Abstract
Metal nanoparticles have been extensively investigated for different types of pharmaceutical applications. However, their use has raised some concerns about their toxicity involving the increase of reactive oxygen species causing cellular apoptosis. Therefore, in this review we summarize the most relevant toxicity mechanisms of gold, silver, copper and copper oxide nanoparticles as well as production methods of metal nanoparticles. Parameters involved in their toxicity such as size, surface charge and concentration are also highlighted. Moreover, a critical revision of the literature about the strategies used to reduce the toxicity of this type of nanoparticles is carried out throughout the review. Additionally, surface modifications using different coating strategies, nanoparticles targeting and morphology modifications are deeply explained.
1. Introduction
Nanotechnology, the science of material manipulation at nanoscale level, is believed to be one the most promising fields for biomedical applications. The use of nanomaterials provides unique properties that are not observed at the macroscale level [1].
In this sense, one of the most novel and studied nanostructured systems are metal nanoparticles (MNPs) [2]. In this nanotechnological area, the development of techniques for the controlled synthesis of well-defined metal NPs constitutes an enormous challenge. Metal NPs exhibit unique electronic, magnetic, catalytic and optical properties that are different from those of bulk metals [3]. The exclusive features associated with NPs are responsible for their multifunctional properties and developing interest for their application in various fields such as medical and pharmaceutical industry. In these areas, drug and gene delivery are of high interest. Moreover, certain metals have distinctive properties, such as the antimicrobial properties of gold and silver [4].
Several chemical and physical methods are employed to synthesize these nanoparticles such as chemical reduction, electrochemical synthesis, laser ablation method, mechanical milling, microwave-assisted synthesis or polyol synthesis. Depending on which one is used for the preparation of nanoparticles, there will be differences on their morphology, stability and physicochemical properties [5].
In this sense, one of the problems associated with metal and metal oxide NPs is their possible toxicity [6,7]. Toxicity values are directly related to nanoparticles’ properties such as morphology, size or zeta potential [8]. For instance, nanometric size is considered crucial in nanomaterial toxicity due to their higher surface area leading to a greater reactivity [8].
Thus, the selection of preparation methods for metal and metal oxide NPs constitutes a critical parameter to be considered leading to relevant physicochemical properties such as chemical composition, size, solubility, shape or electrical properties, among others, which may be the cause of enhanced toxicity issues to the human body that could result in adverse effects on an organ, tissue or cellular level [9].
The study of the toxicity of metal nanoparticles have received increasing interest and here we describe their toxicity mechanisms, which are of extreme relevance in order to reduce cytotoxic effects in humans.
2. Preparation Methods
Two different strategies are used for the preparation of metal and metal oxide NPs, which are bottom up or top down synthesis, depending on the starting material used [10,11]. When the method consists of starting from a bulk material and it is reduced to NPs by different processes, it constitutes a top-down method, whereas if it starts from a single atom or molecules to produce the final formulation it is a bottom-up method [12]. Here, we describe the most commonly used synthesis methods for metal NPs (Table 1).
2.1. Laser Ablation
This is a top-down process of removing portions from the bulk material by irradiation with a focused laser beam, generating vapor and plasma from target metal immersed in a liquid medium [13]. This vapor-plasma plume with high pressure and temperature is cooled by the surrounding liquid medium, leading to the formation of metal nanoparticles via nucleation and growth steps (Figure 1). The most commonly used vapor lasers are Nd:YAG (Neodymium-doped yttrium aluminum garnet) [14,15,16]. This synthesis method allows a versatile design of the final NPs’ properties by modifying the parameters of the process such as the vapor laser used, time or wavelength of laser pulse, ablation time or liquid medium employed. If these parameters are optimized, this method has the capability of yielding to well-dispersed and stable NPs. This is a simple technique involving an easy experimental procedure with endless possibilities of conditions, which allows fabrication of diverse NPs with desired functions. This technique has been used by several researchers for the preparation of AuNPs, AgNPs or ZnONPs [17,18,19].
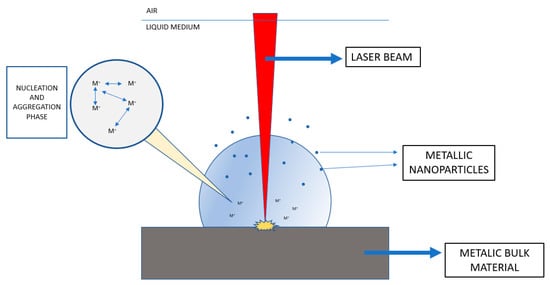
Figure 1.
Laser beam synthesis representation (based on [14,15,16]).
2.2. Spark Discharge
This is a simple process method and one of the most versatile techniques in the gas phase [20,21]. Nanoparticles are obtained by using electrical discharges between two electrodes separated with dielectric gas. Plasma channel between electrodes is formed with dielectric medium breakdown. The generation of current flow is associated with high temperatures that play an important role in removing material from the electrodes. This is followed by a fast-cooling phase by adiabatic expansion and the nanoparticles are formed by nucleation and growth steps, similar to the laser ablation technique. Interestingly, this method does not require the use of chemical precursors and is considered eco-friendly. It has been used to successfully synthesize AuNPs and AgNPs with a controlled size [22,23]. It was also used to synthesize a mixed metallic nanoparticles (Ag-Cu), (Pt-Au) that are immiscible using a combination of the metals in the electrodes [24].
2.3. Evaporation/Condensation
Evaporation/condensation constitutes a synthesis method in gas phase which does not require the use of liquid solvents. In this method the particles are generated by evaporating the material at high temperatures and then decreasing the temperature to condense the metal vapor, forming nanoparticles. This method can be used to control the size of nanoparticles in an accurate manner. Some authors used this method to produce AgNPs with three specific diameters (namely, 50, 90 and 130 nm). The temperature used was 1300–1400 °C. Although AgNPs were successfully obtained, in the collection phase it was observed that some nanoparticles were attached to each other, forming aggregates [25].
Furthermore, other authors were able to eliminate these unwanted aggregates using a silica coating that can avoid flocculation and agglomeration [26].
2.4. Mechanical Milling
Mechanical milling constitutes a top-down process where the starting material is reduced to a nanostructure by mechanical mixing [27,28,29]. When the mill chamber, which is commonly a hollow cylindrical shell partially filled with grinding bodies made of stainless steel or ceramic materials, starts to rotate about a horizontal axis, it produces a rotational motion of the material placed inside this container causing ball drops from the top of the chamber to the bottom, generating impacts and collisions and finally leading to a homogeneous dispersion of NPs from the target material. The main parameters to be controlled which determine the properties of the final NPs are the raw material used, the size of grinding balls, the degree of filling, the rotation speed and the temperature of the process [27].
This synthesis method allows working at low temperatures, this having the advantage of avoiding degradation of thermolabile materials. Moreover, it does not use organic solvents and it is also convenient in order to be employed for large scale production [30]. The main disadvantages to be overcome are the undesired pollution which comes from the milling media and the difficulty of preparing NPs of a small size range [27].
2.5. Electrochemical Synthesis
In the electrochemical synthesis method, it is necessary to use a bulk solution containing metal salts. This technique consists of the electrodeposition of the metal NPs in the substrate surface [31,32]. The main parameters to control in the process are the electrode potential and current density, which will affect the deposition kinetics as well as the nucleation and crystal growth. These parameters are crucial to controlling the properties and morphology of metal NPs. Moreover, nucleation and growth process are important to control in order to synthetize metal NPs with a suitable dispersion [33,34].
2.6. Chemical Reduction Method
Chemical reduction constitutes one of the most popular and used synthesis methods, since it is a simple and low-cost method to obtain controlled particle size with low size dispersion. In this process, a bulk solution containing the metal precursor is used and a surfactant and reducing agent are added during the chemical reaction [35]. This process consists of the reduction of the metal cations contained within the metal salts to form metal atoms [36]. The collisions and aggregations between the formed metal atoms and cations result in the formation of clusters. The nucleation is continuously growing to a critical size when the NPs are stabilized. The rate of nucleation growth is crucial to control the shape and size of metal NPs. This rate can be modified using different surfactants, reducing agents or metal precursors, as well as the pH and the temperature at which the reaction is carried out. The metal precursors are usually salts containing the target metal atom. For instance, AgNPs are usually produced using AgNO3 as a precursor. A surfactant is used in this process to prevent NP aggregation and to disperse them within the solution. Additionally, NaBH4 is widely used as a reducing agent. The use of amine-boranes as reducing agents is being increased [37,38], both for their capability to be used also as stabilizing agents and because they do not require solvent addition [39,40].
One other kind of chemical reduction process is employing polyols that have emerged as a very useful reducing agent. In this process, polyols also act as a solvent of the metal salt precursor. Polyols have some advantages such as their capacity to coordinate particle surfaces, which prevents or minimizes coalescence, its high boiling point and its high viscosity due to the presence of several hydroxyl groups, which allows working at high temperatures and favors the control of particle growth [41]. Several metal NPs have been successfully obtained in the last few years using the polyol synthesis method [42,43,44,45].
2.7. Microwave-Assisted Synthesis
In the last several years, the microwave-assisted synthesis method has received increasing interest due to its effective use of dielectric heating that has demonstrated to reduce the time of reaction and the side reactions, which can lead to enhanced chemical yields and reproducibility of the process. Compared to conventional heat conduction methods, the microwave is more efficient in terms of temperature homogeneity and energy used [46]. This technique is usually combined with the previously mentioned methods. For instance, polyol synthesis can be improved with microwave heating [47,48].
2.8. Green-Synthesis
Nowadays, the development of metal NPs using green-synthesis methods is being intensely studied [49,50,51,52,53]. Application of green chemistry can reduce the use of hazardous solvents or other toxic compounds, improve the energetic efficiency of reactions and processes, and minimize safety issues. The main strategies are based on plant mediated synthesis where a metal salt precursor is obtained from plants. Plant extracts are also rich on alkaloids, flavonoids, terpenoids or phenolic acids that can reduce metallic ions to metal atoms leading to NP formation [54,55,56]. One of the main advantages of this method is the reduction of human and environmental toxicity, since they are more biocompatible and less harmful for health, and the production methods are eco-friendly, using organic natural sources and reducing energy consumption [49].

Table 1.
Production methods of metal nanoparticles [12,57].
Table 1.
Production methods of metal nanoparticles [12,57].
| Synthesis Method | Advantages | Disadvantages |
|---|---|---|
| Laser ablation | Simple and effective Easy to modify nanoparticles properties by changing synthesis parameters | The laser path can be blocked by the portions of material released from the surface, causing reduction in ablation rate |
| Spark discharge | Cost-efficient Environmentally-friendly No impurities | Pure gas is required |
| Evaporation/condensation | Control of size No solvents used | High energy required |
| Mechanical milling | Work at low temperatures No solvent used | High energy required Time consuming method Contamination from milling media |
| Electrochemical synthesis | Simple, fast and inexpensive method Control of size and morphology of nanoparticles | Impurities from liquid media |
| Chemical reduction method | Simple and effective | Impurities from reaction Toxicity issues of reactive agents |
| Microwave-assisted synthesis | More efficient use of energy Higher production rates | Less homogeneity of nanoparticles size and morphology |
| Green synthesis | Eco-friendly Less toxicity Reduction of energy consumption | Use of natural sources Less effective than other methods |
3. Toxicity Mechanisms of Metal Nanoparticles
A large number of drug delivery formulations based on metal NPs are successfully applied in biomedicine, clinics, cosmetics and pharmaceutical industry. While the inclusion of nanomaterials in several products can enhance their performance, on the other hand, there is growing evidence that the small particle size may also induce undesired side effects. The reduced size at the nanoscale level creates complex physicochemical interactions when exposed to a physiological environment [58].
Consequently, despite some unique advantages associated specifically to metal NPs, potential toxic effects should be considered, both for human administration and for the environment after their application [36].
The key to understand the toxicity of metal NPs is that their small size allows them to show an increased penetration rate but also to cause alterations in the cellular redox balance leading to increased production of reactive oxygen species (ROS) and thus causing abnormal cellular functionality, leading to cytotoxic effects (Figure 2) [59,60]. Uncontrolled generation of ROS causes harmful effects on cellular structures such as proteins, lipids and nucleic acids, and some evidence shows that this can also be responsible for the progression of several diseases [61,62,63]. The interaction between lipids and reactive species can cause lipid peroxidation. This process is a chain reaction created by free radicals to form lipid hydroperoxides. The accumulation of hydroperoxides and their subsequent decomposition to alkoxyl and peroxyl radicals accelerates the peroxidation of polyunsaturated fatty acids leading to oxidative damage to cell membranes [64]. It has been shown that the consequences of lipid peroxidation include the decrease of lipid fluidity and a subsequent alteration of its permeability and integrity, leading to a functional loss of cell membranes [65].
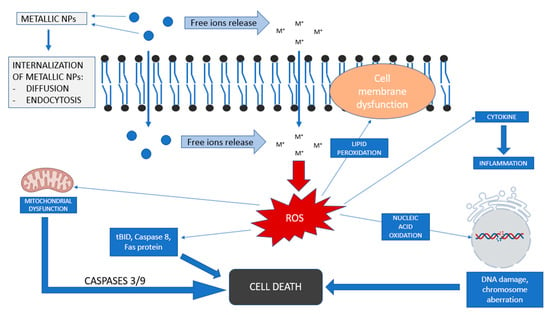
Figure 2.
General view of toxicity mechanisms of metallic nanoparticles (based on [59,60]).
It has also been shown that ROS can also induce DNA damage by several oxidative reactions with DNA bases leading to mutations. Oxidative DNA damage has been demonstrated to play an important role in the initiation and development of cancer [66,67].
Mitochondria also play and important role in cell damage. The overproduction of ROS cause mitochondrial release of apoptogenic signaling molecules inducing caspase cascade responsible for cell death [68,69]. An additional mitochondrial-independent pathway is also induced by ROS by other signaling molecules such as Caspase 8 or Fas protein.
Thus, to ensure the safe development of NP-containing products, scientific knowledge on potential hazards posed by these nanostructured systems needs to be deepened hand-in-hand with the progress in nanotechnological industry. Reaching this ultimate goal will enable us to avoid, at least, synthesizing potential toxic engineered nanomaterials for commercial use. Moreover, understanding the interaction mechanisms of NPs with cells and their consequences is the first defense in hazard prevention also regarding to accidentally produced NPs due to human activities or derived from natural causes.
3.1. Silver Nanoparticles
Silver NPs (AgNPs) are being increasingly used in many different products. They are well known for their antibacterial activity. However, the potential toxicity of AgNPs constitutes a great concern [70,71,72].
AgNPs can induce toxicity mainly by two different pathways. The first one is the release of a Ag+ ions, which are toxic in high concentrations [73,74]. The second one is derived from the nanometric size of AgNPs. These are able to interact with proteins, nucleic acids and carbohydrates of the biological system, modifying their surface properties. However, these surface changes play an important role in the interaction of NPs with cell surface receptors, facilitating the entrance of the NPs via endocytosis. AgNPs’ uptake can also be influenced by their size, shape and concentration [75]. In this area, AgNPs’ concentration-dependent toxicity has been studied in a zebrafish model [76]. In this study, different concentrations (5, 10, 25, 50 and 100 µg/L) of AgNPs were added to zebrafish embryos and incubated for 72 h at 28.5 °C. The mortality of zebrafish embryos increased proportionally to AgNPs’ concentration. The calculated LC50 was stablished between 25–50 µg/L and at 100 µg/L only 15–30% were alive. Hearth rate and hatching rate were also induced by AgNP in a dose-dependent manner. In a preclinical study the skin toxicity of AgNPs was evaluated [77]. Three different concentrations of AgNPs (0.34, 3.4 and 34 µg/mL) were topically administered to a pig animal model. Significant differences of focal inflammations and edemas between 0.34 and 34 µg/mL were observed, being the latter the concentration with higher toxic effects [77]. AgNPs can permeate cell membranes and produce higher levels of intracellular Ag+, causing cytotoxic and genotoxic effects. The cytotoxic effects of AgNPs have mostly been characterized in terms of oxidative stress, DNA damage and modulation of cytokine production. The cellular uptake of AgNPs can stimulate the production of radical oxygen species (ROS), resulting in oxidative stress. ROS can induce cell death either by apoptosis or necrosis [78,79,80].
Regarding genotoxicity, the increased generation of ROS produced by AgNPs can cause DNA damage by decreasing ATP production, which is associated with mitochondrial damage, impairing energy-dependent DNA repair mechanisms [81]. Direct DNA damage by Ag+ or by Ag NPs themselves have also been reported [82,83].
Moreover, in vitro studies have shown the toxic effects of AgNPs in different cells lines. AgNPs have been exposed to murine macrophage cell RAW 264.7 [84], where an increase of cytotoxicity caused by citrate coated 20 nm AgNPs compared with those of 110 nm was observed by causing a more intense acute pulmonary inflammation. The results also showed a reduction of toxicity when AgNPs were coated with PVP avoiding Ag+ complexation.
Other studies have also demonstrated size-dependent toxicity of AgNPs. In this sense, Gliga and colleagues used bronchial epithelial cells (BEAS-2B) that were exposed to AgNPs of different average sizes [85]. The results showed a higher cytotoxicity in the smallest size of AgNPs (10 nm) with higher release of free silver ions [86]. Other studies using alveolar epithelial cells (A549) and human epidermal keratocytes (HEKs) revealed a clear influence of the surface properties of AgNPs, the AgNPs coated with carbon being less cytotoxic [87]. Finally, in an interesting study, human mesenchymal cells exposed to AgNPs show a release of interleukins 6 and 8 and vascular endothelial growth factor (VEGF) secretion. Moreover, DNA damage was evaluated with a COMET assay and chromosomal aberration test, showing significant damage after 1 h and a concentration-dependent toxicity [88]. These results showed that the toxicity of AgNPs strongly depends on size and shape, surface properties and concentration.
3.2. Gold Nanoparticles
Interest in gold nanoparticles (AuNPs) has increased due to their ease of synthesis and their unique properties with a potential use in drug delivery [89,90] and in biological imaging, which makes them attractive tools for cancer detection and therapy [91,92].
It has been found that the toxicity of AuNPs is closely related to their physicochemical parameters that can influence their biological activity and cellular interactions.
Depending on the synthesis method or functionalization processes, an AuNP’s surface can also be modified, being a crucial factor for toxicity due to the fact that different molecular interaction can occur. For instance, Goodman and co-workers found that modifying the surface charge of AuNPs caused different levels of toxicity. They determined that cationic AuNPs were moderately toxic, whereas anionic AuNPs showed no evidence of toxicity [93]. In contrast, in the study developed by Schaeublin and colleagues [94], both anionic and cationic AuNPs caused toxicity, observing in the anionic AuNPs even higher toxicity. This can be explained by the different synthesis methods, using different chemical groups to modulate the surface charge. Moreover, other studies can be found demonstrating the influence of the ligand agent used to modify AuNPs surface [95].
Currently there are very few published studies that examine the risks and side effects of AuNPs, but they indicate that the cause of toxicity can be related to ROS production. In this sense, Jia et al. found out that when the concentration of AuNPs is increased, the NO-released levels were also elevated thus highlighting that a dose-dependent response in reactive nitrogen species (RNS) exists between RNS and AuNPs [96]. This can be due to the fact that NO can react with superoxide producing peroxynitrite species which can interact with DNA, proteins or lipids via oxidative reactions. This reaction may cause oxidative injury, leading to necroptosis or apoptosis [97]. Li et al. also demonstrated that there is a clear evidence that the presence of AuNPs induce oxidative DNA damage [98]. Moreover, other authors also evidence the direct relationship between AuNPs and ROS concluding that at high doses such as 40–50 μg of AuNPs, a significant ROS induction was detected. Moreover, the latter study also showed that ROS generation can be related to production of TNF-α that could be involved in cell death [99]. Further studies showed that the LC50, calculated with Daphnia Magna model, ranged from 65 mg/L to 75 mg/L showing a significance reduction with bimetallic Ag-Au NPs (15 mg/L) [100]. It has also been found that AuNPs can induce autophagy process. Autophagy is a lysosome-dependent degradative pathway that plays an major role in maintaining cellular homeostasis. Ma et al. demonstrated that AuNPs can cause autophagosome accumulation by blocking autophagic flux in a size-dependent manner, showing that 50 nm AuNPs increase the autophagosome accumulation compared to 25 nm and 10 nm [101]. In addition, surface functionalization of AuNPs constitutes a critical aspect to induce autophagy. This has been demonstrated with CTAB-coated Au-nanorods, which possessed higher toxicity than Au-nanorods coated with PSS and PDDAC [102]. This could be interesting for future biomedical applications since recently some researchers have focused on autophagy mechanism as a possible treatment for cancer [103,104].
3.3. Copper/Copper Oxide Nanoparticles
As previously mentioned for Au and AgNPs, ROS and RNS production also play an important role in copper/copper oxide nanoparticles (CuNPs/CuONPs) toxicity [105,106,107]. In this area, Sarkar et al. observed that CuNPs exposure induced the production of ROS and NO, which decreases the activity of antioxidant enzymes [108]. A TUNEL assay was performed to confirm that higher levels of ROS also induce a cascade pathway leading to apoptotic cell death in kidney tissue. This study also demonstrated that the presence of CuNPs induces the release of cytochrome C in the cytosol due to the reduction in mitochondrial membrane potential caused by the alteration of the Bcl/Bax ratio. The Bcl family controls the intrinsic apoptotic pathway. The Bcl2 protein has an antiapoptotic effect, contrary to Bax protein which has pro-apoptotic activity, so the Bcl2/Bax ratio is a key factor to control the caspase cascade leading to a programed cell death [109]. The loss of mitochondrial membrane potential induces the loss of level expressions in Caspases 9 and 3 causing cell death via mitochondria-dependent pathway. In addition, the extrinsic pathway where the exposure of CuNPs produced an increase in the cellular levels of Fas protein, caspase 8 and tBID was studied. It suggests the involvement of a mitochondrial-independent pathway. As such, this study suggests the involvement of two apoptotic pathways to control cell death [108]. Those results are in agreement with other studies investigating the induction of oxidative stress in nanotoxicity. Specifically, Assadian et al. found that the generation of ROS results in the alteration levels of glutathione and peroxidase damage to membrane lipids which can cause cell death in human lymphocytes [110]. Moreover, it has also been described that CuNPs induce apoptosis in HepG2 cells via mitochondrial pathway, evidenced by the modification of Bcl2/Bax ratio originating the caspases 9 and 3 activation [111]. Moreover, it has been reported that the LD50 of CuNPs (23.5 nm) is around 413 mg/Kg. However, this is still controversial. In this area, an interesting study exposed male rats to several CuNPs concentrations (5, 10 and 100 mg/Kg) and after 2, 7 and 14 days the histological changes and hepatic enzymes level were evaluated. The results show that all concentrations induced toxicity as well as modifications in histopathology of liver and lung tissues [112]. In a different study, CuNPs concentrations ranged between 50 and 200 mg/Kg/day were also applied to rats for 5 days. In this case, the results showed lower hepatotoxicity with 50 and 100 mg/Kg/day than at doses of 200 mg/Kg/day [113].
3.4. Zinc/Zinc Oxide Nanoparticles
Zinc and zinc oxide nanoparticles (ZnNPs, ZnONPs) can enter the cell by two different pathways: diffusion of free Zn2+ ions dissolved in extracellular medium and direct internalization of ZnNPs [114]. The dissolution of ZnNPs to release Zn2+ ions has a strong dependence on pH of the medium. The presence of other components in the medium, the UV radiation, the concentration and NPs size are other factors that can have an influence in the release of Zn2+ [115].
ZnO + 2NaOH → Na2ZnO2 + H2O
ZnO + 2HCl → ZnCl2 + H2O
ZnCl2 → Zn2+ + 2Cl−
The internalization of ZnNPs is carried out via endocytosis mediated by membrane receptor or particular endocytic pathways such as the Claritin-dependent pathway, caveolae-independent pathway or caveoloae-dependent pathway [116].
Free Zn2+ generated from ZnNPs has an important effect on toxicity. Studies investigating ZnNPs’ toxicity indicate that a correlation between the concentration of free ions and cell viability exists. Moreover, in this case the consequence is that LDH is released, causing cell membrane damage [117].
Furthermore, the internalized ZnNPs induce the generation of ROS [118], leading to cytokines prompting inflammation that can result in cell death [119].
The toxicity effects of 20 nm ZnONPs were investigated at different doses (5, 50, 100, 300, 1000 and 2000 mg/Kg) administered to rats by oral route [120]. Surprisingly, lower doses showed higher toxicity effects. This might be caused by less agglomeration of ZnO NPs at lower doses that allows them to penetrate into the cells easily [120]. However, future research needs to be focused on lower doses in order to determine the LD50 of Zn and ZnONPs.
3.5. Iron Oxide Nanoparticles
The toxicity of iron oxide nanoparticles (FeONPs) is closely related to their size, characteristics of the surface, shape and concentration. Fe2O3 (maghemite) and Fe3O4 (magnetite) are the most widely used iron materials for NPs synthesis. However, maghemite is preferred to be used since Fe3+ can already be found in the human body and is less likely to cause toxic effects. They differ in the oxidation state which modifies their physicochemical properties [121]. The release of iron in cellular medium can produce free radicals by Fenton reaction, in which the combination of iron ions and hydrogen peroxide (H2O2) generates hydroxyl radicals [122]. The accumulation of hydroxyl radicals can induce lipid peroxidation processes which leads to ferroptosis causing cell death. Recently, iron-based NPs have been studied in cancer therapy due to this phenomenon [123].
Moreover, FeONPs are used as MRI contrast agents at a concentration of 0.56 mg/Kg. Furthermore, concentration-dependent toxicity of FeONPs has been also studied in vitro using murine macrophage cell line J774 at concentrations ranging between 25 and 500 µg/L [124]. Cell viability at 25 µg/L was 95–100% and decreased up to 55–65% at 500 µg/L. Apoptosis index at 25 µg/L was 1.9 and increased up to 26.8 at 500 µg/L [124].
Another in vitro cellular study showed that FeONPs were able to cause cellular membrane damage in erythrocytes, altering their mechanical properties and inducing oxidative stress [125]. The main sources of ROS production are direct generation from their surface, via FeONPs disintegration, from organelle dysfunction or some induction of cell signaling pathways [126,127,128].
Another study showed that internalized FeONPs can induce ROS production in a dose and time-dependent manner. As concentration of FeONPs increased, higher ROS formation was also observed. No further increase in ROS generation was observed at higher concentrations (2 mg/mL, 3 mg/mL), which may be due to saturation in the cellular surface, blocking the uptake of additional FeONPs. Therefore, cellular uptake was at maximum during the first 3 h of exposure, and no increase was found after 4 h, probably due to saturation of the cellular entrance mechanisms [129].
4. Strategies to Reduce Metal Nanoparticles Toxicity
In spite of their great potential for pharmacological use, metal NPs are associated with toxicity issues. The main toxicity problems are closely linked with the release of free ions, their reactivity with biological molecules, tendency to agglomerate, or oxidative damage.
Toxicity can be modified depending on the characteristics of metal NPs, such as size and shape, surface charge or composition, which, at the same time, are closely related to the synthesis method. The development and optimization of synthetic routes are crucial to reduce or eliminate the toxicity issue. Moreover, reduction toxicity strategies are summarized in Table 2.
4.1. Surface Functionalization
In order to stabilize metal NPs, in addition to enhancing their uptake and biocompatibility, organic functional groups have been used to modify NPs’ surfaces.
One of the most commonly used organic groups for functionalization is polyethylene glycol (PEG), which produces a steric barrier preventing the attachment of phagocytes and shielding the surface from aggregation and opsonization, leading to a prolonged time on the systemic circulation (Figure 3) [8,130,131]. These neutral, flexible, and hydrophilic PEG chains help in minimizing adverse immunological effects [132]. PEG weights range from hundreds to several thousands of Daltons. Additionally, low-weight PEGs are highly soluble in water but PEG solubility decreases with increasing molecular weight [133]. In general terms, to achieve the necessary stealth properties, the most suitable molecular weight of PEG has been reported between 1500–5000 Da [133]. However, with ZnO, TiO2 and Cu2O it was observed that low-molecular-weight PEG has a smaller steric effect than long-chain PEGs [133].
Moreover, the terminal OH group of PEG may be selectively oxidized to functionalize PEG with various terminal end groups or to attach large ligands [133]. The use of PEG with additional amino (PEG-NH2) and thiol (PEG-SH) groups has been advocated as useful strategy [134]. AuNPs with average size ranged between of 1.5 and 5.9 nm have been produced and functionalized with PEG-SH and also with PEG-NH2 groups. These NPs show better physical and biological properties for drug delivery applications and no cytotoxic effects were observed in AuNPs-PEG-SH, in contrast with AuNPs-PEG/NH2 and AuNPs-PEG [134]. Using AuNPs, it has also been reported that the spacer length of the PEG (the link between the NP surface and the PEG) also plays a major role, especially when fluorescent tags are also attached to the NPs surface [133].
In this area, a significant reduction of cytotoxicity was also observed in ZnNPs functionalized with PEG. The use of PEG reduced the formation of the protein corona, leading to lower toxicity compared to uncoated ZnNPs [135]. Moreover, Wuelfing et al. reported that a monolayer thiol PEGylation of surface metallic AuNPs with octaedral shape by CH3O–PEG–SH significantly improved their dispersion stability in aqueous milieu due to the steric repulsion effects of ethered PEG strands [132,136].
In addition, several potential safety concerns have been recently raised from the repeated use of PEG-related products since PEG is not biodegradable. Usually, lower molecular weight PEGs are preferable for biomedical applications since high molecular weight PEG can be acccumulated on some tissues. Moreover, under exogenous stress conditions such as heat, radiation or mechanical forces, PEG showed degradation [132]. Furthermore, PEG’s potential synthetic impurities such as 1,4-dioxane, formaldehyde and cyclic dimer of the ethylene oxide emphasize the usage of a highly technical grade of PEG for biomedical applications [132].
Surface charge is another relevant factor to consider, since it has influence in the NPs’ blood stream solubility and interaction with biological molecules and cell membranes. Therefore, a really interesting computational study showed the effects of AuNPs functionalization with carboxylic groups. These may to be spontaneously protonated on the AuNP’s surface, leading to a more controlled and less disruptive interaction with cell membranes [137]. However, in vivo results will be necessary in order to corroborate this result.
4.2. Antibody Functionalization
Antibody-functionalized metal NPs constitute a novel and promising strategy to reduce toxicity by enhancing targeting and effectiveness of drug delivery. In this area, an interesting study using ranibizumab, showed significant improvements in reduction of toxicity in hybrid Au/Fe3O4NPs. Ranibizumab-coated Au/Fe3O4NPs were successfully prepared with a non-covalent binding synthesis technique. The MTT assay showed no toxicity of Ranibizumab-coated Au/Fe3O4NPs [138].
In this area, the use of metal NPs coated with antibodies has a huge potential for cancer treatment, providing better targeting and control of the drug release in specific tumor areas [139].
For instance, AuNPs were conjugated with cetuximab antibody by Li and coworkers to enhance the specificity of AuNPs to human cancer cells through the conjugation to tumor-specific ligands such as EGFR, which is expected to be a promising candidate for cancer therapy [140]. They use the combination of AuNPs charged with H+ particles functionalized with antibodies for radiosensitizing therapy. The results showed a good binding capcity between cetuximab and and EGFR in a concentration-dependent manner leading to a rapid and efficient cell uptake. An enhancement of the effect by proton radiation was observed compared with non-targeted AuNPs [141].
4.3. Coating Modification
The primary purpose of coating modifications is to reduce metal ion release and agglomerations and avoid oxidation processes.
Within this field, silica-based coatings are the most frequently used. In this sense, ZnNPs have been prepared by Chia et al and coated with silica to prevent the release of Zn+2. The results showed that the presence of the silica coating effectively reduced cytotoxicity in addition to retaining the antimicrobial properties of ZnNPs [142]. Another study carried out in vitro using FeONPs coated with thin silica shell in A549 and HeLa cells showed a significant reduction of ROS production in Fe3O4/SiO2 compared to uncoated NPs, leading to a decrease of cytotoxicity [143]. Moreover, a reduction of DNA damage was also observed [143]. Other authors also found that the use of silica coating in AgNPs effectively reduced their toxicity. SiO2 was demonstrated to prevent the direct interaction of cells with AgNP surfaces and the liberation of Ag2+ ions [144,145].
Chitosan, a natural alkalyne polysaccharide with good biocompatibility and biodegradability, constitutes a widely used compound for NPs coating. This long chain carbohydrate can avoid surface oxidation and control the disintegration of metal NPs, reducing their potential toxicity. Chitosan coating was shown to effectively reduce toxicity of CuNPs in an in vitro study using human A549 cells. This toxicity reduction appears to be linked to decreased ROS generation, suggesting that chitosan coating enhances the release control of copper ions [146]. In a different study, chitosan-coated AuNPs were synthesized to evaluate the uptake, cytotoxicity and immunological responses. The results showed that chitosan can modullate the interaction between AuNPs and proteins in cellular culture medium, modifying cellular responses. The human monocytic cell line was used to assess the uptake and cytotoxicity of chitosan coated AuNPs. Cellular internalization capacity of chitosan coated AuNPs has been compared with citrated funtionalized AuNPs. The positive charge of chitosan has been shown to associate with negatively charged lipid bilayers leading to a greater and faster internalization compared with anionic coatings. Cationic surface has also shown to enhance the interaction with serum proteins since most of them are anionic improving phyisicochemical properties of AuNPs. However, it has to be taken into account the observed cellular inflammatory responses induced by chitosan due to the enhanced particle interaction [146,147]. Figure 3 summarizes the surface and coating modification strategies of metal and metal oxide nanoparticles.
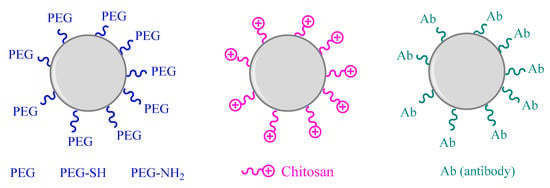
Figure 3.
Surface and coating modification strategies of metal and metal oxide nanoparticles (based on [134,138,146]).
4.4. Morphology
4.4.1. Size
The average size of synthesized metal NPs ranges from 1 to 100 nm. This parameter confers different properties compared to the bulk material form. The higher surface area enhances their reactivity, with higher number of molecules interaction in cellular system. Moreover, the reduction of size also allows for enhancing the cellular uptake of NPs, leading to a higher concentration inside the cell [148]. Thus, size plays an important role in determining the toxicity of nanomaterials. Some studies demonstrate the size-dependent toxicity of metal nanoparticles. For instance, a citotoxicity study was carried out exposing different AgNP sizes (20 nm, 80 nm, 113 nm) to RAW 264.7 and L929 cells. The results showed significant differences in ROS production and extracellular LDH activity, being higher in the case of the smallest AgNPs [149].
However, not all studies show higher uptake and toxicity with the smallest NPs. A study developed with AuNPs was performed, exposing different AuNPs sizes (namely, 14, 30, 50, 74 and 100 nm) to cells. Results showed that the greater uptake was obtained by 50 nm AuNPs with no significant difference in toxicity among the populations assesed [150]. On the other hand, an interesting in vivo study showed highest adverse effects in mice of AuNPs with sizes ranging from 8 to 37 nm, while nanoparticles of 3, 5, 50 and 100 nm did not show any cytotoxic effects [151].
Although the majority of the evidence points out a trend towards increased toxicity related with smaller NP size, further studies will be necessary. However, it is sure that size-dependent toxicity has to be taken into account when developing metal and metal oxide NPs in order to reduce their possible side effects.
4.4.2. Shape
Shape is also a critical factor contributing to toxic effects. Different NP shapes have influence in the interaction with biological molecules and to cross through biological barriers. Metal NPs can be synthesized in many different shapes such as spheres, triangles, rods, stars and cubic prisms (Figure 4) [149,152].
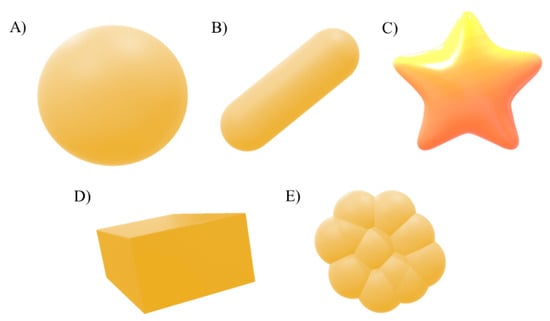
Figure 4.
Common shapes of AuNPs; sphere (A), rod (B), star (C) triangles, rods, stars and cubic (D) and triangle-like (E) prisms (based on [155]).
Nanospheres, nanostars and nanorods based on AuNPs have been synthesized to investigate the influence of shape [153]. Even though the results showed the highest uptake with the nanospherical shape and the lowest with nanostars, the cytotoxicity of nanospheres was also the lowest, suggesting that higher uptake does not always induce higher toxicity [153]. Recently, it has been demonstrated that crystal orientation of metal nanocrystals has a considerable influence in cytotoxicity [154]. The results of this study showed that 100 Pd nanocrystals show less toxicity than 111 Pd nanocrystals. These results seem to be related to the stronger oxygen-binding capacity of 100 Pd nanocrystals compared to 111, resulting in less hydroxyl radical generation and reducing the oxidative damage.

Table 2.
Summary of reduction of toxicity strategies employed in metal nanoparticles and the techniques used to assed their characterization and therapeutic efficacy (ND: no data available).
Table 2.
Summary of reduction of toxicity strategies employed in metal nanoparticles and the techniques used to assed their characterization and therapeutic efficacy (ND: no data available).
| Stragey Employed | Type of Metal NP | Functionalitzation Stragey | Physicochemical Characteritzation | In Vitro Studies | In Vivo Studies | References |
|---|---|---|---|---|---|---|
| Surface functionalization | Gold nanoparticles | PEG-SH and PG-NH2 groups | TEM/HRTEM, UV-Vis spectroscopy | Citotoxicity assay in SAOS-2 cell line cultivated in McCoy’s 5A medium with 15% heat-inactivated FBS, penicillin and streptomycin | ND | [134] |
| Surface functionalization | Zinc nanoparticles | PEG | FTIR | Citotoxicity assay in THP-1 immune cells | ND | [135] |
| Surface functionalization | Gold nanoparticles | Anionic ligands | ND | ND | ND | [137] |
| Antibody funtionalization | Gold coated magnetite | Antibody raanibizumab | SEM, DLS, XRD, TGA | Citotoxicity test by MTT assay | ND | [138] |
| Coating modification | Zinc nanoparticles | Silica coating | TEM, XPS, EDX, FTIR | Citotoxicity assessment in both colorectal epithelial cell lines (SW480 and DLD-1) | ND | [142] |
| Coating modification | Iron oxide nanoparticles | Silica coating | TEM, DLS and potential measurements | Citotoxicity assay in HeLa and A549 cells | ND | [143] |
| Coating modification | Silver nanoparticles | Silica coating | TEM and SEM images, optical absortion | Toxicity evaluation with E. coli bacteria | ND | [26] |
| Coating modification | Copper nanoparticles | Chitosan coating | XPS, XRD, TEM, DLS | Citotoxicity with human alveolar epithelial cell (A549) using standard MTS assay | In vivo study using mice by nasal administration to investigate inflammatory responses | [146] |
| Size modification | Silver Nanoparticles | ND | TEM, DLS, Z-potential | Citotoxicity study with murine peritoneal macrophage cell line (RAW 264.7) and L929 fibroblasts | ND | [149] |
| Size modification | Gold nanoparticles | ND | TEM, ICP-MS | In vitro study with HeLa cells by MTT assay | Mice intraperitoneal injection into BALB/C at a dose of 8 mg/kg/week | [151] |
| Shape modification | Gold nanoparticles | chitosan | HR Tem images | In vitro study into four cancer cell lines: AGS, HepG2, HT29, HeLa by MTT assay | ND | [156] |
5. Conclusions
In summary, metal and metal oxide NPs show unique properties that allow them to be a promising tool for the therapeutic treatment of several diseases. Since the use of these nanosystems are of increasing interest, more experts are focusing on the investigation of their abilities to be used for biomedical applications. However, toxicity mechanisms need to be deeply understood in order to be able to reduce their risks. In general terms, the most critical issues are the release of free metal ions, the intracellular uptake and the oxidative reactions leading to inflammatory responses.
The optimization of synthesis methods and the reduction of nanotoxicity are interlocking goals that need to be pursued in parallel, since it is clear that the morphology and physicochemical properties of NPs are closely linked with toxicity. Importantly, metals are not biodegradable materials and it is of crucial relevance to find the most adequate surface functionalization in order to increase their biocompatibility and improve their targeting, trying to avoid the generation of harmful species in body systemic circulation. Optimization of size and shape combined with functionalitzation of NPs by surface coating or ligands such as antibodies attached to their surface consitutes a successful tool to reduce the toxicity of metal NPs.
Author Contributions
V.G.-T., A.C. (Amanda Cano), M.E. (Marta Espina) and M.E. (Miren Ettcheto) contributed for the conceptualization, methodology, validation, formal analysis and investigation and for the writing—original draft preparation; A.C. (Antoni Camins), E.B., E.S.-L., M.V.-C., M.L.G. and E.B.S. contributed for the supervision, writing the second version, editing the revision, project administration, resources and funding acquisition. All authors have made a substantial contribution to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Portuguese Science and Technology Foundation (FCT) from the Ministry of Science and Technology (MCTES), European Social Fund (FSE) of EU, through the project UIDB/04469/2020 (CEB strategic fund), co-funded by European Funds (PRODER/COMPETE) and FEDER, under the Partnership Agreement PT2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Sachin, K.; Karn, S.K. Microbial Fabricated Nanosystems: Applications in Drug Delivery and Targeting. Front. Chem. 2021, 9, 617353. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Combined Toxicity of Metal Nanoparticles: Comparison of Individual and Mixture Particles Effect. In Protein Kinase-mediated Decisions Between Life and Death; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 165–193. ISBN 978-3-030-49844-3. [Google Scholar]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Parsai, T.; Kumar, A. Weight-of-evidence process for assessing human health risk of mixture of metal oxide nanoparticles and corresponding ions in aquatic matrices. Chemosphere 2021, 263, 128289. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Isaacoff, B.P.; Brown, K.A. Progress in Top-Down Control of Bottom-Up Assembly. Nano Lett. 2017, 17, 6508–6510. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Zhang, J.; Claverie, J.; Chaker, M.; Ma, D. Colloidal Metal Nanoparticles Prepared by Laser Ablation and their Applications. ChemPhysChem 2017, 18, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Alhamid, M.Z.; Hadi, B.S.; Khumaeni, A. Synthesis of silver nanoparticles using laser ablation method utilizing Nd:YAG laser. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2202, p. 020013. [Google Scholar] [CrossRef]
- Vahabzadeh, E.; Torkamany, M.J. Iron Oxide Nanocrystals Synthesis by Laser Ablation in Water: Effect of Laser Wavelength. J. Clust. Sci. 2014, 25, 959–968. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of nanoparticles by laser ablation: A review. KONA Powder Part. J. 2017, 2017, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Al-Nassar, S.I.; Hussein, F.I.; Ma, A.K. The effect of laser pulse energy on ZnO nanoparticles formation by liquid phase pulsed laser ablation. J. Mater. Res. Technol. 2019, 8, 4026–4031. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Laser ablation synthesis of gold nanoparticles in organic solvents. J. Phys. Chem. B 2006, 110, 7232–7237. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; Del Val, J.; Pou, J. Production of silver nanoparticles by laser ablation in open air. Appl. Surf. Sci. 2015, 336, 108–111. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Ullmann, M.; Vons, V.A.; Lafont, U.; Schmidt-Ott, A. Generation of nanoparticles by spark discharge. J. Nanoparticle Res. 2009, 11, 315–332. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ghosh, A. A thermo-electric model of material removal during electric discharge machining. Int. J. Mach. Tools Manuf. 1999, 39, 669–682. [Google Scholar] [CrossRef]
- Messing, M.E.; Dick, K.A.; Wallenberg, L.R.; Deppert, K. Generation of size-selected gold nanoparticles by spark discharge—For growth of epitaxial nanowires. Gold Bull. 2009, 42, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.-C.; Jung, J.-H.; Park, H.-H.; Ji, J.-H.; Kim, S.-S. Generation of Silver Nanoparticles by Spark Discharge Aerosol Generator Using Air as a Carrier Gas. Trans. Korean Soc. Mech. Eng. B 2006, 30, 170–176. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Xu, Q.; Van Der Pers, N.M.; Schmidt-Ott, A. Generation of mixed metallic nanoparticles from immiscible metals by spark discharge. J. Nanoparticle Res. 2010, 12, 247–259. [Google Scholar] [CrossRef]
- Harra, J.; Mäkitalo, J.; Siikanen, R.; Virkki, M.; Genty, G.; Kobayashi, T.; Kauranen, M.; Mäkelä, J.M. Size-controlled aerosol synthesis of silver nanoparticles for plasmonic materials. J. Nanoparticle Res. 2012, 14, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiriou, G.A.; Sannomiya, T.; Teleki, A.; Krumeich, F.; Vörös, J.; Pratsinis, S.E. Non-toxic dry-coated nanosilver for plasmonic biosensors. Adv. Funct. Mater. 2010, 20, 4250–4257. [Google Scholar] [CrossRef] [Green Version]
- Prasad Yadav, T.; Manohar Yadav, R.; Pratap Singh, D. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef] [Green Version]
- Mancillas-Salas, S.; Hernández-Rodríguez, P.; Reynosa-Martínez, A.C.; López-Honorato, E. Production of aluminum nanoparticles by wet mechanical milling. MRS Adv. 2020, 5, 3133–3140. [Google Scholar] [CrossRef]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of iron oxide nanoparticles by mechanical milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Jeun, Y.E.; Baek, B.; Lee, M.W.; Ahn, H.S. Surfactant-free electrochemical synthesis of metallic nanoparticles via stochastic collisions of aqueous nanodroplet reactors. Chem. Commun. 2018, 54, 10052–10055. [Google Scholar] [CrossRef] [PubMed]
- McDarby, S.P.; Wang, C.J.; King, M.E.; Personick, M.L. An Integrated Electrochemistry Approach to the Design and Synthesis of Polyhedral Noble Metal Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21322–21335. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical synthesis of metal oxides and hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Nasretdinova, G.R.; Kokorekin, V.A. Mediated electrochemical synthesis of metal nanoparticles. Russ. Chem. Rev. 2018, 87, 1080–1110. [Google Scholar] [CrossRef]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of Metal Nanoparticles and Their Pharmacological Applications: Present Status and Application Prospects. J. Nanostruct. Chem. 2018, 12, 217–254. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A.O. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef]
- Lidor-Shalev, O.; Zitoun, D. Reaction mechanism of “amine-borane route” towards Sn, Ni, Pd, Pt nanoparticles. RSC Adv. 2014, 4, 63603–63610. [Google Scholar] [CrossRef]
- Pelletier, F.; Ciuculescu, D.; Mattei, J.G.; Lecante, P.; Casanove, M.J.; Yaacoub, N.; Greneche, J.M.; Schmitz-Antoniak, C.; Amiens, C. On the use of amine-borane complexes to synthesize iron nanoparticles. Chem. Eur. J. 2013, 19, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, U.; Jagirdar, B.R. Metal and alloy nanoparticles by amine-borane reduction of metal salts by solid-phase synthesis: Atom economy and green process. Inorg. Chem. 2012, 51, 13023–13033. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S.B.; Sanyal, U.; Jagirdar, B.R. Chemical synthesis of metal nanoparticles using amine-boranes. ChemSusChem 2011, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D. From formation mechanisms to synthetic methods toward shape-controlled oxide nanoparticles. Nanoscale 2013, 5, 9455–9482. [Google Scholar] [CrossRef]
- Carroll, K.J.; Reveles, J.U.; Shultz, M.D.; Khanna, S.N.; Carpenter, E.E. Preparation of elemental Cu and Ni nanoparticles by the polyol method: An experimental and theoretical approach. J. Phys. Chem. C 2011, 115, 2656–2664. [Google Scholar] [CrossRef]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ungelenk, J.; Speldrich, M.; Dronskowski, R.; Feldmann, C. Polyol-mediated low-temperature synthesis of crystalline tungstate nanoparticles MWO4 (M = Mn, Fe, Co, Ni, Cu, Zn). Solid State Sci. 2014, 31, 62–69. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gómez, M. Palladium Nanoparticles in Polyols: Synthesis, Catalytic Couplings, and Hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis—A critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Blosi, M.; Albonetti, S.; Dondi, M.; Martelli, C.; Baldi, G. Microwave-assisted polyol synthesis of Cu nanoparticles. J. Nanoparticle Res. 2011, 13, 127–138. [Google Scholar] [CrossRef]
- Li, D.; Komarneni, S. Microwave-assisted polyol process for synthesis of Ni nanoparticles. J. Am. Ceram. Soc. 2006, 89, 1510–1517. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Alayli, A.; Nadaroğlu, H.; Alayli Güngör, A.; İnce, S. Synthesis of Nanoparticles by Green Synthesis Method. Int. J. Innov. Res. Rev. 2017, 1, 6–9. [Google Scholar]
- Nasrollahzadeh, M.; Ghorbannezhad, F.; Issaabadi, Z.; Sajadi, S.M. Recent Developments in the Biosynthesis of Cu-Based Recyclable Nanocatalysts Using Plant Extracts and their Application in the Chemical Reactions. Chem. Rec. 2019, 19, 601–643. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Daculsi, R.; De Portal, C.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxic effects and cellular oxidative mechanisms of metallic nanoparticles on renal tubular cells: Impact of particle solubility. Toxicol. Res. 2015, 4, 409–422. [Google Scholar] [CrossRef]
- Lozano, T.; Rey, M.; Rojas, E.; Moya, S.; Fleddermann, J.; Estrela-Lopis, I.; Donath, E.; Wang, B.; Mao, Z.; Gao, C.; et al. Cytotoxicity effects of metal oxide nanoparticles in human tumor cell lines. J. Phys. Conf. Ser. 2011, 304, 012046. [Google Scholar] [CrossRef] [Green Version]
- Taniyama, Y.; Griendling, K.K. Reactive Oxygen Species in the Vasculature: Molecular and Cellular Mechanisms. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A. Formation of free radicals and mechanisms of action in normal biochemical processes and pathological states. New Compr. Biochem. 1994, 28, 131–153. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Li, X.M.; Gugiu, B.G.; Gu, X.; Qin, J.; Salomon, R.G.; Hazen, S.L. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 2008, 283, 2385–2396. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Oberley, T.D. Commentary Oxidative Damage and Cancer. Am. J. Pathol. 2002, 160, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zou, H.; Luo, T.; Long, M.; Bian, J.; Liu, X.; Gu, J.; Yuan, Y.; Song, R.; Wang, Y.; et al. Caspase-dependent and caspase-independent pathways are involved in cadmium-induced apoptosis in primary rat proximal tubular cell culture. PLoS ONE 2016, 11, e0166823. [Google Scholar] [CrossRef] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef]
- Smith, J.N.; Thomas, D.G.; Jolley, H.; Kodali, V.K.; Littke, M.H.; Munusamy, P.; Baer, D.R.; Gaffrey, M.J.; Thrall, B.D.; Teeguarden, J.G. All that is silver is not toxic: Silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part. Fibre Toxicol. 2018, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Mizuta, Y.; Akagi, J.I.; Toyoda, T.; Sone, M.; Ogawa, K. Size-dependent acute toxicity of silver nanoparticles in mice. J. Toxicol. Pathol. 2018, 31, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Gaillet, S.; Rouanet, J.M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. 2015, 77, 58–63. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- DiFonzo, N.; Bordia, P. Reproduced with permission of the copyright owner. Further reproduction prohibited without. J. Allergy Clin. Immunol. 1998, 130, 556. [Google Scholar]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Almofti, M.R.; Ichikawa, T.; Yamashita, K.; Terada, H.; Shinohara, Y. Silver ion induces a cyclosporine A-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome c. J. Biochem. 2003, 134, 43–49. [Google Scholar] [CrossRef]
- Hwang, M.G.; Katayama, H.; Ohgaki, S. Inactivation of Legionella pneumophila and Pseudomonas aeruginosa: Evaluation of the bactericidal ability of silver cations. Water Res. 2007, 41, 4097–4104. [Google Scholar] [CrossRef]
- Uygur, B.; Craig, G.; Mason, M.D.; Ng, A.K. Cytotoxicity and genotoxicity of silver nanomaterials. NSTI Nanotechnol. 2009, 2, 383–386. [Google Scholar]
- Chi, Z.; Liu, R.; Zhao, L.; Qin, P.; Pan, X.; Sun, F.; Hao, X. A new strategy to probe the genotoxicity of silver nanoparticles combined with cetylpyridine bromide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 577–581. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 2014, 10, 385–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliga, A.R.; Di Bucchianico, S.; Lindvall, J.; Fadeel, B.; Karlsson, H.L. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci. Rep. 2018, 8, 6668. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health Perspect. 2010, 118, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. J. Deliv. Target. Ther. Agents 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Durr, N.J.; Larson, T.; Smith, D.K.; Korgel, B.A.; Sokolov, K.; Ben-yakar, A. Two-Photon Luminescence Imaging of Cancer Cells Using Molecularly Targeted Gold Nanorods. Nano Lett. 2007, 7, 941–945. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Wang, Z. Gold Nanoparticles With Special Shapes: Controlled Synthesis, Surface-enhanced Raman Scattering, and The Application in Biodetection. Sensors 2007, 7, 3299–3311. [Google Scholar]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J. Am. Chem. Soc. 2009, 131, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Chaicherd, S.; Killingsworth, M.C.; Pissuwan, D. Toxicity of gold nanoparticles in a commercial dietary supplement drink on connective tissue fibroblast cells. SN Appl. Sci. 2019, 1, 336. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Whaley Bishnoi, S. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Cao, W.; Ma, B.; Ma, X.; Jin, S.; Zhang, J.; Wang, P.C.; Li, F.; Liang, X.J. Anchoring effects of surface chemistry on gold nanorods: Modulating autophagy. J. Mater. Chem. B 2015, 3, 3324–3330. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; Arias, A.; Martínez-García, D.; Pérez-Tomás, R.; Quesada, R.; Soto-Cerrato, V. Targeting Autophagy for Cancer Treatment and Tumor Chemosensitization. Cancers 2019, 11, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Forouhar Vajargah, M.; Mohamadi Yalsuyi, A.; Hedayati, A.; Faggio, C. Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc. Res. Tech. 2018, 81, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of Copper Oxide (CuO) Nanoparticles on Human Blood Lymphocytes. Biol. Trace Elem. Res. 2018, 184, 350–357. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [Green Version]
- Doudi, M.; Setorki, M. Acute effect of nano-copper on liver tissue and function in rat Acute effect of nano-copper on liver tissue and function in rat ffect of nano-copper on function and tissue liver of rat E. Nanomed. J. 2015, 1, 331–338. [Google Scholar]
- Lei, R.; Wu, C.; Yang, B.; Ma, H.; Shi, C.; Wang, Q.; Wang, Q.; Yuan, Y.; Liao, M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008, 232, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xin, H.; Yang, S.; Guo, M.; Malkoske, T.; Yin, D.; Xia, S. Environmental risks of ZnO nanoparticle exposure on Microcystis aeruginosa: Toxic effects and environmental feedback. Aquat. Toxicol. 2018, 204, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wei, Y.; Zhao, H.; Chen, S.; Bu, X.; Lu, F.; Qu, D.; Yao, L.; Zheng, J.; Zhang, J. The effect of Fe2O3 and ZnO nanoparticles on cytotoxicity and glucose metabolism in lung epithelial cells. J. Appl. Toxicol. 2015, 35, 651–664. [Google Scholar] [CrossRef]
- Pasupuleti, S.; Alapati, S.; Ganapathy, S.; Anumolu, G.; Pully, N.R.; Prakhya, B.M. Toxicity of zinc oxide nanoparticles through oral route. Toxicol. Ind. Health 2012, 28, 675–686. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Chapter 3—Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Zhang, Z.; Dong, D.; Shen, Y.; Fang, Y.; Hu, L.; Liu, M.; Dai, C.; Peng, S.; et al. Iron and magnetic: New research direction of the ferroptosis-based cancer therapy. Am. J. Cancer Res. 2018, 8, 1933–1946. [Google Scholar]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, Q.; Xiang, Y.; Liu, Y.; Xiang, L.; Li, F.; Deng, X.; Xiao, Y.; Chen, L.; Chen, L.; Li, Z. Eryptosis Indices as a Novel Predictive Parameter for Biocompatibility of Fe3O4 Magnetic Nanoparticles on Erythrocytes. Sci. Rep. 2015, 5, 16209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soenen, S.J.H.; Nuytten, N.; De Meyer, S.F.; De Smedt, S.C.; De Cuyper, M. High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small 2010, 6, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.B.; Olzinski, A.R.; Bernard, R.E.; Aravindhan, K.; Mirabile, R.C.; Boyce, R.; Willette, R.N.; Jucker, B.M. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. Part A 2011, 96, 186–195. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen biodegradable nanoparticles: One step closer towards a better ocular interaction study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Jose, A.; Sunaja Devi, K.R.; Pinheiro, D.; Lakshmi Narayana, S. Electrochemical synthesis, photodegradation and antibacterial properties of PEG capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 187, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wuelfing, W.P.; Gross, S.M.; Miles, D.T.; Murray, R.W. Nanometer gold clusters protected by surface-bound monolayers of thiolated poly(ethylene glycol) polymer electrolyte. J. Am. Chem. Soc. 1998, 120, 12696–12697. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated inorganic nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Reznickova, A.; Slavikova, N.; Kolska, Z.; Kolarova, K.; Belinova, T.; Hubalek Kalbacova, M.; Cieslar, M.; Svorcik, V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 26–34. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.A.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Salassi, S.; Canepa, E.; Ferrando, R.; Rossi, G. Anionic nanoparticle-lipid membrane interactions: The protonation of anionic ligands at the membrane surface reduces membrane disruption. RSC Adv. 2019, 9, 13992–13997. [Google Scholar] [CrossRef] [Green Version]
- Ayata, N.; Sezer, A.D.; Bucak, S.; Turanlı, E.T. Preparation and in vitro characterization of monoclonal antibody ranibizumab conjugated magnetic nanoparticles for ocular drug delivery. Braz. J. Pharm. Sci. 2020, 56, 1–15. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 553–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Bouchy, S.; Penninckx, S.; Marega, R.; Fichera, O.; Gallez, B.; Feron, O.; Martinive, P.; Heuskin, A.C.; Michiels, C.; et al. Antibody-functionalized gold nanoparticles as tumor-Targeting radiosensitizers for proton therapy. Nanomedicine 2019, 14, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Leong, D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef] [Green Version]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [Green Version]
- Soumbo, M.; Scarangella, A.; Villeneuve-Faure, C.; Bonafos, C.; Roques, C.; Makasheva, K. Combined effect of proteins and AgNPs on the adhesion of yeast Candida albicans on solid silica surfaces. In Proceedings of the 2020 IEEE 20th International Conference on Nanotechnology (IEEE-NANO), Montreal, QC, Canada, 29–31 July 2020; pp. 242–245. [Google Scholar] [CrossRef]
- Santo-Orihuela, P.L.; Foglia, M.L.; Targovnik, A.M.; Miranda, V.M.; Desimone, M.F. Nanotoxicological Effects of SiO2 Nanoparticles on Spodoptera frugiperda Sf9 Cells. Curr. Pharm. Biotechnol. 2016, 17, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Worthington, K.L.S.; Adamcakova-Dodd, A.; Wongrakpanich, A.; Mudunkotuwa, I.A.; Mapuskar, K.A.; Joshi, V.B.; Allan Guymon, C.; Spitz, D.R.; Grassian, V.H.; Thorne, P.S.; et al. Chitosan coating of copper nanoparticles reducesin vitrotoxicity and increases inflammation in the lung. Nanotechnology 2013, 24, 395101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyles, M.S.P.; Kristl, T.; Andosch, A.; Zimmermann, M.; Tran, N.; Casals, E.; Himly, M.; Puntes, V.; Huber, C.G.; Lütz-Meindl, U.; et al. Chitosan functionalisation of gold nanoparticles encourages particle uptake and induces cytotoxicity and pro-inflammatory conditions in phagocytic cells, as well as enhancing particle interactions with serum components. J. Nanobiotechnol. 2015, 13, 84. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Ahn, E.Y.; Park, Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, K.; Feng, Y.; Cheng, Y.; Zhang, M.; Wang, Z.; Wu, Z.; Zhang, H. Achievement of safer palladium nanocrystals by enlargement of {100} crystallographic facets. Nanotoxicology 2017, 11, 907–922. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).