Occurrence and Human Health Risk Assessment of Pharmaceuticals and Hormones in Drinking Water Sources in the Metropolitan Area of Turin in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Compounds
2.2. Reagents and Chemicals
2.3. Study Area and Sampling
2.4. Sample Preparation and Analysis
2.5. Calculations
2.5.1. Validation Study
2.5.2. Average Concentrations in Water
2.5.3. Human Health Risk Assessment
3. Results
3.1. Validation Results
3.2. Screening Assessment in the Study Area
3.3. Occurrence of Pharmaceuticals in Treated/Drinking Water
3.4. Human Health Risk Assessment
3.4.1. Individual Compounds
| Compounds | Log Kow | ADI (μg/kg bw/day) | Source | pGLV (μg/L) | MEC (ng/L) | RQiaverage | RQimax |
|---|---|---|---|---|---|---|---|
| Atenolol | −0.03 | 2 | [48] | 7 | 2.29 | 3.27 × 10−4 | 6.91 × 10−2 |
| Azithromycin | 3.24 | Not considered | N/A | N/A | Not considered | N/A | N/A |
| Caffeine | 0.16 | 1510 | [49] | 5285 | 3.52 | 6.65 × 10−7 | 1.25 × 10−5 |
| Carbamazepine | 2.25 | 0.34 | [48] | 1.19 | 2.63 | 2.21 × 10−3 | 1.54 × 10−1 |
| Clarithromycin | 3.18 | Not considered | N/A | N/A | Not considered | N/A | N/A |
| Ciprofloxacin | −0.001 | 12 | [48] | 42 | 0.37 | 8.75 × 10−6 | 1.67 × 10−4 |
| Cyclophosphamide | 0.97 | 33 | [48] | 115.5 | 0.11 | 9.80 × 10−7 | 9.52 × 10−6 |
| Diclofenac | 0.57 | 200 | N/A | 700 | 1.5 | 2.14 × 10−6 | 1.74 × 10−4 |
| Erythromycin | 2.48 | 0.7 | [50] | 2.45 | 0.06 | 0 | 0 |
| Ketoprofen | 3.00 | 20 | [36] | 70 | 4.07 | 5.81 × 10−5 | 2.18 × 10−3 |
| Ofloxacin | −0.20 | 0.02 | [48] | 0.07 | 0.12 | 0 | 0 |
| Sulfamethoxazole | 0.48 | 510 | [48] | 1785 | 1.39 | 7.78 × 10−7 | 5.57 × 10−5 |
| Trimethoprim | 0.73 | 190 | [48] | 665 | 1.78 | 2.68 × 10−6 | 1.31 × 10−4 |
| 17-beta Estradiol | 3.94 | Not considered | N/A | N/A | Not considered | N/A | N/A |
| Estrone | 3.43 | Not considered | N/A | N/A | Not considered | N/A | N/A |
| Ibuprofen | 3.79 | 400 | [36] | 1400 | 0.2 | 1.52 × 10−7 | 7.53 × 10−6 |
3.4.2. Risk Assessment of Combined Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Compound | Chemical Group | Regulation Status | Drinking Water Value |
|---|---|---|---|
| Atenolol | β-Blockers | NORMAN framework prioritization 1 | N/A |
| Azithromycin | Macrolide Antibiotic | EU Watch List | N/A |
| Clarithromycin | EU Watch List | N/A | |
| Erythromycin | EU Watch List | N/A | |
| Caffeine | Stimulant | NORMAN framework prioritization | N/A |
| Carbamazepine | Anticonvulsant | NORMAN framework prioritization | N/A |
| Ciprofloxacin | Fluoroquinolones antibiotics | NORMAN framework prioritization | N/A |
| Ofloxacin | NORMAN framework prioritization | N/A | |
| Cyclophosphamide | Alkylating agent | NORMAN framework prioritization | N/A |
| Diclofenac | Analgesics anti-inflammatory drugs | EU Watch List | N/A |
| Ketoprofen | NORMAN framework prioritization | N/A | |
| Ibuprofen | NORMAN framework prioritization | N/A | |
| Sulfamethoxazole | Antibacterial sulfonamides | NORMAN framework prioritization | N/A |
| Trimethoprim | NORMAN framework prioritization | N/A | |
| 17-beta Estradiol | Estrogens | EU Watch List | 1 ng/L (reference value) |
| Estrone | NORMAN framework prioritization | N/A |
| HPLC Method | Ionization Mode | Compounds |
|---|---|---|
| 0.1% Formic Acid in MilliQ Water and Methanol, total run time: 10 min, flow rate: 0.200 mL/min | Positive ESI | Atenolol |
| Azithromycin | ||
| Caffeine | ||
| Carbamazepine | ||
| Clarithromycin | ||
| Ciprofloxacin | ||
| Cyclophosphamide | ||
| Diclofenac | ||
| Erythromycin | ||
| Ketoprofen | ||
| Ofloxacin | ||
| Sulfamethoxazole | ||
| Trimethoprim | ||
| 0.02% Ammonia in MilliQ Water and Methanol, total run time: 10 min, flow rate: 0.400 mL/min | Negative ESI | 17-beta Estradiol |
| Estrone | ||
| 0.1% Formic Acid and 0.1% Ammonium Acetate in MilliQ Water and Methanol, total run time: 10 min, flow rate: 0.400 mL/min | Negative ESI | Ibuprofen |
References
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2020, 92, 473–505. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Sanjiv, T. Influences of Natural and Anthropogenic Factors on Surface and Groundwater Quality in Rural and Urban Areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Fuoco, I.; Figoli, A.; Criscuoli, A.; Brozzo, G.; De Rosa, R.; Gabriele, B.; Apollaro, C. Geochemical Modeling of Chromium Release in Natural Waters and Treatment by RO/NF Membrane Processes. Chemosphere 2020, 254, 126696. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.-H.; Ok, Y.S. Occurrence of Contaminants in Drinking Water Sources and the Potential of Biochar for Water Quality Improvement: A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Baken, K.A.; Sjerps, R.M.A.; Schriks, M.; van Wezel, A.P. Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern. Environ. Int. 2018, 118, 293–303. [Google Scholar] [CrossRef]
- Castiglioni, S.; Fanelli, R.; Calamari, D.; Bagnati, R.; and Zuccato, E. Methodological approaches for studying pharmaceuticals in the environment by comparing predicted and measured concentrations in River Po, Italy. Regul. Toxicol. Pharmacol. 2004, 39, 25–32. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Minero, C. Fate of selected pharmaceuticals in river waters. Environ. Sci. Pollut. Res. 2013, 20, 2262–2270. [Google Scholar] [CrossRef]
- Houtman, C.J.; Kroesbergen, J.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data. Sci. Total Environ. 2014, 496, 54–62. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Jelic, A.; Petrović, M.; Barceló, D. Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: A case study of a catchment area in the Po Valley (Italy). Sci. Total Environ. 2014, 470–471, 844–854. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Fanelli, R.; Reitano, G.; Bagnati, R.; Chiabrando, C.; Pomati, F.; Rosseti, C.; Calamari, D. Pharmaceuticals in the environment in Italy: Causes, occurrence, effects and control. Environ. Sci. Pollut. Res. Int. 2006, 13, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Patrolecco, L.; Capri, S.; Ademollo, N. Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ. Sci. Pollut. Res. Int. 2015, 22, 5864–5876. [Google Scholar] [CrossRef] [PubMed]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelò, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef]
- Santos, A.V.; Couto, C.F.; Lebron, Y.A.R.; Foureaux, A.F.S.; Reis, E.O.; Santos, L.V.; de Andrade, L.H.; Amaral, M.C.S.; Lange, L.C. Occurrence and risk assessment of pharmaceutically active compounds in water supply systems in Brazil. Sci. Total Environ. 2020, 746, 141011. [Google Scholar] [CrossRef]
- Pedrouzo, M.; Borrull, F.; Pocurull, E.; Marcé, R.M. Presence of Pharmaceuticals and Hormones in Waters from Sewage Treatment Plants. Water Air Soil Pollut. 2011, 217, 267–281. [Google Scholar] [CrossRef]
- Pal, P. Treatment and Disposal of Pharmaceutical Wastewater: Toward the Sustainable Strategy. Sep. Purif. Rev. 2018, 47, 179–198. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, Y.; Liu, L.; He, Y.; Li, W.; Huang, J.; Yang, H.; Yu, G. PPCPs in a drinking water treatment plant in the Yangtze River Delta of China: Occurrence, removal and risk assessment. Front. Environ. Sci. Eng. 2019, 13, 27. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C.; Grenier-Loustalot, M.-F. Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ. Chem. Lett. 2011, 9, 103–114. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A. Removal of EDCs and Pharmaceuticals in Drinking and Reuse Treatment Processes; IWA Publishing: London, UK, 2007. [Google Scholar]
- Lagesson, A.; Fahlman, J.; Brodin, T.; Fick, J.; Jonsson, M.; Byström, P.; Klaminder, J. Bioaccumulation of five pharmaceuticals at multiple trophic levels in an aquatic food web—Insights from a field experiment. Sci. Total Environ. 2016, 568, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hilaire, S.; Xia, K. Veterinary Pharmaceuticals, Pathogens and Antibiotic Resistance. In Animal Manure, 1st ed.; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 385–407. [Google Scholar]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- EUR-Lex, European Union Strategic Approach of Pharmaceuticals in the Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52019DC0128&from=EN (accessed on 28 January 2021).
- EUR-Lex, DIRECTIVE (EU) 2020/2184. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32020L2184&from=EN (accessed on 28 January 2021).
- Binetti, R.; Calza, P.; Costantino, G.; Morgillo, S.; Papagiannaki, D. Perfluoroalkyl Substance Assessment in Turin Metropolitan Area and Correlation with Potential Sources of Pollution According to the Water Safety Plan Risk Management Approach. Separations 2019, 6, 17. [Google Scholar] [CrossRef]
- ISO. ISO 5667 Water Quality—Sampling; International Standards Organisation: Geneva, Switzerland, 2009. [Google Scholar]
- EPA Method 1694. Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS; United States Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- ISO. ISO/IEC 17025 General Requirements for the Competence of Testing and Calibration Laboratories; International Standards Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- US EPA. Estimation Programs Interface Suite™ for Microsoft® Windows; v 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Schriks, M.; Heringa, M.B.; van der Kooi, M.M.E.; de Voogt, P.; van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 2010, 44, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- de Jongh, C.M.; Kooij, P.J.F.; de Voogt, P.; ter Laak, T.L. Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci. Total Environ. 2012, 427–428, 70–77. [Google Scholar] [CrossRef]
- Pais, M.C.N.; Nascimento, E.D.S. Guideline values and human risk assessment for the presence of anti-inflammatory drugs remaining in drinking water after lab scale treatment. Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Qin, L.-T.; Pang, X.-R.; Zeng, H.-H.; Liang, Y.-P.; Mo, L.-Y.; Wang, D.-Q.; Dai, J.-F. Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian karst wetland in Guilin, China. Sci. Total Environ. 2020, 708, 134552. [Google Scholar] [CrossRef]
- Guideline, ICH Harmonised Tripartite. Validation of Analytical Procedures: Text and Methodology Q2 (R1); International Conference on Harmonization: Geneva, Switzerland, 2005; pp. 11–12. [Google Scholar]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Data on occurrence and fate of emerging contaminants in a urbanised area. Data Brief 2018, 17, 533–543. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schwesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and Pharmaceuticals in Groundwater Used As a Source of Drinking Water Across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Chen, Y.; Ye, B.; Han, J.; Jin, N. Occurrence and distribution of pharmaceuticals in raw, finished, and drinking water from seven large river basins in China. J. Water Health 2019, 17, 477–489. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Teresa, O.L.d.V.M.; Jessica, A.-L.; Isaura, Y.-N. Assessing the Estrogenic Activity of EDCs and Human Risks of Groundwater after Ozonation and Chlorination. Ozone Sci. Eng. 2020, 42, 244–254. [Google Scholar] [CrossRef]
- Bruce, G.M.; Pleus, R.C.; Snyder, S.A. Toxicological Relevance of Pharmaceuticals in Drinking Water. Environ. Sci. Technol. 2010, 44, 5619–5626. [Google Scholar] [CrossRef]
- Caffeine—ECHA. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/10085/7/6/2 (accessed on 28 January 2021).
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 28 January 2021).
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]


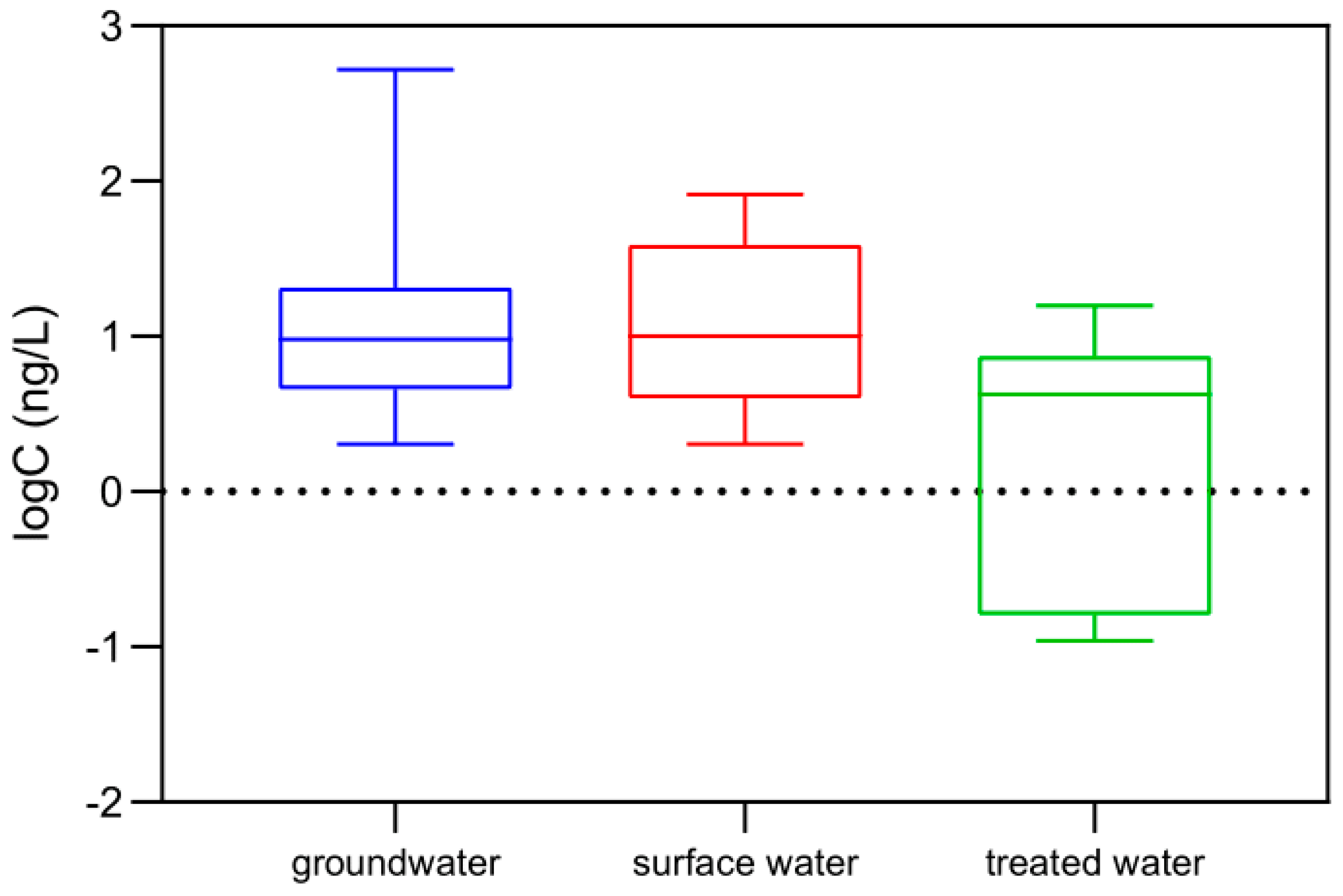
| Compounds | Conc. (ng/L) | Trueness % n = 15 | Uncertainty % n = 15 | Linearity | LOD (ng/L) | LOQ (ng/L) |
|---|---|---|---|---|---|---|
| Atenolol | 4000 | −3.977 | 3.047 | 0.9996 | 0.196 | 0.655 |
| Azithromycin | 4000 | −10.090 | 7.290 | 0.9951 | 0.736 | 2.454 |
| Caffeine | 4000 | −1.300 | 1.912 | 0.9991 | 0.322 | 1.073 |
| Carbamazepine | 4000 | −14.831 | 9.761 | 0.9999 | 0.066 | 0.219 |
| Clarithromycin | 4000 | −3.519 | 2.786 | 0.9996 | 0.031 | 0.074 |
| Ciprofloxacin | 4000 | −1.603 | 0.892 | 0.9996 | 0.788 | 2.625 |
| Cyclophosphamide | 4000 | −0.161 | 2.563 | 0.9996 | 0.010 | 0.034 |
| Diclofenac | 4000 | −7.627 | 2.531 | 0.9998 | 0.376 | 1.254 |
| Erythromycin | 4000 | −4.793 | 3.114 | 0.9998 | 0.244 | 0.814 |
| Ketoprofen | 4000 | −10.221 | 4.476 | 0.9999 | 0.115 | 0.385 |
| Ofloxacin | 4000 | −1.1769 | 2.735 | 0.9978 | 0.493 | 1.644 |
| Sulfamethoxazole | 4000 | −4.823 | 2.202 | 0.9983 | 0.110 | 0.366 |
| Trimethoprim | 4000 | −7.457 | 5.497 | 0.9998 | 3.492 | 11.369 |
| 17-beta Estradiol | 4000 | −7.129 | 6.546 | 0.9972 | 0.303 | 1.010 |
| Estrone | 4000 | −23.144 | 3.655 | 0.9971 | 0.400 | 1.333 |
| Ibuprofen | 4000 | −1.599 | 1.770 | 0.9969 | 0.412 | 1.375 |
| Compounds | QF * n = 325 | Cmin (ng/L) | Cmax (ng/L) | Caverage (ng/L) | Cmedian (ng/L) | Q1 (ng/L) | Q3 (ng/L) |
|---|---|---|---|---|---|---|---|
| Atenolol | 12.00% | 1.07 | 483.94 | 18.73 | 3.96 | 1.64 | 7.93 |
| Azithromycin | 4.00% | 2.55 | 82.46 | 14.84 | 3.28 | 2.64 | 14.63 |
| Caffeine | 61.23% | 1.15 | 65.92 | 5.69 | 3.53 | 2.21 | 5.51 |
| Carbamazepine | 37.84% | 0.23 | 183.49 | 6.93 | 2.44 | 1.07 | 5.24 |
| Clarithromycin | 22.46% | 0.10 | 101.30 | 7.57 | 1.48 | 0.40 | 4.60 |
| Ciprofloxacin | 4.30% | 2.86 | 7.00 | 4.16 | 3.25 | 2.88 | 5.33 |
| Cyclophosphamide | 9.23% | 0.08 | 1.10 | 0.31 | 0.26 | 0.19 | 0.34 |
| Diclofenac | 11.38% | 1.26 | 121.46 | 12.41 | 3.62 | 2.22 | 11.89 |
| Erythromycin | 0.00% | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Ketoprofen | 48.92% | 0.4 | 152.88 | 8.28 | 2.58 | 1.40 | 7.31 |
| Ofloxacin | 0.00% | <LOD | <LOQ | <LOD | <LOD | <LOD | <LOD |
| Sulfamethoxazole | 27.69% | 0.41 | 99.47 | 4.94 | 1.91 | 0.92 | 3.68 |
| Trimethoprim | 2.46% | 12.87 | 87.16 | 37.80 | 31.02 | 22.07 | 41.71 |
| 17-beta Estradiol | 35.07% | 1.08 | 9.00 | 1.28 | 1.18 | 1.45 | 2.04 |
| Estrone | 36.00% | 1.35 | 125.97 | 7.69 | 3.20 | 2.12 | 5.71 |
| Ibuprofen | 3.07% | 1.46 | 10.54 | 3.77 | 3.15 | 1.78 | 3.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagiannaki, D.; Morgillo, S.; Bocina, G.; Calza, P.; Binetti, R. Occurrence and Human Health Risk Assessment of Pharmaceuticals and Hormones in Drinking Water Sources in the Metropolitan Area of Turin in Italy. Toxics 2021, 9, 88. https://doi.org/10.3390/toxics9040088
Papagiannaki D, Morgillo S, Bocina G, Calza P, Binetti R. Occurrence and Human Health Risk Assessment of Pharmaceuticals and Hormones in Drinking Water Sources in the Metropolitan Area of Turin in Italy. Toxics. 2021; 9(4):88. https://doi.org/10.3390/toxics9040088
Chicago/Turabian StylePapagiannaki, Dimitra, Stefania Morgillo, Gianluca Bocina, Paola Calza, and Rita Binetti. 2021. "Occurrence and Human Health Risk Assessment of Pharmaceuticals and Hormones in Drinking Water Sources in the Metropolitan Area of Turin in Italy" Toxics 9, no. 4: 88. https://doi.org/10.3390/toxics9040088
APA StylePapagiannaki, D., Morgillo, S., Bocina, G., Calza, P., & Binetti, R. (2021). Occurrence and Human Health Risk Assessment of Pharmaceuticals and Hormones in Drinking Water Sources in the Metropolitan Area of Turin in Italy. Toxics, 9(4), 88. https://doi.org/10.3390/toxics9040088







