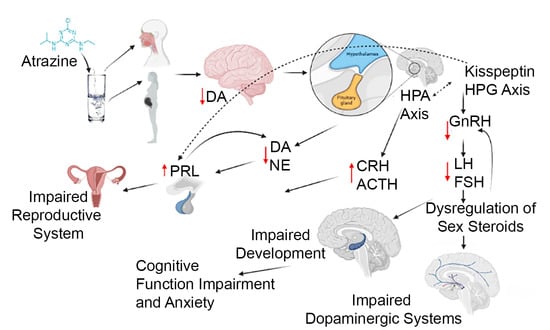
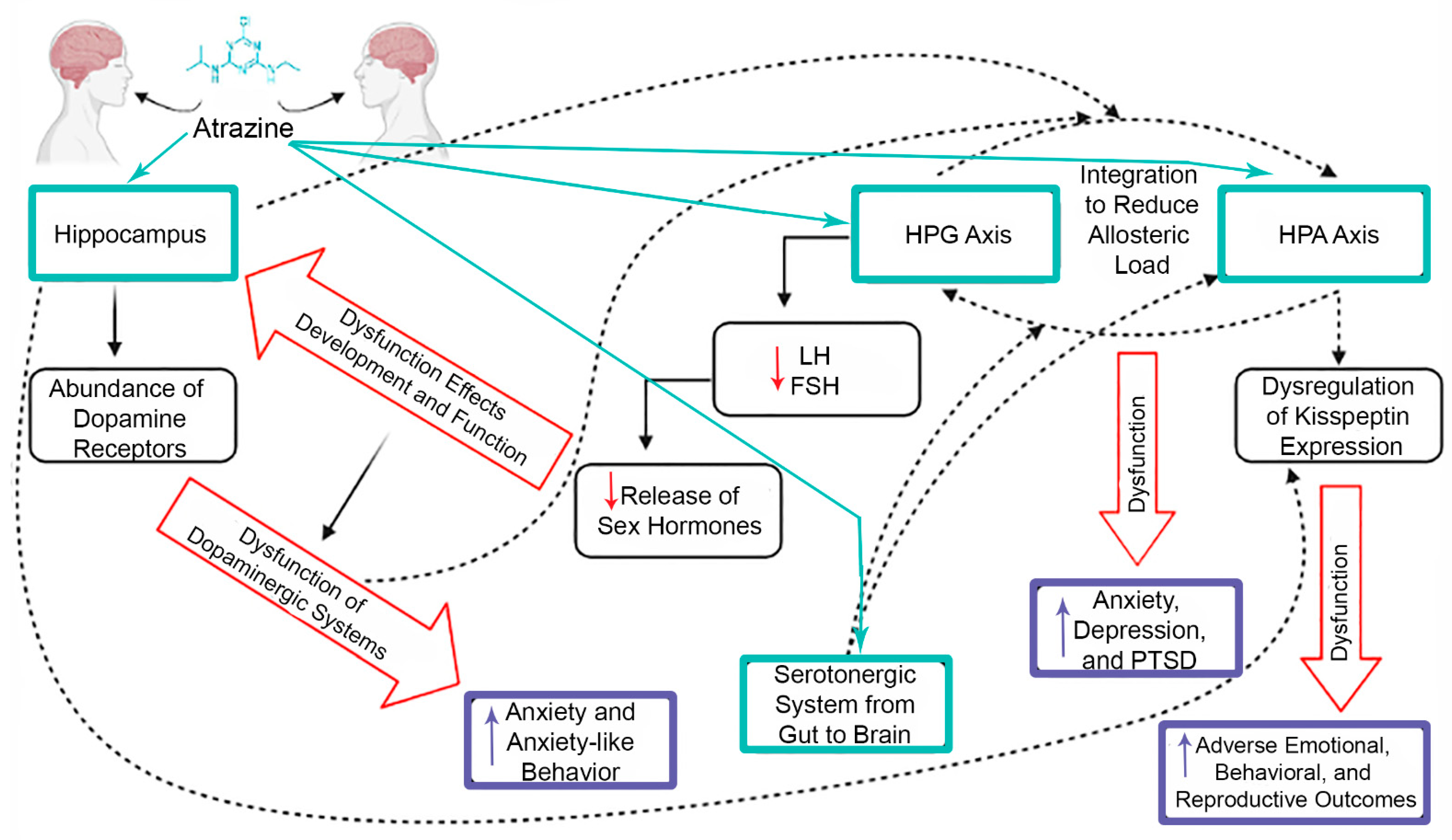
Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine
Abstract
1. Introduction
2. Role of Major Neuroendocrine Hormones Regulated by the Hypothalamic-Pituitary Axes
2.1. Hypothalamic-Pituitary-Gonadal Axis
2.1.1. Gonadotropin-Releasing Hormone (GnRH)
2.1.2. Luteinizing Hormone (LH)
2.1.3. Follicle-Stimulating Hormone (FSH)
2.2. Hypothalamic-Pituitary-Adrenal Axis
2.2.1. Corticotropin-Releasing Hormone (CRH)
2.2.2. Adrenocorticotropic Hormone (ACTH)
3. Effects of Atrazine on Major Neuroendocrine Hormones
3.1. HPG Axis
3.2. HPA Axis
4. Role of Major Neurotransmitters in the Hypothalamus
4.1. Dopamine
4.2. Serotonin
4.3. Norepinephrine
4.4. Gamma-Aminobutyric Acid
4.5. Glutamate
4.6. Acetylcholine
5. Effects of Atrazine on Major Neurotransmitters
5.1. Dopamine
| Reference | Species | Atrazine Exposure a | Length of Exposure | Results |
|---|---|---|---|---|
| Das et al., 2000 [81] | PC12 Cells | 12.5, 25, 50, 100, or 200 µM | 6, 12, 8, 24, 48 h | Decrease in intracellular DA, concentration dependent outcomes, NE reduction at 100 and 200 µM |
| Coban and Filipov, 2007 [31] | Male Juvenile C57BL/6 Mice | 5, 25, 125, or 250 mg/kg | 14 days, gavage | Dose-dependent decrease of DA in striatum up to one week after exposure |
| Walters et al., 2015 [83] | Male Sprague-Dawley Rats | 0.1 or 10 mg/kg | Dams treated Gestational Day 1- Postnatal Day 21, Male offspring continued 6 months upon weening | Male offspring showed decrease in DA and DOPAC levels at low and high concentrations, 10 mg/kg showed disruptions in motor function |
| Li et al., 2018 [53] | Sprague Dawley Rats | 10 or 100 mg/kg | 30 consecutive days starting at Postnatal Day 28, gavage | DA levels increased in hippocampus in low dose group, D1DR expression levels decreased in dose-dependent manner |
| Li et al., 2015 [84] | Pubertal Male Sprague Dawley Rats | 50, 100, or 200 mg/kg | 28 days, gavage, postnatal day 27–54 | Exposure to higher doses led to decreased levels of DA and decreased expression of Nurr1 |
| Sun et al., 2014 [85] | Sprague Dawley Rats | Pregnant Dams received 10 µL/g body weight or vehicle, offspring received 0, 25, or 50 mg/kg/day | Pregnant dams starting GD 5, offspring until PND 22, gavage | Ventral midbrains examined and found DA concentrations and mRNA of Nurr1 decreases in offspring at both concentrations |
| Li et al., 2014 [86] | Sprague Dawley Rats | 25 or 50 mg/kg/day | Pregnant dams received ATR from GD 0, and offspring received ATR until PND 1 | 6 months after treatment DA and expression of Nurr1 were decreased in striatum and substantia nigra |
| Li et al., 2019 [87] | Male Sprague Dawley Rats | 10 or 100 mg/kg | 30 days, starting at PND 35 | Impairment of memory after exposure, downregulation of protein and mRNA expression levels associated with MEK/ERK/CREB pathway at low and high concentrations |
| Song et al., 2015 [88] | Male Wistar Rats | 10, 50, or 100 mg/kg | 3 months, gavage | Nigrostriatal dopaminergic system pathways examined and found micromorphology suggesting neuronal apoptosis and mitochondrial autophagy of DA neurons at all concentrations, increasing in severity with increasing dose |
| Filipov et al., 2007 [89] | Adult Male Sprague Dawley Rats | up to 500 µM | Striatal slices incubated for 4 h | Tissue DA levels decreased dose-dependent at 100 µM or greater, exposure interferes with the uptake and storage of DA |
| Hossain and Filipov, 2008 [90] | Striatal synaptosomes and vesicles from Adult Male Sprague Dawley Rats | 1–250 µM | 15 min | Atrazine and two metabolites caused inhibition of DA uptake dose-dependently |
| Bardullas et al., 2011 [92] | Male Sprague Dawley Rat | 10 mg/kg | 1 year | Impaired motor coordination, greater spontaneous locomotor activity, decrease in striatal DA |
| Rodríguez et al., 2013 [93] | Adult Male Sprague Dawley Rat | 100 mg/kg | 6 IP injections over 2 weeks | Hypoactivity following injection that lasted for 5 days, reductions in striatal DA, amphetamine 2 months post exposure caused significant stimulation |
| Rodriguez et al., 2017 [94] | Male Sprague Dawley Rats | 100 mg/kg | Single injection | Significant decrease in locomotor activity, cells other than DA neurons cause locomotor dysfunction |
| Belloni et al., 2011 [95] | Juvenile and Adult CD1 Mice | 0.001 or 0.1 mg/kg | Dams exposed GD 14 through PND 21 | Feminization of behavioral profile in male offspring, learning performance alterations in adults at both concentrations |
5.2. Serotonin
5.3. Gaba and Glutamate
5.4. Acetylcholine
6. Relevance to Human Exposure
7. Crosstalk between CNS and Endocrine System
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rinsky, J.L.; Hopenhayn, C.V.; Golla, S.; Bush, H.M. Atrazine Exposure in Public Drinking Water and Preterm Birth. Public Health Rep. 2012, 127, 72–80. [Google Scholar] [CrossRef]
- Almberg, K.S.; Turyk, M.E.; Jones, R.M.; Rankin, K.; Freels, S.; Stayner, L.T. Atrazine Contamination of Drinking Water and Adverse Birth Outcomes in Community Water Systems with Elevated Atrazine in Ohio, 2006–2008. Int. J. Environ. Res. Public Health 2018, 15, 1889. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Acuña, H.; Frankenberger, J.; Hahn, L.; Carbajo, C. Drinking-Water Herbicide Exposure in Indiana and Prevalence of Small-for-Gestational-Age and Preterm Delivery. Environ. Health Perspect. 2009, 117, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Work Group on the Evaluation of Carcinogenic Risks to Humans. Atrazine; International Agency for Research on Cancer: Lyon, France, 1991. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499610/ (accessed on 13 June 2021).
- Bethsass, J.; Colangelo, A. European Union Bans Atrazine, While the United States Negotiates Continued Use. Int. J. Occup. Environ. Health 2006, 12, 260–267. [Google Scholar] [CrossRef]
- Atrazine: Interim Registration Review Decision Case: 0062. 2020. Available online: https://www.epa.gov/sites/default/files/2020-09/documents/atrazine-id-signed-final.pdf (accessed on 13 June 2021).
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450. [Google Scholar] [CrossRef]
- Foradori, C.D.; Zimmerman, A.D.; Hinds, L.R.; Zuloaga, K.L.; Breckenridge, C.B.; Handa, R.J. Atrazine Inhibits Pulsatile Gonadotropin-Releasing Hormone (GnRH) Release Without Altering GnRH Messenger RNA or Protein Levels in the Female Rat1. Biol. Reprod. 2013, 88, 1–7. [Google Scholar] [CrossRef]
- Foradori, C.D.; Hinds, L.R.; Hanneman, W.H.; Handa, R.J. Effects of Atrazine and Its Withdrawal on Gonadotropin-Releasing Hormone Neuroendocrine Function in the Adult Female Wistar Rat1. Biol. Reprod. 2009, 81, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Reidenbach, L.S.; Thanki, D.H.; Winchester, A.E.; Qualizza, B.A.; Ryan, G.A.; Egan, K.E.; Hedrick, V.E.; Sobreira, T.J.P.; Peterson, S.M.; et al. Embryonic atrazine exposure elicits proteomic, behavioral, and brain abnormalities with developmental time specific gene expression signatures. J. Proteom. 2018, 186, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Lin, L.F.; Taslakjian, B.; Yuan, C.; Freeman, J.L. Embryonic atrazine exposure and later in life behavioral and brain transcriptomic, epigenetic, and pathological alterations in adult male zebrafish. Cell Biol. Toxicol. 2021, 37, 421–439. [Google Scholar] [CrossRef]
- Ochoa-Acuña, H.; Carbajo, C. Risk of limb birth defects and mother’s home proximity to cornfields. Sci. Total Environ. 2009, 407, 4447–4451. [Google Scholar] [CrossRef]
- Winchester, P.D.; Huskins, J.; Ying, J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009, 98, 664–669. [Google Scholar] [CrossRef]
- Chevrier, C.; Limon, G.; Monfort, C.; Rouget, F.; Garlantézec, R.; Petit, C.; Durand, G.; Cordier, S. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ. Health Perspect. 2011, 119, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Munger, R.; Isacson, P.; Hu, S.; Burns, T.; Hanson, J.; Lynch, C.F.; Cherryholmes, K.; Van Dorpe, P.; Hausler, W.J., Jr. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ. Health Perspect. 1997, 105, 308–314. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Durand, G.; Coutté, M.B.; Chevrier, C.; Cordier, S. Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestational-age status. Occup. Environ. Med. 2005, 62, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Cragin, L.A.; Kesner, J.S.; Bachand, A.M.; Barr, D.B.; Meadows, J.W.; Krieg, E.F.; Reif, J.S. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ. Res. 2011, 111, 1293–1301. [Google Scholar] [CrossRef]
- Swan, S.H. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int. J. Androl. 2006, 29, 62–68. [Google Scholar] [CrossRef]
- Laws, S.C. The Effects of Atrazine on Female Wistar Rats: An Evaluation of the Protocol for Assessing Pubertal Development and Thyroid Function. Toxicol. Sci. 2000, 58, 366–376. [Google Scholar] [CrossRef]
- Rayner, J.L.; Enoch, R.R.; Fenton, S.E. Adverse Effects of Prenatal Exposure to Atrazine During a Critical Period of Mammary Gland Growth. Toxicol. Sci. 2005, 87, 255–266. [Google Scholar] [CrossRef]
- Hovey, R.C.; Coder, P.S.; Wolf, J.C.; Sielken Jr, R.L.; Tisdel, M.O.; Breckenridge, C.B. Quantitative Assessment of Mammary Gland Development in Female Long Evans Rats Following In Utero Exposure to Atrazine. Toxicol. Sci. 2011, 119, 380–390. [Google Scholar] [CrossRef]
- Foradori, C.D.; Hinds, L.R.; Quihuis, A.M.; Lacagnina, A.F.; Breckenridge, C.B.; Handa, R.J. The Differential Effect of Atrazine on Luteinizing Hormone Release in Adrenalectomized Adult Female Wistar Rats1. Biol. Reprod. 2011, 85, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Murr, A.S.; Best, D.S.; Fraites, M.J.; Zorrilla, L.M.; Narotsky, M.G.; Stoker, T.E.; Goldman, J.M.; Cooper, R.L. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod. Toxicol. 2011, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.G.; Chen, H.; Folmer, J.; Liu, J.; Papadopoulos, V.; Zirkin, B.R. Gestational Exposure to Atrazine: Effects on the Postnatal Development of Male Offspring. J. Androl. 2008, 29, 304–311. [Google Scholar] [CrossRef]
- Fraites, M.J.; Narotsky, M.G.; Best, D.S.; Stoker, T.E.; Davis, L.K.; Goldman, J.M.; Hotchkiss, M.G.; Klinefelter, G.R.; Kamel, A.; Qian, Y.; et al. Gestational atrazine exposure: Effects on male reproductive development and metabolite distribution in the dam, fetus, and neonate. Reprod. Toxicol. 2011, 32, 52–63. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Sepúlveda, M.S.; Lin, T.-L.; Jannasch, A.S.; Freeman, J.L. An embryonic atrazine exposure results in reproductive dysfunction in adult zebrafish and morphological alterations in their offspring. Sci. Rep. 2016, 6, 21337. [Google Scholar] [CrossRef]
- Foradori, C.D.; Healy, J.E.; Zimmerman, A.D.; Kemppainen, R.J.; Jones, M.A.; Read, C.C.; White, B.D.; Yi, K.D.; Hinds, L.R.; Lacagnina, A.F.; et al. Characterization of Activation of the Hypothalamic-Pituitary-Adrenal Axis by the Herbicide Atrazine in the Female Rat. Endocrinology 2018, 159, 3378–3388. [Google Scholar] [CrossRef]
- Fraites, M.J.P.; Cooper, R.L.; Buckalew, A.; Jayaraman, S.; Mills, L.; Laws, S.C. Characterization of the Hypothalamic-Pituitary-Adrenal Axis Response to Atrazine and Metabolites in the Female Rat. Toxicol. Sci. 2009, 112, 88–99. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef]
- Graceli, J.B.; Dettogni, R.S.; Merlo, E.; Niño, O.; da Costa, C.S.; Zanol, J.F.; Ríos Morris, E.A.; Miranda-Alves, L.; Denicol, A.C. The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol. Cell Endocrinol. 2020, 518, 110997. [Google Scholar] [CrossRef] [PubMed]
- Coban, A.; Filipov, N.M. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J. Neurochem. 2007, 100, 1177–1187. [Google Scholar] [CrossRef]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Bruno, J.P.; Parikh, V. Abnormal Neurotransmitter Release Underlying Behavioral and Cognitive Disorders: Toward Concepts of Dynamic and Function-Specific Dysregulation. Neuropsychopharmacology 2007, 32, 1452–1461. [Google Scholar] [CrossRef]
- Alvarez-Bolado, G. Development of neuroendocrine neurons in the mammalian hypothalamus. Cell Tissue Res. 2019, 375, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Grattan, D.R. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef]
- Meethal, S.V.; Atwood, C.S. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol. Life Sci. 2005, 62, 257–270. [Google Scholar] [CrossRef]
- Hiller-Sturmhöfel, S.; Bartke, A. The endocrine system: An overview. Alcohol Health Res. World 1998, 22, 153–164. [Google Scholar]
- Harrison, G.S.; Wierman, M.E.; Nett, T.M.; Glode, L.M. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr. Relat. Cancer 2004, 11, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Skorupskaite, K.; George, J.T.; Anderson, R.A. Physiology of GNRH and Gonadotropin Secretion. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK279070/ (accessed on 31 May 2021).
- Nedresky, D.; Singh, G. Physiology, Luteinizing Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539692/ (accessed on 31 May 2021).
- Orlowski, M.; Sarao, M.S. Physiology, Follicle Stimulating Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535442/ (accessed on 31 May 2021).
- Slominski, A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br. J. Dermatol. 2009, 160, 229–232. [Google Scholar] [CrossRef]
- Allen, M.J.; Sharma, S. Physiology, Adrenocorticotropic Hormone (ACTH). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK500031/ (accessed on 27 May 2021).
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441996/ (accessed on 5 May 2021).
- Goldman, J.M.; Davis, L.K.; Murr, A.S.; Cooper, R.L. Atrazine-induced elevation or attenuation of the LH surge in the ovariectomized, estrogen-primed female rat: Role of adrenal progesterone. Reproduction 2013, 146, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ishii, M.N.; Seki, N.; Sakai, Y.; Yamashita, T.; Awatsuji, H.; Kanda, K.; Matsumoto, K.; Matsui, H. Reduction of Kiss1 expression in the anteroventral periventricular nucleus is associated with atrazine-induced attenuation of the luteinizing hormone surge in female rats. Biol. Reprod. 2019, 100, 41–48. [Google Scholar] [CrossRef]
- Trentacoste, S.V.; Friedmann, A.S.; Youker, R.T.; Breckenridge, C.B.; Zirkin, B.R. Atrazine Effects on Testosterone Levels and Androgen-Dependent Reproductive Organs in Peripubertal Male Rats. J. Androl. 2001, 22, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Sharifi, E.; Soltani, A. Effects of Atrazine Toxin on Levels of LH, FSH and Testosterone Hormones in Adult Male Rat. Nat. Environ. Pollut. Technol. 2011, 10, 6. [Google Scholar]
- Hotchkiss, M.G.; Best, D.S.; Cooper, R.L.; Laws, S.C. Atrazine does not induce pica behavior at doses that increase hypothalamic–pituitary–adrenal axis activation and cause conditioned taste avoidance. Neurotoxicol. Teratol. 2012, 34, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Riffle, B.W.; Klinefelter, G.R.; Cooper, R.L.; Winnik, W.M.; Swank, A.; Jayaraman, S.; Suarez, J.; Best, D.; Laws, S.C. Novel molecular events associated with altered steroidogenesis induced by exposure to atrazine in the intact and castrate male rat. Reprod. Toxicol. 2014, 47, 59–69. [Google Scholar] [CrossRef]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2006, 15, R154–R158. [Google Scholar] [CrossRef]
- Shahid, Z.; Asuka, E.; Singh, G. Physiology, Hypothalamus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535380/ (accessed on 14 June 2021).
- Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. Int. J. Mol. Sci. 2018, 19, 2241. [Google Scholar] [CrossRef] [PubMed]
- Money, K.M.; Stanwood, G.D. Developmental origins of brain disorders: Roles for dopamine. Front. Cell Neurosci. 2013, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Olguín, H.J.; Guzmán, D.C.; García, E.H.; Mejía, G.B. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid. Med. Cell Longev. 2015, 2016, e9730467. [Google Scholar] [CrossRef]
- Stagkourakis, S.; Kim, H.; Lyons, D.J.; Broberger, C. Dopamine Autoreceptor Regulation of a Hypothalamic Dopaminergic Network. Cell Rep. 2016, 15, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Smidt, M.P.; Burbach, J.P.H. Development and Engineering of Dopamine Neurons; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of Projection Pathways from the Arcuate Nucleus of the Hypothalamus to Hypothalamic Regions Implicated in the Neural Control of Feeding Behavior in Mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A Review Article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef]
- Frazer, A.; Hensler, J.G. Serotonin Involvement in Physiological Function and Behavior. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27940/ (accessed on 27 May 2021).
- Frazer, A.; Hensler, J.G. Serotonin. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK28150/ (accessed on 27 May 2021).
- Kling, A. 5-HT2A a Serotonin Receptor with a Possible Role in Joint Diseases; Umeå Universitet: Umeå, Sweden, 2013; Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-64013urn:nbn:se:umu:diva-64013 (accessed on 27 May 2021).
- Andrews, P.W.; Bharwani, A.; Lee, K.R.; Fox, M.; Thomson, J.A. Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci. Biobehav. Rev. 2015, 51, 164–188. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, J. Regulation of Appetite: Role of Serotonin and Hypothalamus. Iran. J. Pharmacol. Ther. 2013, 11, 73–79. [Google Scholar]
- Jørgensen, H.; Knigge, U.; Kjær, A.; Møller, M.; Warberg, J. Serotonergic Stimulation of Corticotropin-Releasing Hormone and Pro-Opiomelanocortin Gene Expression. J. Neuroendocrinol. 2002, 14, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Andrews, A.M. Differentiating Siblings: The Case of Dopamine and Norepinephrine. ACS Chem. Neurosci. 2017, 8, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Schmeichel, B.E.; España, R.A. Noradrenergic Modulation of Wakefulness/Arousal. Sleep Med. Rev. 2012, 16, 187–197. [Google Scholar] [CrossRef]
- Atzori, M.; Cuevas-Olguin, R.; Esquivel-Rendon, E.; Garcia-Oscos, F.; Salgado-Delgado, R.C.; Saderi, N.; Miranda-Morales, M.; Treviño, M.; Pineda, J.C.; Salgado, H. Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic Neurosci. 2016, 8, 2016. [Google Scholar] [CrossRef]
- Levy, B.H.; Tasker, J.G. Synaptic regulation of the hypothalamic–pituitary–adrenal axis and its modulation by glucocorticoids and stress. Front. Cell Neurosci. 2012, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Itoi, K.; Suda, T.; Tozawa, F.; Dobashi, I.; Ohmori, N.; Sakai, Y.; Abe, K.; Demura, H. Microinjection of norepinephrine into the paraventricular nucleus of the hypothalamus stimulates corticotropin-releasing factor gene expression in conscious rats. Endocrinology 1994, 135, 2177–2182. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Clarke, I.J. Noradrenaline and Dopamine Regulation of Prolactin Secretion in Sheep: Role in Prolactin Homeostasis but not Photoperiodism. J. Neuroendocrinol. 2012, 14, 36–44. [Google Scholar] [CrossRef]
- Allen, M.J.; Sabir, S.; Sharma, S. GABA Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK526124/ (accessed on 29 May 2021).
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef]
- Decavel, C.; Pol, A.N.V.D. GABA: A dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 1990, 302, 1019–1037. [Google Scholar] [CrossRef]
- Stratton, M.S.; Searcy, B.T.; Tobet, S.A. GABA Regulates Corticotropin Releasing Hormone Levels in the Paraventricular Nucleus of the Hypothalamus in Newborn Mice. Physiol. Behav. 2011, 104, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A.; Trombley, P. Glutamate neurons in hypothalamus regulate excitatory transmission. J. Neurosci. 1993, 13, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Sam, C.; Bordoni, B. Physiology, Acetylcholine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557825/ (accessed on 31 May 2021).
- Belousov, A.B.; O’Hara, B.F.; Denisova, J.V. Acetylcholine Becomes the Major Excitatory Neurotransmitter in the Hypothalamus In Vitro in the Absence of Glutamate Excitation. J. Neurosci. 2001, 21, 2015–2027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, P.C.; McElroy, W.K.; Cooper, R.L. Differential Modulation of Catecholamines by Chlorotriazine Herbicides in Pheochromocytoma (PC12) Cells in Vitro. Toxicol. Sci. 2000, 56, 324–331. [Google Scholar] [CrossRef]
- Cooper, R.L.; Stoker, T.E.; Tyrey, L.; Goldman, J.M.; McElroy, W.K. Atrazine Disrupts the Hypothalamic Control of Pituitary-Ovarian Function. Toxicol. Sci. 2000, 53, 297–307. [Google Scholar] [CrossRef]
- Walters, J.L.; Lansdell, T.A.; Lookingland, K.J.; Baker, L.E. The effects of gestational and chronic atrazine exposure on motor behaviors and striatal dopamine in male Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2015, 289, 185–192. [Google Scholar] [CrossRef]
- Li, Y.-S.; He, X.; Ma, K.; Wu, Y.-P.; Li, B.-X. The Effect of Exposure to Atrazine on Dopaminergic Development in Pubertal Male SD Rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2015, 104, 184–189. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.-S.; Yang, J.-W.; Yu, J.; Wu, Y.-P.; Li, B.-X. Exposure to Atrazine during Gestation and Lactation Periods: Toxicity Effects on Dopaminergic Neurons in Offspring by Downregulation of Nurr1 and VMAT2. Int. J. Mol. Sci. 2014, 15, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Yang, J.; Wu, Y.; Yu, J.; Li, B. Age-dependent dopaminergic dysfunction following fetal exposure to atrazine in SD rats. Environ. Toxicol. Pharmacol. 2014, 37, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Bi, H.; Li, B. The MEK/ERK/CREB signaling pathway is involved in atrazine induced hippocampal neurotoxicity in Sprague Dawley rats. Ecotoxicol. Environ. Saf. 2019, 170, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Y.; Li, J.-N.; Wu, Y.-P.; Zhang, B.; Li, B.-X. Atrazine Causes Autophagy- and Apoptosis-Related Neurodegenerative Effects in Dopaminergic Neurons in the Rat Nigrostriatal Dopaminergic System. Int. J. Mol. Sci. 2015, 16, 13490–13506. [Google Scholar] [CrossRef] [PubMed]
- Filipov, N.M.; Stewart, M.A.; Carr, R.L.; Sistrunk, S.C. Dopaminergic toxicity of the herbicide atrazine in rat striatal slices. Toxicology 2007, 232, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Filipov, N.M. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology 2008, 248, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lin, L.; Sánchez, O.F.; Bryan, C.; Freeman, J.L.; Yuan, C. Pre-differentiation exposure to low-dose of atrazine results in persistent phenotypic changes in human neuronal cell lines. Environ. Pollut. 2021, 271, 116379. [Google Scholar] [CrossRef] [PubMed]
- Bardullas, U.; Giordano, M.; Rodríguez, V.M. Chronic atrazine exposure causes disruption of the spontaneous locomotor activity and alters the striatal dopaminergic system of the male Sprague–Dawley rat. Neurotoxicol. Teratol. 2011, 33, 263–272. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Limón-Pacheco, J.H.; Mendoza-Trejo, M.S.; González-Gallardo, A.; Hernández-Plata, I.; Giordano, M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. NeuroToxicology 2013, 34, 82–94. [Google Scholar] [CrossRef]
- Rodriguez, V.M.; Mendoza-Trejo, M.S.; Hernandez-Plata, I.; Giordano, M. Behavioral effects and neuroanatomical targets of acute atrazine exposure in the male Sprague-Dawley rat. NeuroToxicology 2017, 58, 161–170. [Google Scholar] [CrossRef]
- Belloni, V.; Dessì-Fulgheri, F.; Zaccaroni, M.; Di Consiglio, E.; De Angelis, G.; Testai, E.; Santochirico, M.; Alleva, E.; Santucci, D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology 2011, 279, 19–26. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Weber, G.J.; Sepúlveda, M.S.; Xiao, C.; Cannon, J.R.; Freeman, J.L. Developmental origins of neurotransmitter and transcriptome alterations in adult female zebrafish exposed to atrazine during embryogenesis. Toxicology 2015, 333, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dodd, C.A.; Xiao, S.; Krishna, S.; Ye, X.; Filipov, N.M. Gestational and Lactational Exposure to Atrazine via the Drinking Water Causes Specific Behavioral Deficits and Selectively Alters Monoaminergic Systems in C57BL/6 Mouse Dams, Juvenile and Adult Offspring. Toxicol. Sci. 2014, 141, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.D.; Chia, J.H.Z.; Johnson, S.L. Paternal exposure to a common herbicide alters the behavior and serotonergic system of zebrafish offspring. PLoS ONE 2020, 15, e0228357. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Pichardo, M.E.; Reyes-Bravo, D.Y.; Mendoza-Trejo, M.S.; Marín-López, A.G.; Giordano, M.; Hernández-Chan, N.; Domínguez-Marchan, K.; Ortega-Rosales, L.C.; Rodríguez, V.M. Brain alterations in GABA, glutamate and glutamine markers after chronic atrazine exposure in the male albino rat. Arch. Toxicol. 2020, 94, 3217–3230. [Google Scholar] [CrossRef]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.; et al. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef]
- Biomonitoring Summary CDC. 6 May 2019. Available online: https://www.cdc.gov/biomonitoring/Atrazine_BiomonitoringSummary.html (accessed on 26 July 2021).
- Barr, D.B.; Panuwet, P.; Nguyen, J.V.; Udunka, S.; Needham, L.L. Assessing Exposure to Atrazine and Its Metabolites Using Biomonitoring. Environ. Health Perspect. 2007, 115, 1474–1478. [Google Scholar] [CrossRef]
- Atrazine—Draft Human Health Risk Assessment for Registration Review. EPA-HQ-OPP-2013-0266-1159; 2018. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2013-0266-1159 (accessed on 26 July 2021).
- Jablonowski, N.D.; Schäffer, A.; Burauel, P. Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environ. Sci. Pollut. Res. 2011, 18, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.K.; Jones, T.L.; Filipov, N.M. Disposition of the Herbicide 2-Chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine (Atrazine) and Its Major Metabolites in Mice: A Liquid Chromatography/Mass Spectrometry Analysis of Urine, Plasma, and Tissue Levels. Drug Metab. Dispos. 2009, 37, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Document Display, NEPIS, US EPA. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100SFAF.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000036%5CP100SFAF.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL# (accessed on 18 August 2021).
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, e00037. [Google Scholar] [CrossRef]
- Zarrindast, M.-R.; Khakpai, F. The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch. Iran. Med. 2015, 18, 591–603. [Google Scholar] [PubMed]
- Comninos, A.N.; Dhillo, W.S. Emerging Roles of Kisspeptin in Sexual and Emotional Brain Processing. Neuroendocrinology 2018, 106, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Leite, C.M.; Kalil, B.; Franci, C.R.; Anselmo-Franci, J.A.; Szawka, R.E. Kisspeptin Regulates Tuberoinfundibular Dopaminergic Neurones and Prolactin Secretion in an Oestradiol-Dependent Manner in Male and Female Rats. J. Neuroendocrinol. 2015, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Aquino, N.S.S.; Kokay, I.C.; Perez, C.T.; Ladyman, S.R.; Henriques, P.C.; Silva, J.F.; Broberger, C.; Grattan, D.R.; Szawka, R.E. Kisspeptin Stimulation of Prolactin Secretion Requires Kiss1 Receptor but Not in Tuberoinfundibular Dopaminergic Neurons. Endocrinology 2019, 160, 522–533. [Google Scholar] [CrossRef]
- Mills, E.G.A.; Dhillo, W.S.; Comninos, A.N. Kisspeptin and the control of emotions, mood and reproductive behaviour. J. Endocrinol. 2018, 239, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, V.; Djolai, M.; Matavulj, M. Alterations in jejunal morphology and serotonin-containing enteroendocrine cells in peripubertal male rats associated with subchronic atrazine exposure. Ecotoxicol. Environ. Saf. 2011, 74, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.-R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef]
- Heisler, L.K.; Pronchuk, N.; Nonogaki, K.; Zhou, L.; Raber, J.; Tung, L.; Yeo, G.S.; O’Rahilly, S.; Colmers, W.F.; Elmquist, J.K.; et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J. Neurosci. 2007, 27, 6956–6964. [Google Scholar] [CrossRef]


| Reference | Species | Atrazine Exposure a | Length of Exposure | Results |
|---|---|---|---|---|
| Foradori et al. [8] | Ovariectomized Adult Female Wistar Rats | 200 mg/kg | 4 days, gavage | Reduction in GnRH pulse frequency at 200 mg/kg |
| Foradori et al. [9] | Ovariectomized Adult Female Wistar Rats | 50, 100, or 200 mg/kg | 4 days, gavage | Reduction in LH and FSH surges at all concentrations and at 200 mg/kg, respectively due to decrease in GnRH neurons in 100 mg/kg and 200 mg/kg concentrations, 4 days after treatment GnRH neuronal function returned to normal |
| Goldman et al. [45] | Ovariectomized, Estrogen-Primed Long Evans Hooded Female Rats | 10, 30, or 100 mg/kg | 4 days or single administration | Suppressed LH surge after four daily treatments at 100 mg/kg, elevations in LH surge after single administration of 10, 30, and 100 mg/kg |
| Kimura et al. [46] | Ovariectomized Female Wistar Rats | 100 mg/kg | 5 days, orally | Reduced LH surge and Kiss1 mRNA expression in AVPV |
| Trentacoste et al. [47] | Peripubertal Male Sprague Dawley Rats | 1–200 mg/kg | Day 22–47, gavage | 100 and 200 mg/kg reduced serum and intratesticular testosterone, reduced serum LH |
| Wirbisky et al. [26] | Zebrafish | 0.3, 3, or 30 ppb (µg/L) | 1–72 hpf | Increase in progesterone at 3 and 30 ppb, increase in follicular atresia |
| Mokhtari et al. [48] | Male Wistar Rats | 100, 200, or 400 mg/kg | 14 days intraperitoneally | Serum LH decreased in 200 and 400 mg/kg group, FSH decreased in 400 mg/kg group, decrease in testosterone in experimental groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stradtman, S.C.; Freeman, J.L. Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine. Toxics 2021, 9, 207. https://doi.org/10.3390/toxics9090207
Stradtman SC, Freeman JL. Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine. Toxics. 2021; 9(9):207. https://doi.org/10.3390/toxics9090207
Chicago/Turabian StyleStradtman, Sydney C., and Jennifer L. Freeman. 2021. "Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine" Toxics 9, no. 9: 207. https://doi.org/10.3390/toxics9090207
APA StyleStradtman, S. C., & Freeman, J. L. (2021). Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine. Toxics, 9(9), 207. https://doi.org/10.3390/toxics9090207