Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Settings, Design and Subjects
2.2. Exposure of Normal-and Chronic Bronchitis-like Bronchial Mucosa Models to Cigarette Smoke Condensate
2.3. Statistics
3. Results
3.1. Study Cohort
3.2. SCGB1A1 Levels in BMS-COPD and TS-COPD Subjects
3.3. SCGB1A1 Levels in BMS-COPD Subjects Classified According to Disease Severity
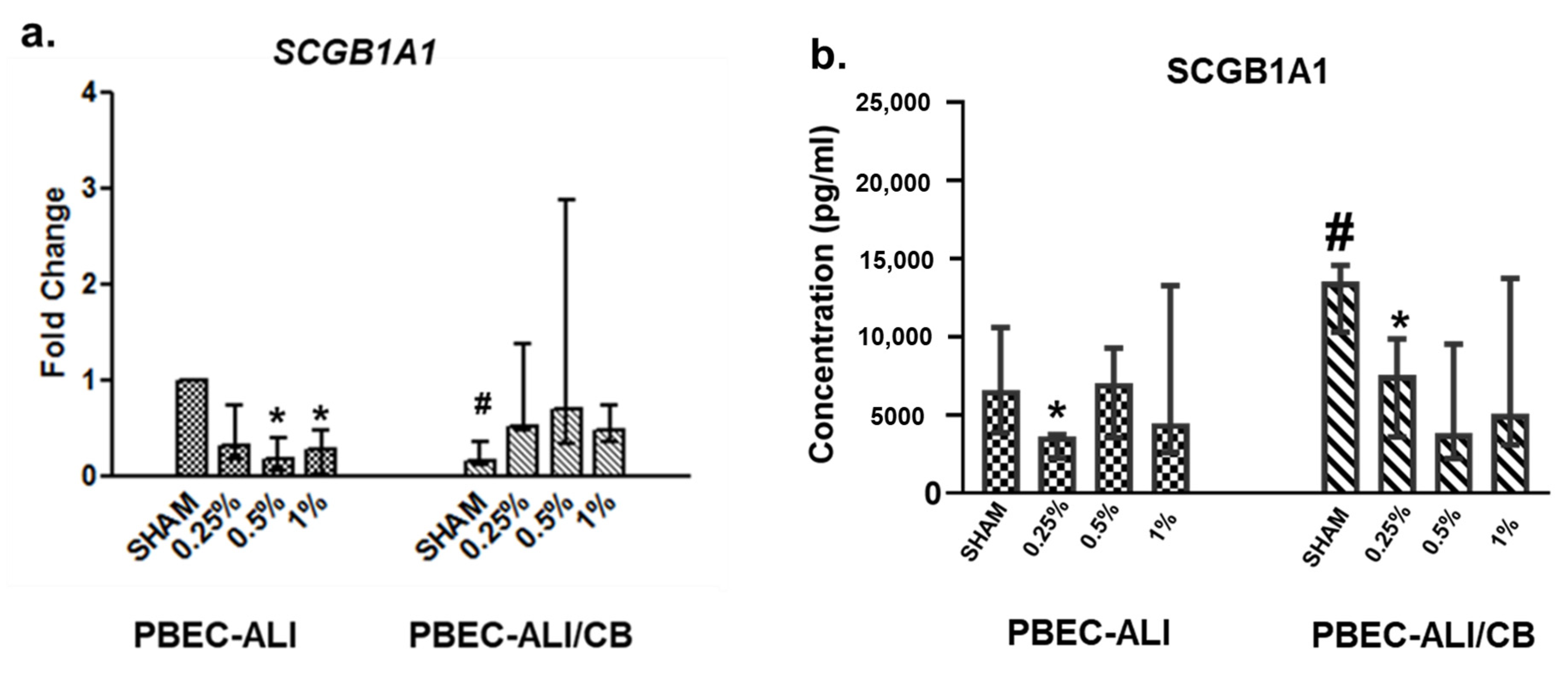
3.4. SCGB1A1 Transcript and Protein Levels Following In Vitro Cigarette Smoke Condensate Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiemstra, P.S.; Bourdin, A. Club cells, CC10 and self-control at the epithelial surface. Eur. Respir. J. 2014, 44, 831–832. [Google Scholar] [CrossRef] [Green Version]
- Dickens, J.A.; Lomas, D.A. CC-16 as a biomarker in chronic obstructive pulmonary disease. COPD 2012, 9, 574–575. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.B.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef] [Green Version]
- India State-Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Glob. Health 2018, 6, e1363–e1374.
- India State-Level Disease Burden Initiative Collaborators. Nations within a nation: Variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet 2017, 390, 2437–2460. [Google Scholar] [CrossRef] [Green Version]
- Salvi, S.; Agrawal, A. India needs a national COPD prevention and control programme. J. Assoc. Physicians India 2012, 60 (Suppl. 5–7). [Google Scholar] [PubMed]
- Rajkumar, P.; Pattabi, K.; Vadivoo, S.; Bhome, A.; Brashier, B.; Bhattacharya, P.; Mehendale, S.M. A cross-sectional study on prevalence of chronic obstructive pulmonary disease (COPD) in India: Rationale and methods. BMJ Open 2017, 7, e015211. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Meguro, M.; Barley, E.; Spencer, S.; Jones, P.W. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest 2007, 132, 456–463. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Knudson, R.J.; Lebowitz, M.D.; Holberg, C.J.; Burrows, B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am. Rev. Respir. Dis. 1983, 127, 725–734. [Google Scholar] [PubMed]
- Behera, D.; Jindal, S.K. Respiratory symptoms in Indian women using domestic cooking fuels. Chest 1991, 100, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; De Oca, M.M.; Mendez, R.A.; Plata, V.P.; Cabral, H. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Hedelin, A.; Malmlöf, M.; Kessler, V.; Seisenbaeva, G.; Gerde, P.; Palmberg, L. Development of Combining of Human Bronchial Mucosa Models with XposeALI® for Exposure of Air Pollution Nanoparticles. PLoS ONE 2017, 12, e0170428. [Google Scholar] [CrossRef]
- Ji, J.; Ganguly, K.; Mihai, X.; Sun, J.; Malmlöf, M.; Gerde, P.; Upadhyay, S.; Palmberg, L. Exposure of normal and chronic bronchitis-like mucosa models to aerosolized carbon nanoparticles: Comparison of pro-inflammatory oxidative stress and tissue injury/repair responses. Nanotoxicology 2019, 13, 1362–1379. [Google Scholar] [CrossRef]
- Ganguly, K.; Nordström, A.; Thimraj, T.A.; Rahman, M.; Ramström, M.; Sompa, S.I.; Lin, E.Z.; O’Brien, F.; Koelmel, J.; Ernstgård, L.; et al. Addressing the challenges of E-cigarette safety profiling by assessment of pulmonary toxicological response in bronchial and alveolar mucosa models. Sci. Rep. 2020, 10, 20460. [Google Scholar] [CrossRef]
- McAuley, D.F.; Matthay, M.A. Clara cell protein CC16. A new lung epithelial biomarker for acute lung injury. Chest 2009, 135, 1408–1410. [Google Scholar] [CrossRef]
- Rong, B.; Fu, T.; Gao, W.; Li, M.; Rong, C.; Liu, W.; Liu, H. Reduced Serum Concentration of CC16 Is Associated with Severity of Chronic Obstructive Pulmonary Disease and Contributes to the Diagnosis and Assessment of the Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 461–470. [Google Scholar]
- Bustos, M.L.; Mura, M.; Hwang, D.; Ludkovski, O.; Wong, A.P.; Keating, A.; Waddell, T.K. Depletion of bone marrow CCSP-expressing cells delays airway regeneration. Mol. Ther. 2015, 23, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knabe, L.; Fort, A.; Chanez, P.; Bourdin, A. Club cells and CC16: Another “smoking gun”? (With potential bullets against COPD). Eur. Respir. J. 2015, 45, 1519–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laucho-Contreras, M.E.; Polverino, F.; Gupta, K.; Taylor, K.L.; Kelly, E.; Pinto-Plata, V.; Divo, M.; Ashfaq, N.; Petersen, H.; Stripp, B.; et al. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur. Respir. J. 2015, 45, 1544–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Di, Y.P.; Wu, R.; Pinkerton, K.E.; Chen, Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS ONE 2015, 10, e0116159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamer, P.I.; Furlong, M.; Lothrop, N.; Guerra, S.; Billheimer, D.; Stern, D.A.; Zhai, J.; Halonen, M.; Wright, A.L.; Martinez, F.D. CC16 Levels into Adult Life Are Associated with Nitrogen Dioxide Exposure at Birth. Am. J. Respir. Crit. Care Med. 2019, 200, 600–607. [Google Scholar] [CrossRef]
- Park, H.Y.; Churg, A.; Wright, J.L.; Li, Y.; Tam, S.; Man, S.F.P.; Tashkin, D.; Wise, R.; Connett, J.E.; Sin, D.D. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Vestbo, J.; Edwards, L.; Scanlon, P.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011, 365, 1184–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rava, M.; Tares, L.; Lavi, I.; Barreiro, E.; Zock, J.-P.; Ferrer, A.; Muniozguren, N.; Nadif, R.; Cazzoletti, L.; Kauffmann, F.; et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J. Allergy Clin. Immunol. 2013, 132, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Vestbo, J.; Anderson, W.; Coxson, H.O.; Crim, C.; Dawber, F.; Edwards, L.; Hagan, G.; Knobil, K.; Lomas, D.A.; MacNee, W.; et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008, 63, 1058–1063. [Google Scholar]
- Guerra, S.; Halonen, M.; Vasquez, M.M.; Spangenberg, A.; Stern, D.A.; Morgan, W.J.; Wright, A.L.; Lavi, I.; Tarès, L.; Carsin, A.E.; et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: A prospective study. Lancet Respir. Med. 2015, 3, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Cho, M.H.; Hersh, C.P.; Lomas, D.A.; Miller, B.E.; Kong, X.; Bakke, P.; Gulsvik, A.; Agustí, A.; Wouters, E.; et al. ECLIPSE, ICGN, and COPDGene Investigators. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 1238–1247. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Bárcenas, J.; Calderón-Moore, A.; Baptista-González, H.; Irles, C. Clara Cell Protein Expression in Mechanically Ventilated Term and Preterm Infants with Respiratory Distress Syndrome and at Risk of Bronchopulmonary Dysplasia: A Pilot Study. Can. Respir. J. 2017, 2017, 8074678. [Google Scholar] [CrossRef]
- Filippone, M.; Baraldi, E. On early life risk factors for COPD. Am. J. Respir. Crit. Care Med. 2011, 183, 415–416. [Google Scholar] [CrossRef]
- Vázquez, J.H.; García, I.A.; Jiménez-García, R.; Meca, A.Á.; de Andrés, A.L.; Ruiz, C.M.; García, M.J.; de Miguel Díez, J. COPD phenotypes: Differences in survival. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2245–2251. [Google Scholar] [CrossRef] [Green Version]
- Petersen, H.; Leng, S.; Belinsky, S.A.; Miller, B.E.; Tal-Singer, R.; Owen, C.A.; Celli, B.; Tesfaigzi, Y. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur. Respir. J. 2015, 46, 1501–1503. [Google Scholar] [CrossRef] [Green Version]
- Capistrano, S.J.; Van Reyk, D.; Chen, H.; Oliver, B.G. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics 2017, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Shim, J.J.; Burgel, P.-R.; Ueki, I.F.; Dao-Pick, T.; Tam, D.C.-W.; Nadel, J.A. IL-13-induced Clara cell secretory protein expression in airway epithelium: Role of EGFR signaling pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L67–L75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, A.; Sheikh, F.; Niimi, T.; DeMayo, F.J.; Keegan, A.D.; Donnelly, R.P.; Kimura, S. Induction of uteroglobin-related protein 2 (Ugrp2) gene expression by the Th2 cytokines IL-4 and IL-13. J. Immunol. 2005, 175, 5708–5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gras, D.; Chanez, P.; Vachier, I.; Petit, A.; Bourdin, A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol. Ther. 2013, 140, 290–305. [Google Scholar] [CrossRef]
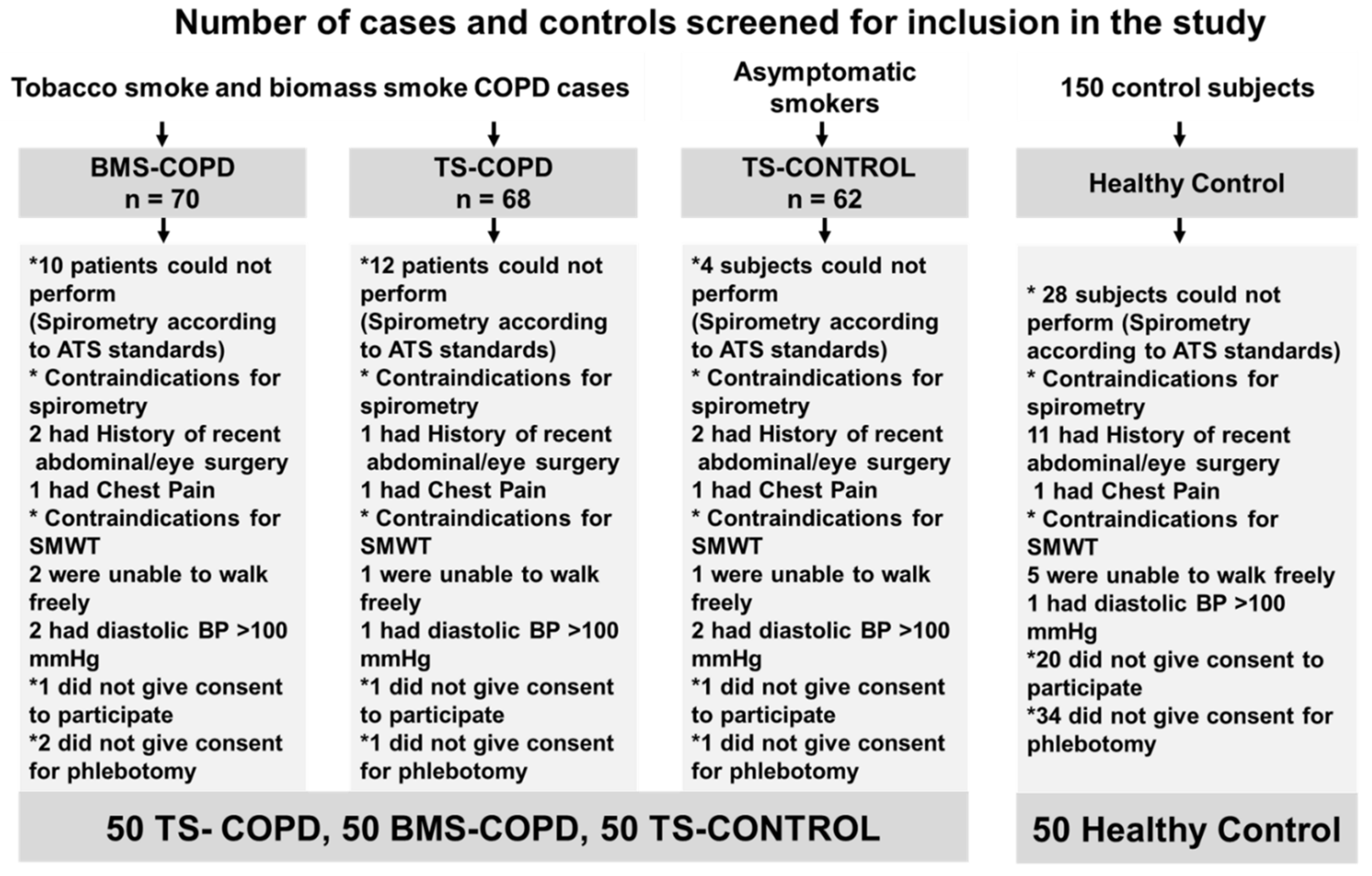
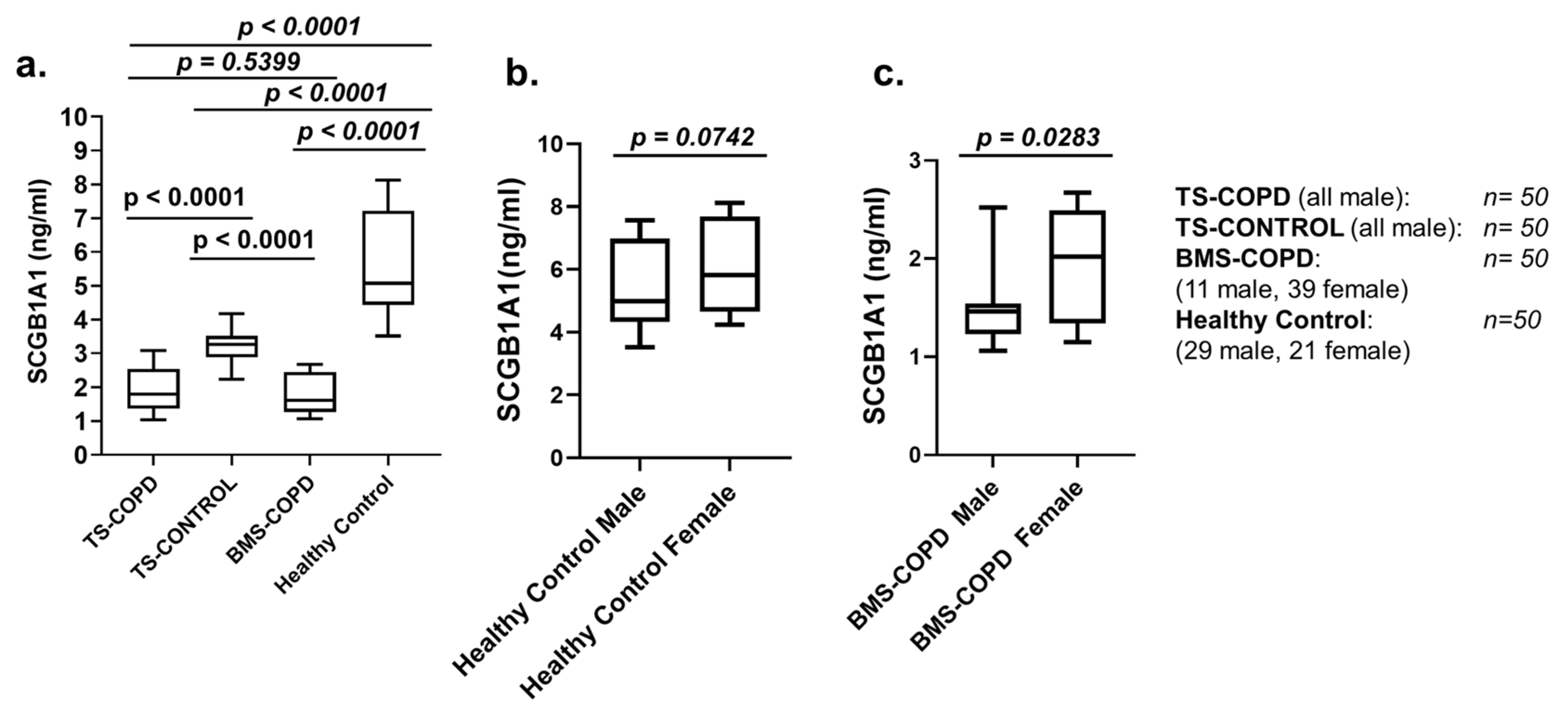
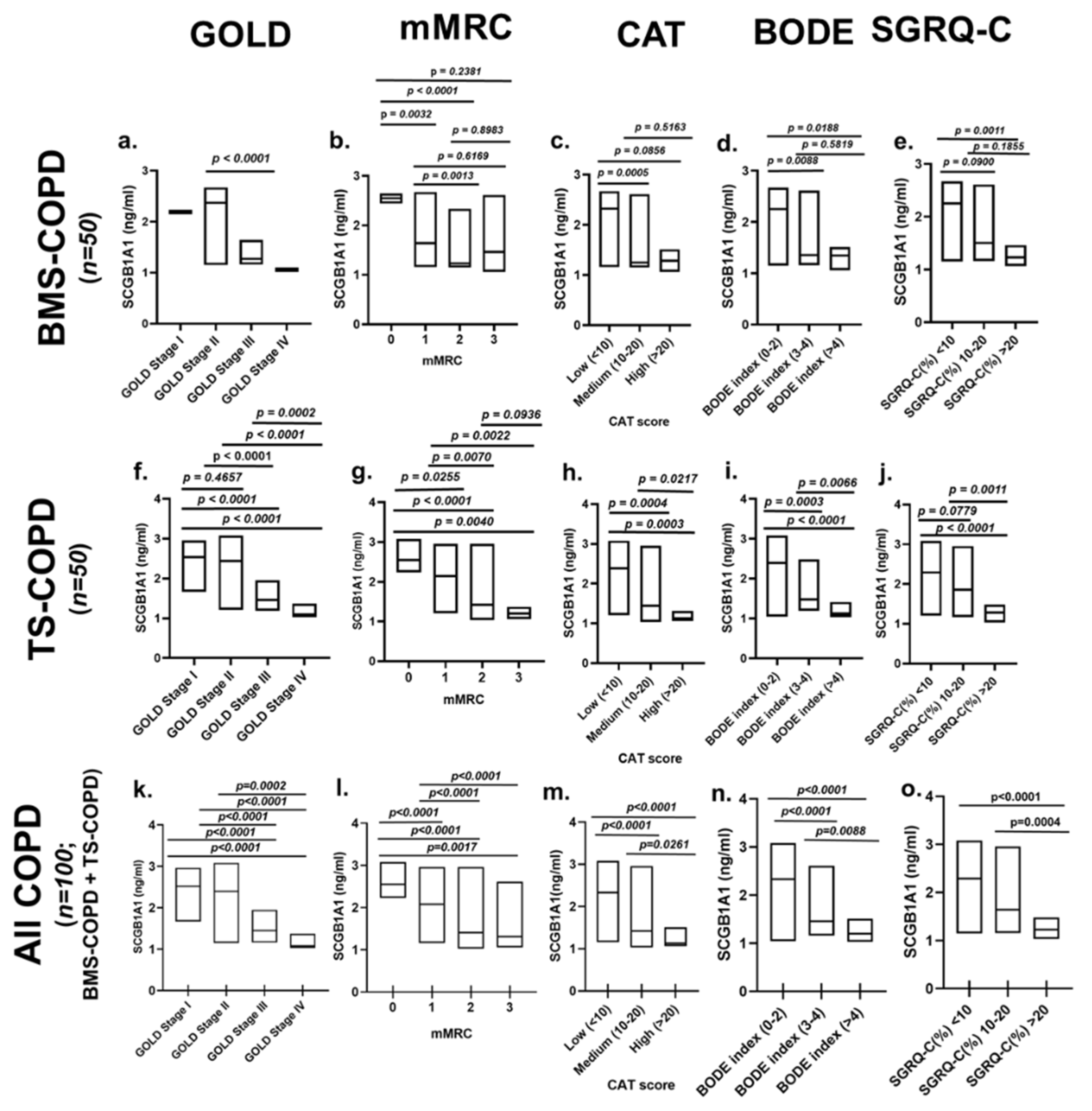
| TS-COPD | TS-CONTROL | BMS-COPD | Healthy Control | |
|---|---|---|---|---|
| Gender | ||||
| Female | 0 | 0 | 39 | 21 |
| Male | 50 | 50 | 11 | 29 |
| Total (n) | 50 | 50 | 50 | 50 |
| Age (years) (mean, range) | 66 (51–85) | 61 (40–85) | 59 (40–84) | 55 (40–76) |
| BMEI (median, IQR) | -(no BMS exposure during cooking) | -(no BMS exposure during cooking) | 75 (60–90) | -(no BMS exposure during cooking) |
| Pack-years (median, IQR) | 30 (26–33.8) | 26 (22–30) | -(non-smoker) | -(non-smoker) |
| Spirometry (pre bronchodilation) (mean ± SD) | ||||
| FEV1% predicted | 50.4 ± 23.1 | 99.8 ± 19.0 | 45.6 ± 11.7 | 97.0 ± 13.1 |
| FVC% predicted | 73.2 ± 26.7 | 102.2 ± 20.3 | 62.1 ± 12.2 | 98.9 ± 14.1 |
| FEV1/FVC | 0.53 ± 0.08 | 0.79 ± 0.05 | 0.60 ± 0.09 | 0.82 ± 0.04 |
| Spirometry (post bronchodilation) (mean ± SD) | ||||
| FEV1% predicted | 57.4 ± 25.9 | 103.4 ± 21.3 | 53 ± 13.6 | 100.7 ± 14.0 |
| FVC% predicted | 81.4 ± 29.5 | 103.3 ± 22.1 | 70.7 ± 13.5 | 99.5 ± 15.3 |
| FEV1/FVC | 0.55 ± 0.09 | 0.80 ± 0.06 | 0.61 ± 0.08 | 0.85 ± 0.05 |
| GOLD obstruction grading (n) | ||||
| I | 10 | - | 1 | - |
| II | 17 | - | 31 | - |
| III | 16 | - | 17 | - |
| IV | 7 | - | 1 | - |
| Exercise capacity (median, IQR) | ||||
| SMWD | 440 (400.5–465.6) | 520.3 (461–559.6) | 435 (396.3–477.5) | 541.5 (510.6–556.4) |
| SMWD (%) | 82 (75.3–90) | 99.5 (96.3–104) | 84 (80–90) | 98 (95.3–101) |
| mMRC dyspnea grading (n) | ||||
| 0 | 7 | 50 | 6 | 50 |
| 1 | 20 | 0 | 26 | 0 |
| 2 | 19 | 0 | 15 | 0 |
| 3 | 4 | 0 | 3 | 0 |
| CAT score (median, IQR) | 9 (6–14) | - | 9 (6–12) | - |
| BODE index (median, IQR) | 2 (1–4) | - | 2 (1–3) | - |
| SGRQ-C (median, IQR) | ||||
| Symptom | 133.2 (65.9–219.2) | - | 99.55 (58.3–156.8) | - |
| Activity | 75.7 (0–81.4) | - | 75.7 (0–81.4) | - |
| Impact | 76.1 (75.1–155.2) | - | 76.1 (75.1–135.4) | - |
| Total score | 285 (144.4–492.2) | - | 231.9 (134.4–363.5) | - |
| SGRQ-C% | 8.9 (5–15) | - | 7 (4–11) | - |
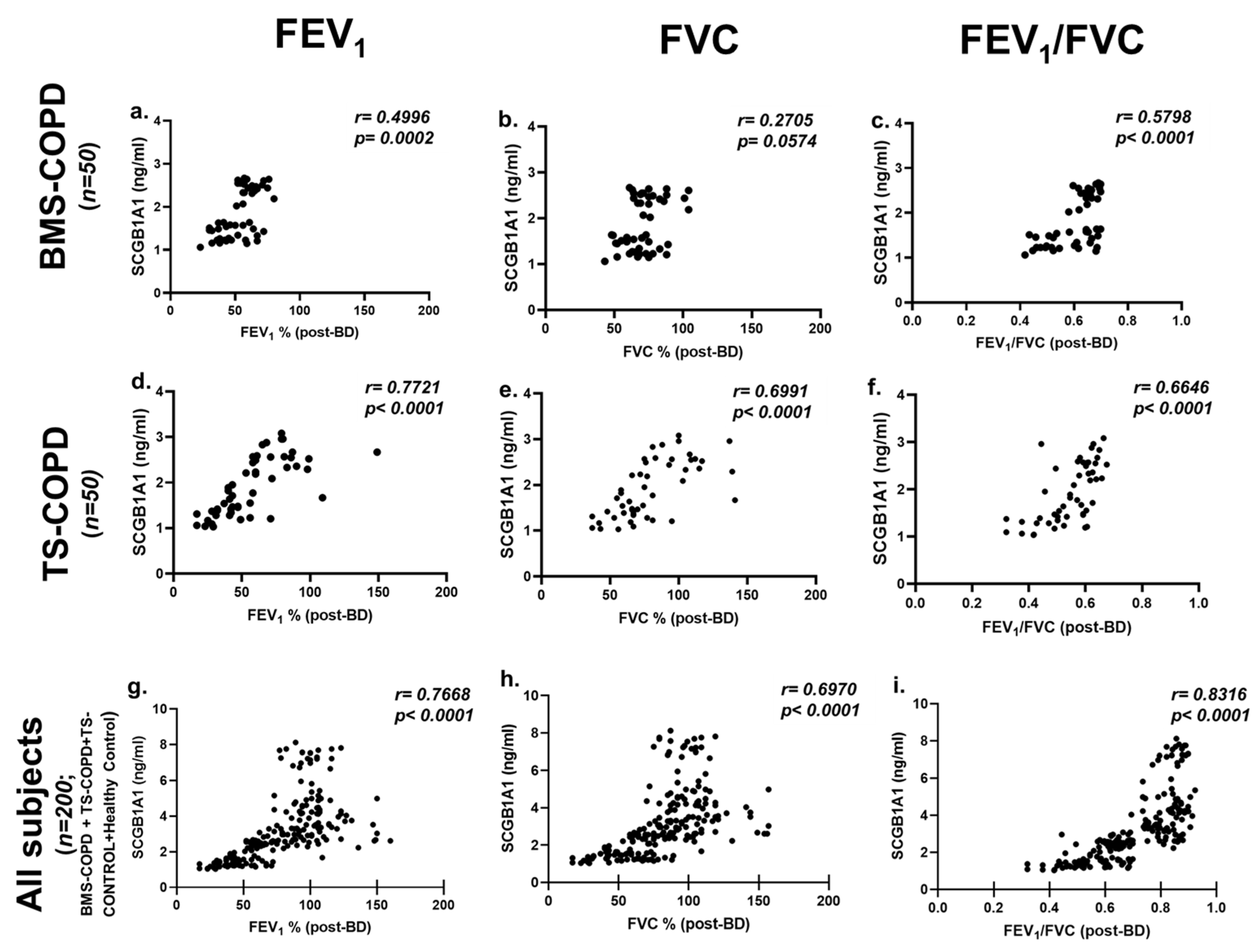
| Category | n | FEV1 | FVC | FEV1/FVC | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-BD | Post-BD | Pre-BD | Post-BD | Pre-BD | Post-BD | |||
| BMS-COPD (all) | 50 | p | 0.0015 | 0.0002 | ns (0.0585) | ns (0.0574) | 0.0038 | <0.0001 |
| r | 0.4377 | 0.4996 | 0.2694 | 0.2705 | 0.4025 | 0.5798 | ||
| BMS-COPD (male) | 11 | p | 0.0012 | ns (0.0842) | 0.0015 | ns (0.3696) | 0.0011 | 0.0116 |
| r | 0.4508 | 0.5484 | 0.4409 | 0.2989 | 0.4526 | 0.7432 | ||
| BMS-COPD (female) | 39 | p | ns (0.0900) | 0.0200 | ns (0.1572) | ns (0.2521) | ns (0.2700) | 0.0041 |
| r | 0.2752 | 0.3712 | 0.2309 | 0.1878 | 0.4490 | 0.1811 | ||
| TS-COPD (all male) | 50 | p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| r | 0.7761 | 0.7721 | 0.6970 | 0.6991 | 0.6357 | 0.6646 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veerapaneni, V.V.; Upadhyay, S.; Thimraj, T.A.; Siddaiah, J.B.; Krishnarao, C.S.; Lokesh, K.S.; Thimmulappa, R.; Palmberg, L.; Ganguly, K.; Anand, M.P. Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease. Toxics 2021, 9, 208. https://doi.org/10.3390/toxics9090208
Veerapaneni VV, Upadhyay S, Thimraj TA, Siddaiah JB, Krishnarao CS, Lokesh KS, Thimmulappa R, Palmberg L, Ganguly K, Anand MP. Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease. Toxics. 2021; 9(9):208. https://doi.org/10.3390/toxics9090208
Chicago/Turabian StyleVeerapaneni, Vivek Vardhan, Swapna Upadhyay, Tania A. Thimraj, Jayaraj Biligere Siddaiah, Chaya Sindaghatta Krishnarao, Komarla Sundararaja Lokesh, Rajesh Thimmulappa, Lena Palmberg, Koustav Ganguly, and Mahesh Padukudru Anand. 2021. "Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease" Toxics 9, no. 9: 208. https://doi.org/10.3390/toxics9090208
APA StyleVeerapaneni, V. V., Upadhyay, S., Thimraj, T. A., Siddaiah, J. B., Krishnarao, C. S., Lokesh, K. S., Thimmulappa, R., Palmberg, L., Ganguly, K., & Anand, M. P. (2021). Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease. Toxics, 9(9), 208. https://doi.org/10.3390/toxics9090208









