Whole-Body Acute Contact Toxicity of Formulated Insecticide Mixtures to Blue Orchard Bees (Osmia lignaria)
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Design
2.1.1. Bees
2.1.2. Experimental Cage Design
2.1.3. Spray Tower
2.1.4. Post Treatment Observations
2.2. Experiment 1: Male O. lignaria Acute Whole-Body Contact Toxicity to Premix Insecticides
2.3. Experiment 2: Female O. lignaria Acute Whole-Body Contact Toxicity to Premix Insecticides
2.4. Experiment 3: Male and Female O. lignaria Acute Whole-Body Contact Toxicity to Individual Active Ingredient Insecticides and 1:1 Binary Combinations
2.5. Statistical Analysis
3. Results
3.1. Experiment 1: Male O. lignaria Acute Whole-Body Contact Toxicity Following Whole Bodily Contact Exposure to Premix Insecticide Sprays
3.2. Experiment 2: Female O. lignaria Acute Contact Toxicity Following Whole-Body Contact Exposure to Premix Insecticides
3.3. Experiment 3: Male and Female O. lignaria Acute Contact Toxicity Following Whole-Body Contact Exposure to Individual Active Ingredient Insecticides and 1:1 Binary Combinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, J.; Kemp, W.P. How to Manage the Blue Orchard Bee: As an Orchard Pollinator; Sustainable Agriculture Network: Beltsville, MD, USA, 2001; p. 88. [Google Scholar]
- Bosch, J.; Kemp, W.P.; Trostle, G.E. Bee population returns and cherry yields in an orchard pollinated with Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol. 2006, 99, 408–413. [Google Scholar] [CrossRef]
- Brittain, C.; Williams, N.; Kremen, C.; Klein, A.-M. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. R. Soc. B Biol. Sci. 2013, 280, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pitts-Singer, T.L.; Artz, D.R.; Peterson, S.S.; Boyle, N.K.; Wardell, G.I. Examination of a managed pollinator strategy for almond production using Apis mellifera (Hymenoptera: Apidae) and Osmia lignaria (Hymenoptera: Megachilidae). Environ. Entomol. 2018, 47, 364–377. [Google Scholar] [CrossRef]
- Belsky, J.; Joshi, N.K. Impact of botic and abiotic stressors on managed and feral bees. Insects 2019, 10, 233. [Google Scholar] [CrossRef]
- Heller, S.; Joshi, N.K.; Chen, J.; Rajotte, E.G.; Mullin, C.; Biddinger, D. Pollinator exposure to systemic insecticides and fungicides applied in the previous fall and pre-bloom period in apple orchards. Environ. Pollut. 2020, 265, 114589. [Google Scholar] [CrossRef]
- Abbott, V.A.; Nadeau, J.L.; Higo, H.A.; Winston, M.L. Lethal and sublethal effects of imidacloprid on Osmia lignaria and clothianidin on Megachile rotundata (Hymenoptera: Megachilidae). J. Econ. Entomol. 2008, 101, 13. [Google Scholar] [CrossRef]
- Ladurner, E.; Bosch, J.; Kemp, W.P.; Maini, S. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Artz, D.R.; Pitts-Singer, T.L. Effects of fungicide and adjuvant sprays on nesting behavior in two managed solitary bees, Osmia lignaria and Megachile rotundata. PLoS ONE 2015, 10, e0135688. [Google Scholar] [CrossRef] [PubMed]
- Ilias, A.; Lagnel, J.; Kapantaidaki, D.E.; Roditakis, E.; Tsigenopoulos, C.S.; Vontas, J.; Tsagkarakou, A. Transcription analysis of neonicotinoid resistance in Mediterranean (MED) populations of B. tabaci reveal novel cytochrome P450s, but no nAChR mutations associated with the phenotype. BMC Genom. 2015, 16, 1–23. [Google Scholar] [CrossRef]
- Højland, D.H.; Nauen, R.; Foster, S.P.; Williamson, M.S.; Kristensen, M. Incidence, spread and mechanisms of pyrethroid resistance in European populations of the cabbage stem flea beetle, Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). PLoS ONE 2015, 10, e0146045. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Adamczyk, J.; Rinderer, T.; Yao, J.; Danka, R.; Luttrell, R.; Gore, J. Spray toxicity and risk potential of 42 commonly used formulations of row crop pesticides to adult honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2015, 108, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Adamczyk, J.; Luttrell, R. Synergistic toxicity and physiological impact of imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera). PLoS ONE 2017, 12, e0176837. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, E.; Toma, C.; Roncaglioni, A.; Kramer, N.; Benfenati, E.; Dorne, C.M.J.-L. Integrating QSAR models predicting acute contact toxicity and mode of action profiling in honey bees (A. mellifera): Data curation using open source databases, performance testing and validation. Sci. Total Environ. 2020, 735, 1–20. [Google Scholar] [CrossRef]
- Bibbs, C.S.; Fulcher, A.; Xue, R.-D. Allethrin-based mosquito control device causing knockdown, morbidity, and mortality in four species of field-caught mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Zoller, H.; Roepke, R.; Zschiesche, E.; Heckeroth, A.R. Fluralaner activity against life stages of ticks using Rhipicephalus sanguineus and Ornithodoros moubata IN in vitro contact and feeding assays. Parasit. Vectors 2015, 8, 90. [Google Scholar] [CrossRef]
- Glazer, I.; Navon, A. Activity and persistence of entomoparasitic nematodes tested against Heliothis armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 1990, 83, 1795–1800. [Google Scholar] [CrossRef]
- Wise, J.C.; Hulbert, D.; Vandervoort, C. Rainfall influences performance of insecticides on the codling moth (Lepidoptera: Tortricidae) in apples. Can. Entomol. 2017, 149, 118–128. [Google Scholar] [CrossRef]
- Phan, N.T.; Joshi, N.K.; Rajotte, E.G.; Lopez-Uribe, M.M.; Zhu, F.; Biddinger, D.J. A new ingestion bioassay protocol for assessing pesticide toxicity to the adult Japanese orchard bee (Osmia cornifrons). Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, H.P.M.; van der Zalm, J.M.; van den Bercken, J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature 1982, 295, 601–603. [Google Scholar] [CrossRef]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef]
- Gough, H.J.; Collins, L.G.; Everett, C.J.; Wilkinson, W. Acute Contact and Oral Toxicity to Honey Bees (Apis mellifera); ICI Americas Inc.: Wilmington, DE, USA, 2018. [Google Scholar]
- EPA. Pesticide Fact Sheet Number 164: Cyfluthrin; Office of Pesticide Programs, EPA U.S. Environmental Protection Agency: Washington, DC, USA, 1987.
- FAO. Specifications and Evaluations for Plant Protection Products: Beta-Cyfluthrin; FAO, Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Schmuck, R.; Schoning, R.; Stork, A.; Schramel, O. Risk posed to honey bees (Apis mellifera L., Hymenoptera) by an imidacloprid seed dressing of suflowers. Pest Manag. Sci. 2001, 57, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Syngenta. Thiamethoxam: Used to formulate Patinum®, Actara®, Centric®, Cruiser®, Flagship®, and Helix®. (Envirofacts: Syngenta Crop Protection Fact Sheet); Syngenta Crop Protection, Inc.: Greensboro, NC, USA, 2005; p. 7. [Google Scholar]
- Biddinger, D.J.; Robertson, J.L.; Mullin, C.; Frazier, J.; Ashcraft, S.A.; Rajotte, E.G.; Joshi, N.K.; Vaughn, M. Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS ONE 2013, 8, e72587. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Russell, R.M.; Preisler, H.K.; Savin, N.E. Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- EPA. Ecological Risk Assessment for Section 3 Registration for Fruit, Vegetable, Selected Field Crop, Turf and Ornamental Uses of Chlorantraniliprole (PC Code 090100); Office of Pesticide Programs, EPA U.S. Environmental Protection Agency: Washington, DC, USA, 2008; p. 37.
- CDPR. California Department of Pesticide Regulation Public Report: Methoxyfenozide; California Department of Pesticide Regulation: Sacramento, CA, USA.
- CDPR. California Department of Pesticide Regulation Public Report: Active Ingredient: Spinetoram; California Department of Pesticide Regulation: Sacramento, CA, USA.
- Smagghe, G.; Deknopper, J.; Meeus, I.; Mommaerts, V. Dietary chlorantraniliprole suppresses reproduction in worker bumblebees. Pest Manag. Sci. 2013, 69, 787–791. [Google Scholar] [CrossRef] [PubMed]
- IRAC. IRAC Mode of Action Classification Scheme; Version 8.4; Insecticide Resistance Action Committee: Brussels, Belgium, 20 May 2018; pp. 1–26. [Google Scholar]
- EPA. EFED Risk Assessment for the Proposed IR-4 Use of the Spinosad Product Entrust® on Pomegranate and Dates; EPA U.S. Environmental Protection Agency: Washington, DC, USA, 2009; pp. 1–85.
- Suiter, D.R.; Scharf, M.E. Insecticide Basics for the Pest Management Professional; University of Georgia: Athens, GA, USA, 2015; p. 1352. [Google Scholar]
- Mommaerts, V.; Sterk, G.; Smagghe, G. Bumblebees can be used in combination with juvenile hormone analogues and ecdysone agonists. Ecotoxicology 2006, 15, 513–521. [Google Scholar] [CrossRef]
- Besard, L.; Mommaerts, V.; Abdu-Alla, G.; Smagghe, G. Lethal and sublethal side-effect assessment supports a more benign profile of spinetoram compared with spinosad in the bumblebee Bombus terrestris. Pest Manag. Sci. 2011, 67, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Shimokawatoko, Y.; Yamaguchi, T.; Tanaka, H. Development of the Novel Insecticide Spinetoram (DIANA®). Sumitomo Kagaku 2012, 2012, 14. Available online: https://www.sumitomo-chem.co.jp/english/rd/report/files/docs/01_2012e.pdf (accessed on 16 March 2021).
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef]
- EPA. Leverage 360® EPA Regulated Insecticide Label; Bayer Crop Science: Leverkusen, Germany, 2015. [Google Scholar]
- James, R.; Pitts-Singer, T.L. Bee Pollination in Agricultural Ecosystems; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Leskey, T.C.; Short, B.D.; Lee, D.-H. Efficacy of insecticide residues on adult Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) mortality and injury in apple and peach orchards: Residual insecticide efficacy on Halyomorpha halys. Pest Manag. Sci. 2013, 70, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Tanigoshi, L.K.; Gerdeman, B.S.; Hollis Spitler, G. Overview of Our First Season’s Experiences to Chemically Manage the Spotted Wing Drosophila through Lab and Field Research on Red Raspberry in Western Washington; Washington State University: Mount Vernon, WA, USA, 2011; pp. 15–16. [Google Scholar]
- Tanigoshi, L.K.; Spitler Hollis, G.; Gerdeman, B.S. Field Efficacy of Severaled Labeled and Experimental Insecticides for Spotted Wing Drosophila Control in Blueberry; Washington State University: Mount Vernon, WA, USA, 2013; pp. 105–107. [Google Scholar]
- Alston, D.; Murray, M. Leafrollers in Fruit Orchards; Utah State University: Logan, UT, USA, 2017; p. 5. [Google Scholar]
- Krawczyk, G.; Biddinger, D. Entomology. In Penn State Tree Fruit Production Guide; Penn State Extension: State College, PA, USA, 2018; p. 436. [Google Scholar]
- Doering, J.; Maus, C.; Schoening, R. Residues of Imidacloprid WG 5 in Blossom and Leaf Samples of Apple Trees After Soil Treatment in the Field; Bayer CropScience AG Report, No. G201819, Application: 2003, Sampling: 2004; Bayer AG: Monheim, Germany, 2004. [Google Scholar]
- Oliver, J.B.; Fare, D.C.; Yousef, N.; Scholl, S.S.; Reding, M.E.; Ranger, C.M.; Moyseenko, J.J.; Halcomb, M.A. Evaluation of a single application of neonicotinoid and multi-application contact insecticides for flatheaded borer management in field grown red maple cultivars. J. Environ. Hortic. 2010, 28, 135–149. [Google Scholar] [CrossRef]
- Mogren, C.L.; Lundgren, J.G. Neonicotinoid-contaminated pollinator strips adjacent to cropland reduce honey bee nutritional status. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Girolami, V.; Mazzon, L.; Squatini, A.; Mori, N.; Marzaro, M.; Dibernardo, A.; Greatti, M.; Giorio, C.; Tapparo, A. Translocation of neonicotinoid insecticides from coated seeds to seedling guttation drops: A novel way of intoxication for bees. J. Econ. Entomol. 2009, 102, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.J.; Castle, S.J. Imidacloprid in melon guttation fluid: A potential mode of exposure for pest and beneficial organisms. J. Econ. Entomol. 2012, 105, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, J.; Code, A.; Vaughan, M.; Biddinger, D.; Shepherd, M.; Black, S.H.; Lee-Mäder, E.; Mazzacano, C. How Neonicotinoids Can Kill Bees: The Science behind the Role These Insecticides Play in Harming Bees, 2nd ed.; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2016; p. 84. [Google Scholar]
- Rodrigo, J.; Herrero, M. Effects of pre-blossom temperatures on flower development and fruit set in apricot. Sci. Hortic. 2002, 92, 125–135. [Google Scholar] [CrossRef]
- Horth, L.; Campbell, L.A. Supplementing small farms with native mason bees increases strawberry size and growth rate. J. Appl. Ecol. 2018, 55, 591–599. [Google Scholar] [CrossRef]
- Herrmann, J.D.; Beye, H.; de la Broise, C.; Hartlep, H.; Diekötter, T. Positive effects of the pollinators Osmia cornuta (Megachilidae) and Lucilia sericata (Calliphoridae) on strawberry quality. Arthropod Plant Interact. 2018, 13, 71–77. [Google Scholar] [CrossRef]
- West, T.; McCutcheon, T.W. Evaluating Osmia cornifrons as pollinators of highbush blueberry. Int. J. Fruit Sci. 2009, 9, 115–125. [Google Scholar] [CrossRef]
- Melito, S.; La Bella, S.; Martinelli, F.; Cammalleri, I.; Tuttolomondo, T.; Leto, C.; Mulas, M. Phenology study of Myrtus communis accessions selected from wild populations of Sicily: Preliminary results. ISHA Acta Hortic. 2015, 1172, 231–236. [Google Scholar]
- Dively, G.P.; Embrey, M.S.; Kamel, A.; Hawthorne, D.J.; Pettis, J.S. Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS ONE 2015, 10, e0118748. [Google Scholar] [CrossRef]
- Sharma, A.; Reddy, G.V.P. IPM and pollinator protection in canola production in the USA. In Integrative Biological Control. Progress in Biological Control; Gao, Y., Hokkanen, H., Menzler-Hokkanen, I., Eds.; Springer: Berlin, Germany, 2020; Volume 20. [Google Scholar]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success: Loss of pollinator fitness. Agric. For. Entomol. 2014, 16, 119–128. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.; Gao, Z.; Zumkier, U. Large-scale monitoring of effects of clothianidin-dressed oilseed rape seeds on pollinating insects in Northern Germany: Effects on red mason bees (Osmia bicornis). Ecotoxicology 2016, 25, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
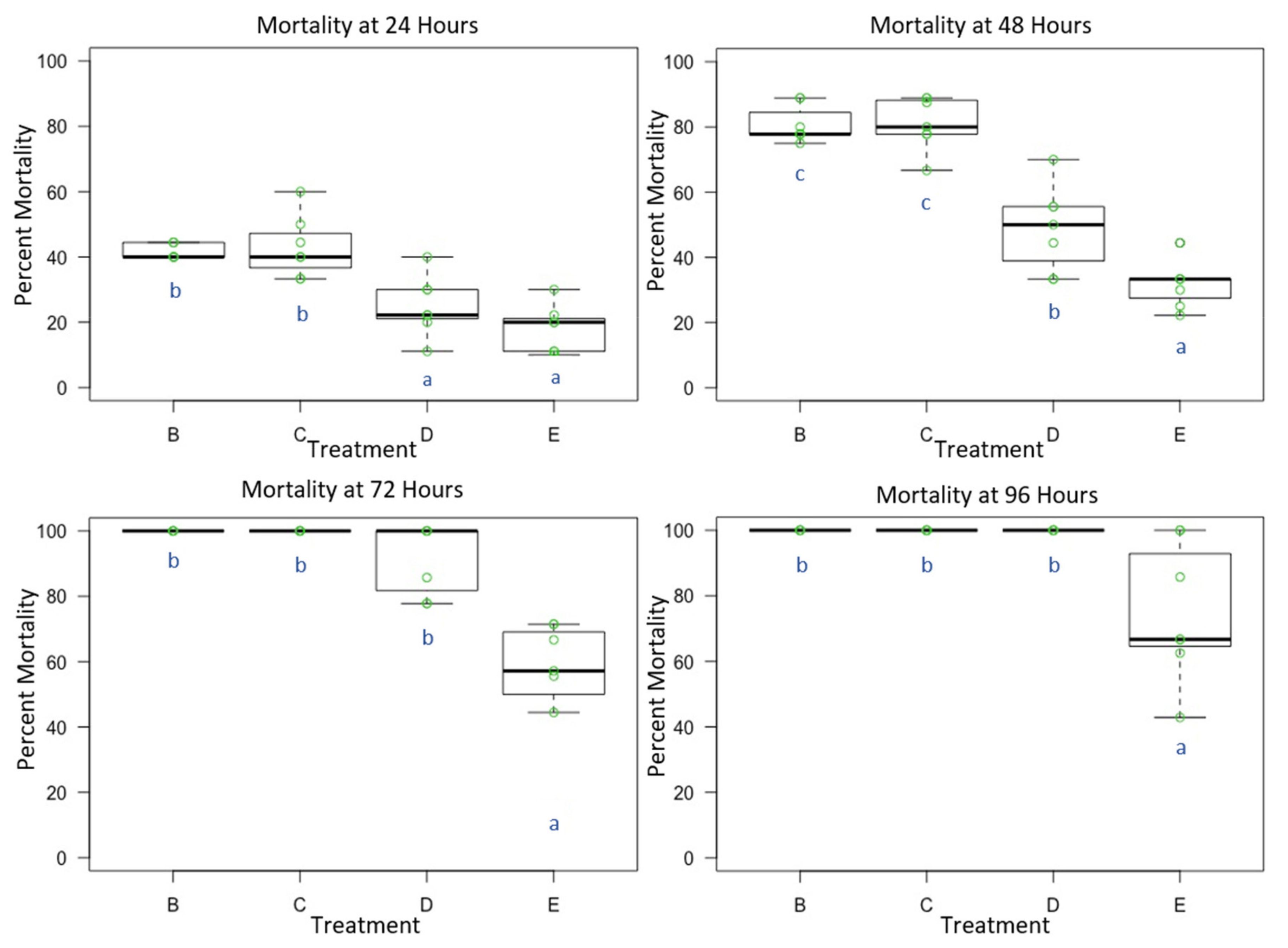
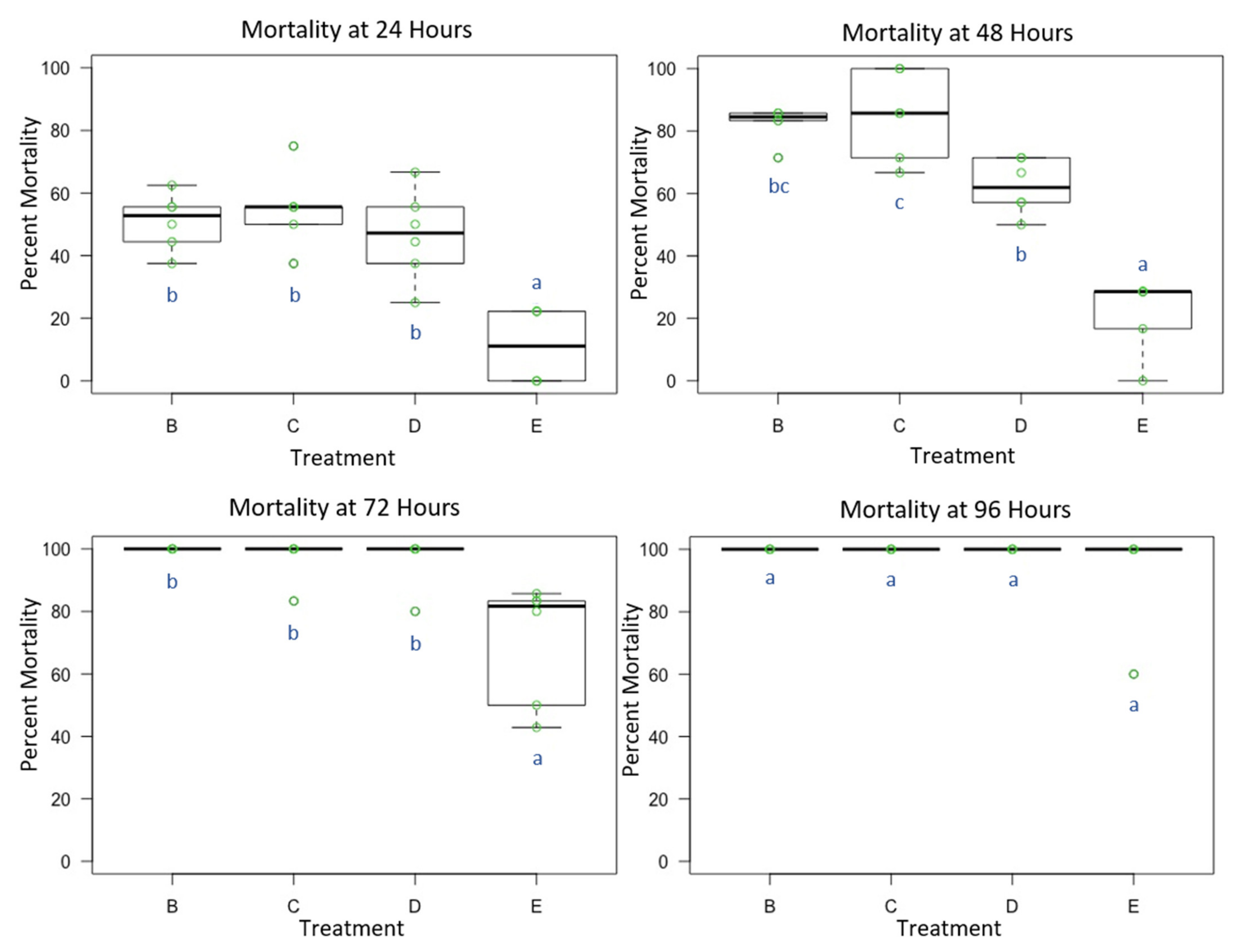
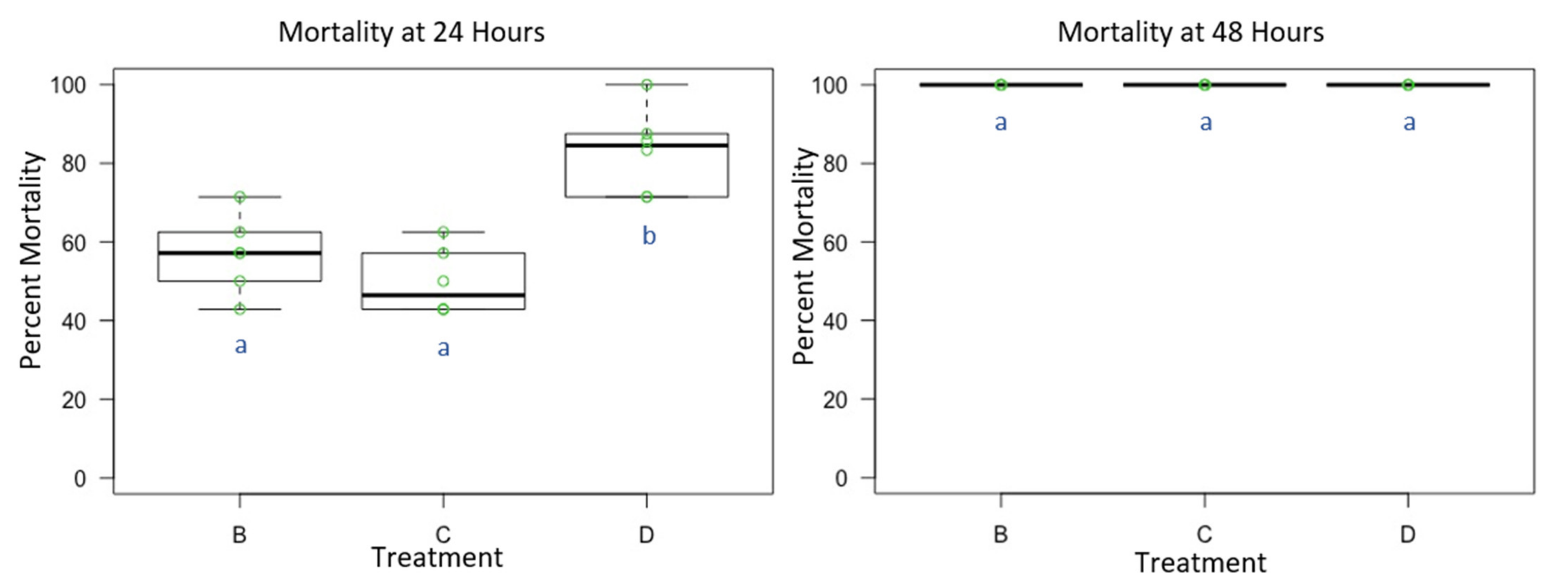
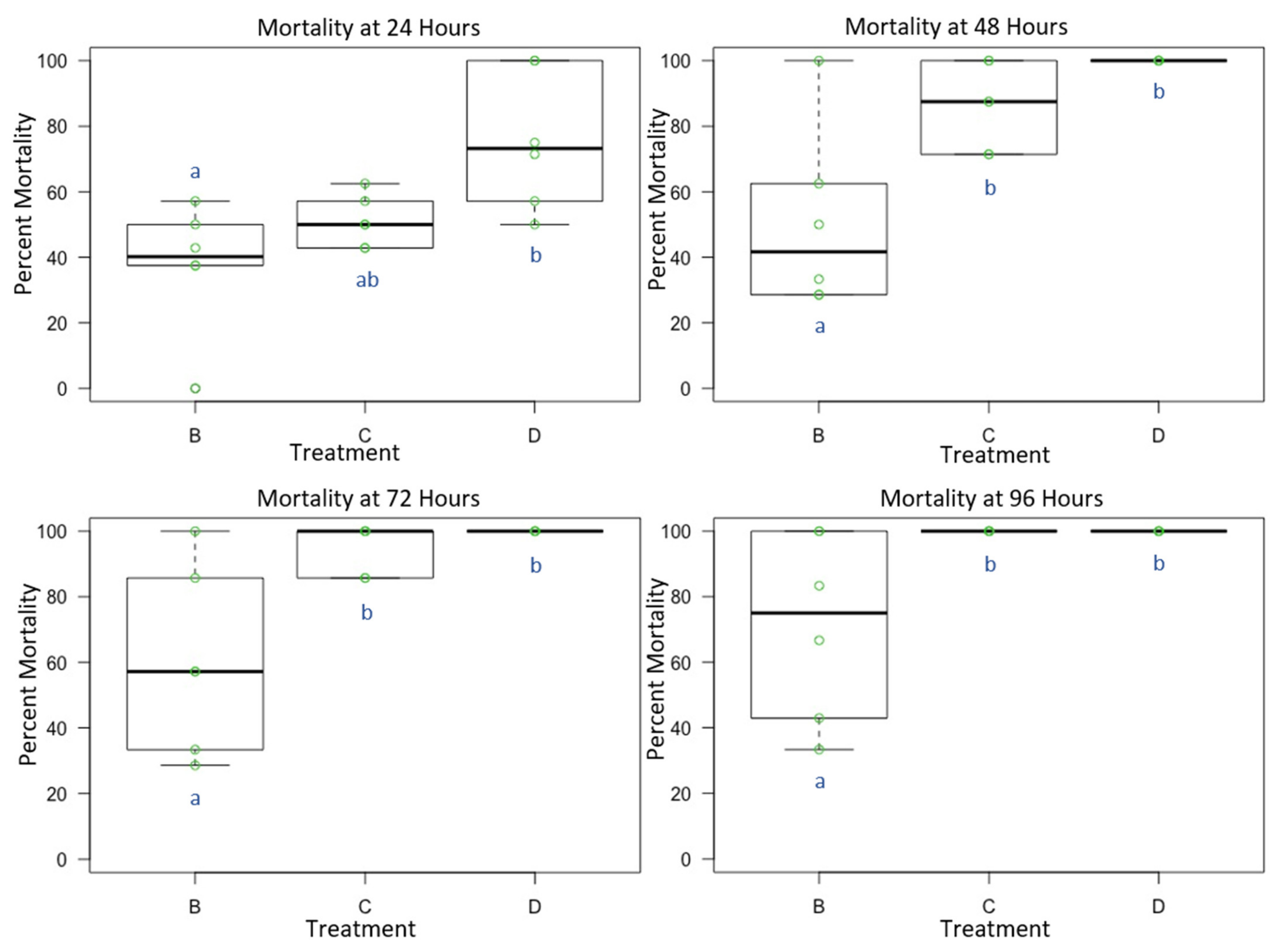
| Treatment | Formulation | Active Ingredient(s) | Mode of Action 1st Insecticide | Mode of Action 2nd Insecticide | Manufacturer |
|---|---|---|---|---|---|
| A | Control (distilled water) | - | - | - | - |
| B | Endigo ZC® | Thiamethoxam (12.6%) + Lambda-cyhalothrin (9.48%) | Thiamethoxam -Nicotinic acetylcholine receptor (nAChR) competitive modulator | Lambda-cyhalothrin -Sodium channel modulator | Syngenta Crop Protection, LLC Greensboro, NC |
| C | Leverage 360® | Imidacloprid (21.0%) + Beta cyfluthrin (10.5%) | Imidacloprid -Nicotinic acetylcholine receptor (nAChR) competitive modulator | Beta-cyfluthrin -Sodium channel modulator | BayerCropScience LP Research Triangle Park, NC |
| D | Besiege® | Chlorantraniliprole (9.26%) + Lambda-cyhalothrin (4.63%) | Chlorantraniliprole -Ryanodine receptor modulator | Lambda-cyhalothrin -Sodium channel modulator | Syngenta Crop Protection, LLC Greensboro, NC |
| E | Intrepid Edge® | Methoxyfenozide (28.3%) + Spinetoram (5.66%) | Methoxyfenozide -Ecdysone receptor agonist (molt accelerating compound) | Spinetoram -Nicotinic acetylcholine receptor (nAChR) allosteric modulator | Dow AgroSciences LLC Indianapolis, IN |
| Treatment | Active Ingredient(s) | Application Concentration Administered (mL/Ha) | Highest Application Concentration on Label (mL/Ha) * | AI Concentration (ppm) in Formulated Product (per L) |
|---|---|---|---|---|
| A | Control (distilled water) | - | - | - |
| B | Thiamethoxam (12.6%) + Lambda-cyhalothrin (9.48%) | 401.76 | 438.28 | Thiamethoxam = 120 Lambda-cyhalothrin = 91 |
| C | Imidacloprid (21.0%) + Beta cyfluthrin (10.5%) | 189.92 | 204.53 | Imidacloprid = 98 Beta-cyfluthrin = 49 |
| D | Chlorantraniliprole (9.26%) + Lambda-cyhalothrin (4.63%) | 657.42 | 876.56 | Chlorantraniliprole = 56 Lambda-cyhalothrin = 28 |
| E | Methoxyfenozide (28.3%) + Spinetoram (5.66%) | 657.42 | 876.56 | Methoxyfenozide = 140 Spinetoram = 28 |
| Treatment | Formulation | Active Ingredient | Insecticide Mode of Action | Manufacturer |
|---|---|---|---|---|
| A | Control (distilled water) | - | - | - |
| B | Admire Pro® | Imidacloprid (42.8%) | Imidacloprid -Nicotinic acetylcholine receptor (nAChR) competitive modulator | BayerCropScience LP Research Triangle Park, NC |
| C | Baythroid XL® | Beta-cyfluthrin (12.7%) | Beta-cyfluthrin -Sodium channel modulator | BayerCropScience LP Research Triangle Park, NC |
| D | (1:1 binary combination by volume) of Treatment B and Treatment C | Imidacloprid + Beta-cyfluthrin | - | - |
| Treatment | Active Ingredient | Application Concentration Administered (mL/Ha) * | Highest Application Concentration on Label (mL/Ha) * | AI Concentration (ppm) in Formulated Product (per L) |
|---|---|---|---|---|
| A | Control (distilled water) | - | - | - |
| B | Imidacloprid (42.8%) | 102.27 | 204.53 | Imidacloprid = 120 |
| C | Beta-cyfluthrin (12.7%) | 102.27 | 204.53 | Beta-cyfluthrin = 26 |
| D ** | Imidacloprid + Beta-cyfluthrin | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belsky, J.; Biddinger, D.J.; Joshi, N.K. Whole-Body Acute Contact Toxicity of Formulated Insecticide Mixtures to Blue Orchard Bees (Osmia lignaria). Toxics 2021, 9, 61. https://doi.org/10.3390/toxics9030061
Belsky J, Biddinger DJ, Joshi NK. Whole-Body Acute Contact Toxicity of Formulated Insecticide Mixtures to Blue Orchard Bees (Osmia lignaria). Toxics. 2021; 9(3):61. https://doi.org/10.3390/toxics9030061
Chicago/Turabian StyleBelsky, Joseph, David J. Biddinger, and Neelendra K. Joshi. 2021. "Whole-Body Acute Contact Toxicity of Formulated Insecticide Mixtures to Blue Orchard Bees (Osmia lignaria)" Toxics 9, no. 3: 61. https://doi.org/10.3390/toxics9030061
APA StyleBelsky, J., Biddinger, D. J., & Joshi, N. K. (2021). Whole-Body Acute Contact Toxicity of Formulated Insecticide Mixtures to Blue Orchard Bees (Osmia lignaria). Toxics, 9(3), 61. https://doi.org/10.3390/toxics9030061






