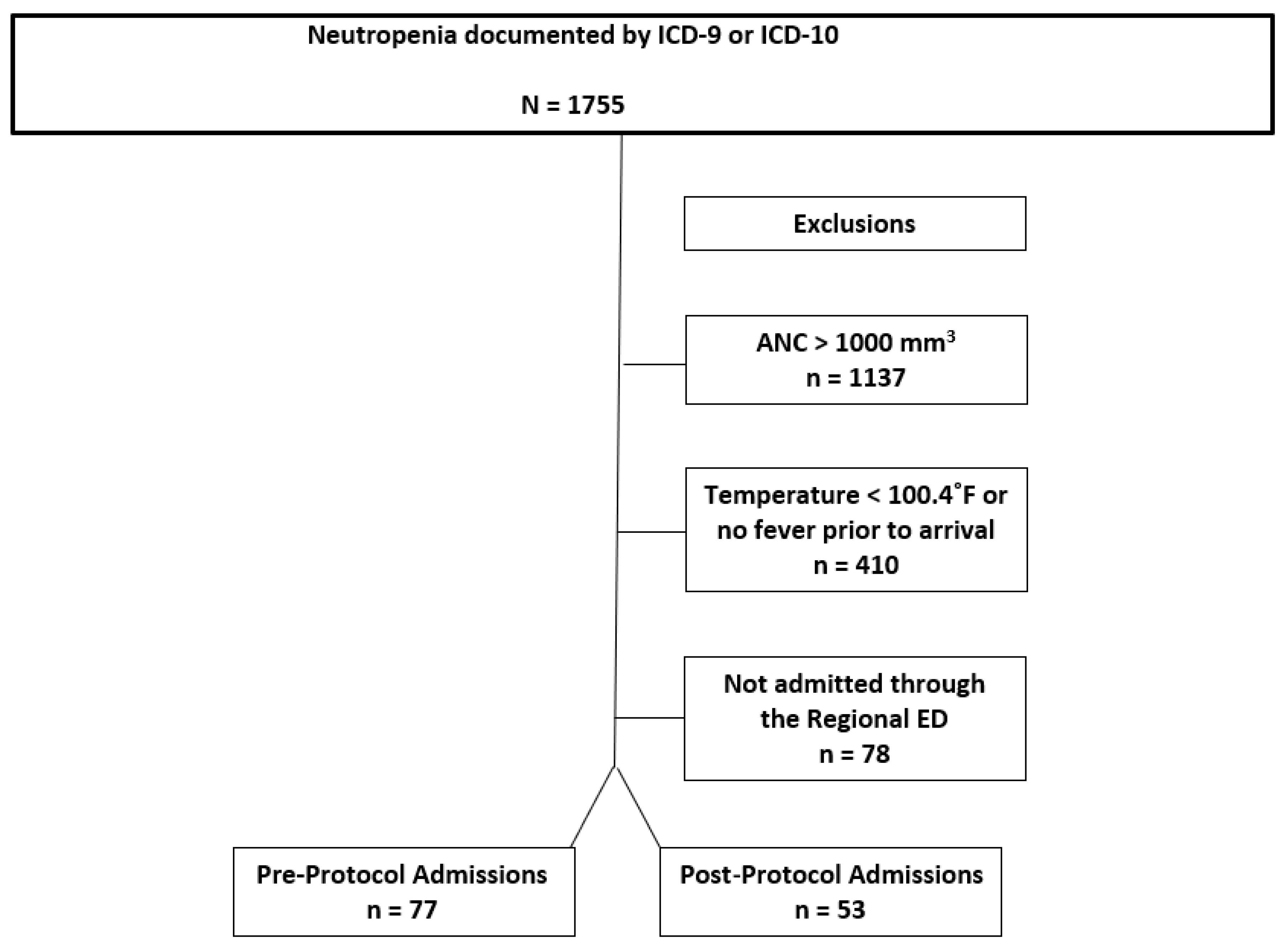
We identified 1755 ED visits by qualifying ICD-9 or ICD-10 codes (
Figure 1) from April 2014 to April 2017. There were 1137 patients excluded who were not neutropenic by a priori definition, 410 were excluded because they did not meet fever criteria, and 78 were excluded because they were not admitted through the emergency department (
Figure 4). Therefore 130 patients were included in the analysis: 77 as pre-protocol admissions and 53 as post-protocol admissions. The characteristics for all episodes of FN are shown in
Table 1. The mean age of our study population was 59 (SD 16) in the pre-protocol and 55 (SD 20) in the post-protocol group. Both groups were predominantly composed of Caucasian males. One notable observed characteristic was that there was a higher percentage (49%) of post-protocol patients with profound neutropenia. Also, the neutropenic triage screen was successfully completed by nursing in 81% of triaged patients. Roughly 30% of the culture results were positive for both groups, and the predominant positive source was blood for both groups. Of note, patients could have more than one organism isolated from more than one source. It was observed that
Pseudomonas aeruginosa was only identified in four results, MRSA was only identified in one result, and only two results of
Enterococcus spp. were seen over the 3-year study period.
3.2. Main Results
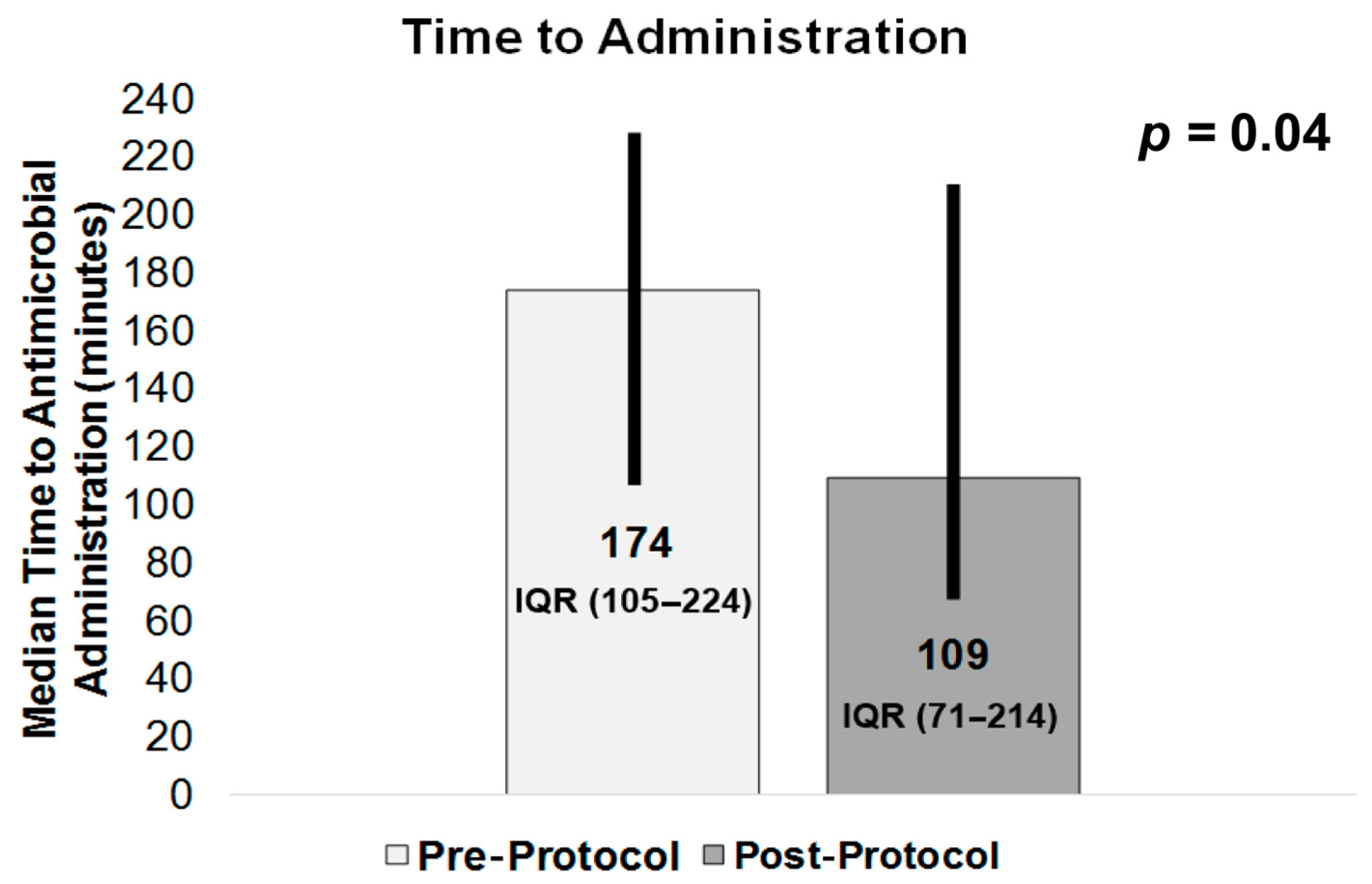
TTA, our primary outcome (shown in
Figure 5), was 174 min (IQR 105–224) in the pre-protocol group vs. 109 min (IQR 71–214) in the post-protocol group (
p = 0.04), with TTA assumed a priori to be reduced post-protocol. Among our secondary outcomes, the results for 30-day mortality (
Table 2) were 14 deaths (18%) in the pre-protocol group vs. 4 deaths (8%) in the post-protocol group (
p = 0.12). Median (IQR) hospital length, in days, was 5 (3–8) for the pre-protocol group vs. 4 (3–7) for the post-protocol group (
p = 0.003). ED median (IQR) length of stay was 301 min (231–339) for the pre-protocol group and 365 min (265–445) for the post-protocol group (
p = 0.003).
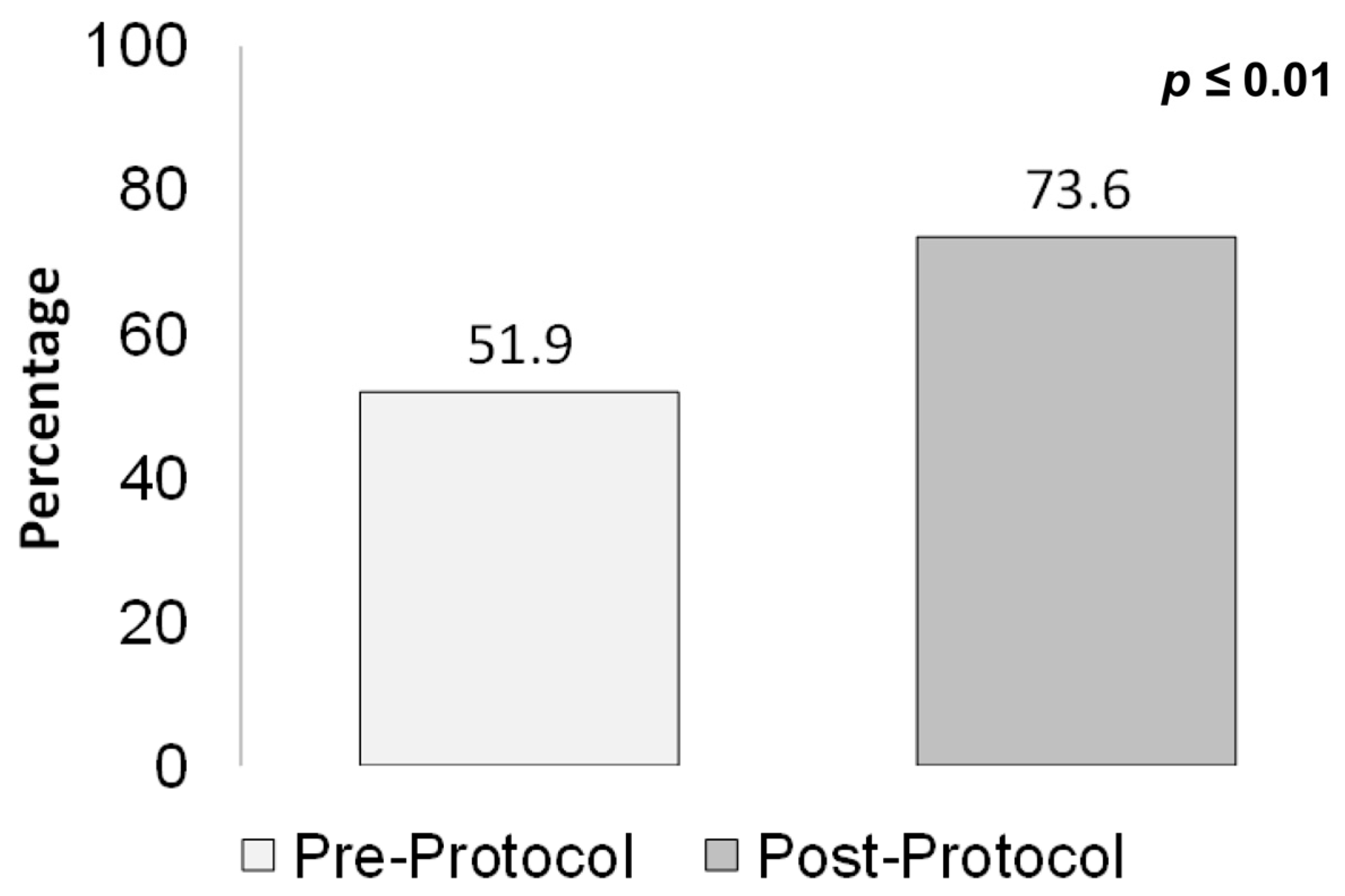
The proportion of patients who were administered a first dose of anti-pseudomonal beta-lactams (
Figure 6), in accordance with guideline recommendations, was 52% pre-protocol vs. 74% post-protocol (
p ≤ 0.01).
The data was further analyzed with a subgroup analysis of those patients who screened positive on the triage screen. A positive triage screen was equal to a score of ≥two on the neutropenic triage screening tool. Time to administration in this subgroup analysis, shown in
Table 3, was a TTA of 87.5 min in the positive triage screen group vs. 174 min in the pre-protocol group (
p < 0.01). Additionally, 30-day mortality in this group showed no mortalities in those who screened positive on the triage screen vs. 14 mortalities in the pre-protocol group (
p = 0.04).
4. Limitations
This study had several limitations. Because this study was a single-center retrospective chart review, there was a potential for bias, particularly in the way patients were identified. ICD-9 or ICD-10 code documentation is included in the diagnostic process, and charting may have deviated in some instances, which could have led to fewer patients being identified. Also, the performance of the triage screen relied on patients answering the questions, and the results may have deviated based on education level, ability to communicate, or interpretation/medical understanding of the questions, predominantly by the patient self-reporting, family or friend reporting, or emergency medical services report. The number of false-positive triage screens that may have occurred was also not assessed. This may have led to the inclusion of patients who were not truly febrile, and if they screened positive, then they should not have been included, and their inclusion may have resulted in an overestimation of the consult effect. Further, for this study, the diagnostic performance of the screening tool was not assessed. Thus, a lack of sensitivity could lead to a decrease in the administration of needed prescriptions, while there is a possibility of high rates of false-positive results, which could lead to unnecessary prescriptions and potential secondary effects, toxicities, side effects like Clostridioides difficile colitis, antimicrobial resistance development, and/or increased healthcare costs. Further studies that explicitly assess the diagnostic performance of such triage screening tools are needed.
5. Discussion
This retrospective, quasi-experimental study on febrile neutropenic patients presenting to the ED allowed for the identification of an effective pharmacy- and nursing-driven protocol utilized at triage to decrease time to administration of antibiotics. In the ED, some of the most difficult tasks in the treatment of these patients are the identification of these patients and the administration of antibiotics within the guideline-recommended time. This study showed a 39% decrease in TTA overall and a 49% reduction in TTA in those who had a positive screen and pharmacy consult. Although not statistically significant, mortality went from 18% pre-protocol to 8% post-protocol. No mortalities were seen in the subgroup that screened positive and received a pharmacy consult, with 18% in the pre-protocol group vs. 0% in those who received a pharmacy consult (p = 0.04). Other secondary outcomes were not as impactful, with an increased ED LOS and no difference in hospital LOS. However, the increased ED LOS was thought to be likely due to ED boarding patients during the post-protocol phase.
Documentation, which plays a key role in our protocol, revealed that the neutropenic triage screen was documented in 81% of post-protocol admissions. A review of the 19% of admissions where it was not documented revealed that most appeared to be in the 6-week period just after implementation of the protocol. Furthermore, a review of the negative triage screens (those that did not score ≥2 on the screen, with 1 point being from the fever question) revealed that only 65% were documented accurately. In several instances, this seemed to have occurred due to the completion of the screen before receiving patient information from emergency medical services (as identified as a possible limitation above). This contributed to a decrease in the number of patients receiving pharmacy consults and could have impacted their TTA.
One notable finding in our analysis was that positive culture results revealed that only 2.3% of all patients would have benefited from the administration of vancomycin, with only three patients growing MRSA or
Enterococcus spp., and empiric administration of vancomycin is not recommended in all patients per IDSA and ASCO guidelines [
2,
3].
Future directions are aimed at improving the utilization and accuracy of the neutropenic triage screening tool. This includes collaborating with emergency services and nursing education. Also, recently, anti-pseudomonal antibiotics were added to automated dispensing machines in the ED, which will allow for the assessment of the impact on TTA, with a possible further reduction in TTA. Furthermore, criteria are being developed for vancomycin administration because organisms warranting this addition to the protocol were rarely identified. Lastly, updates to the protocol may allow pharmacists to immediately order antibiotics after receipt of the pharmacy consult instead of waiting the previously required 15 min.
In summary, future methods aimed at reducing TTA, inappropriate empiric antibiotics, any delays in provider evaluation, and possible workflow issues, as well as decreasing adverse events in patients with febrile neutropenia, are currently being investigated. No specific nationally utilized protocol has been identified for these patients, and our pre-protocol TTA of 174 min was reduced to 109 min after the implementation of the protocol. A further reduction to 87.5 min was seen with those who screened positive on the triage screen and received a pharmacy consult. Thus, a pharmacist- and nursing-driven protocol significantly improves TTA and will be continued in the ED with the aim of improving patient outcomes for patients with febrile neutropenia.
Author Contributions
Conceptualization, H.T., B.T., J.C. and J.T.; methodology, S.S.-S.; software, S.S.-S.; validation, J.T.; formal analysis, S.S.-S., H.T. and J.T.; investigation, S.S.-S.; data curation, S.S.-S. and J.T.; writing—original draft, S.S.-S.; writing—review and editing, S.S.-S., H.T. and J.T.; project administration, H.T. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University of Nevada Reno, 1295724-1, on 11 August 2018.
Informed Consent Statement
Due to the retrospective nature of the study, as per local legislation, informed consent was exempted/waived.
Data Availability Statement
The datasets presented in this article are not readily available because of HIPAA. Requests to access the datasets should be directed to Stefanie Stafford-Stramel.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tai, E.; Guy, G.P.; Dunbar, A.; Richardson, L.C. Cost of Cancer-Related Neutropenia or Fever Hospitalizations, United States, 2012. J. Oncol. Pract. 2017, 13, e552–e561. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.; et al. Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Cancer Prevention and Control. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/cancer-preventing-infections/patients/neutropenia.html (accessed on 11 August 2018).
- National Hospital Ambulatory Medical Care Survey: CDC. Available online: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf (accessed on 20 April 2025).
- Keng, M.K.; Thallner, E.A.; Elson, P.; Ajon, C.; Sekeres, J.; Wenzell, C.M.; Seastone, D.J.; Gallagher, E.M.; Weber, C.M.; Earl, M.A.; et al. Reducing Time to Antibiotic Administration for Febrile Neutropenia in the Emergency Department. J. Oncol. Pract. 2015, 11, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; King, A.R.; Lin, C.P.M.; Friedman, G.K.; Monroe, K.; Kutny, M. Protocol for Reducing Time to Antibiotics in Pediatric Patients Presenting to an Emergency Department with Fever and Neutropenia: Efficacy and Barriers. Pediatr. Emerg. Care 2016, 32, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Benner, C.A.; Mora, E.; Mueller, E.; Seagull, F.J.; Walkovich, K.; Johnson, K.; Halverson, S.; Rothman, E.; Hucks, G.; Younger, J.G.; et al. Making Improvements in the ED: Does ED Busyness Affect Time to Antibiotics in Febrile Pediatric Oncology Patients Presenting to the Emergency Department? Pediatr. Emerg. Care 2018, 34, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Botten, J.; Beard, J.; Zorzi, A.; Thompson, A. A simple intervention to improve antibiotic treatment times for neutropenic sepsis. Acute Med. J. 2016, 15, 3–6. [Google Scholar] [CrossRef]
- Yoshida, H.; Leger, K.J.; Xu, M.; Migita, R.T.; Rutledge, J.C.; Pollard, J.A.; Kroon, L.; Mazor, S.; Moon, A.; Rutman, L.; et al. Improving Time to Antibiotics for Pediatric Oncology Patients with Suspected Infections: An Emergency Department-Based Quality Improvement Intervention. Pediatr. Emerg. Care 2018, 34, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fillon, M. CDC launches infection awareness initiative for cancer patients. J. Natl. Cancer Inst. 2012, 104, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, J.R.; Jones, C.M.; Williamson, J.; Chou, J.; Currie-Coyoy, M.; Jackson, T. Pharmacist involvement in a multidisciplinary initiative to reduce sepsis-related mortality. Am. J. Health Pharm. 2016, 73, 143–149. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).