Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, Sampling, and Survey
2.2. Measurement of Urinary Bisphenols
2.3. Outcome Variables
2.4. Possible Confounders
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as environmental triggers of thyroid dysfunction: Clues and evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Glausiusz, J. Toxicology: The plastics puzzle. Nature 2014, 508, 306–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.T. Human health risk on environmental exposure to Bisphenol-A: A review. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 225–255. [Google Scholar] [CrossRef]
- Björnsdotter, M.K.; de Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.L.; Zheng, Z.G.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Tian, X.; Takamoto, M.; Sugane, K. Bisphenol A promotes IL-4 production by Th2 cells. Int. Arch. Allergy. Immunol. 2003, 132, 240–247. [Google Scholar] [CrossRef]
- Yoshino, S.; Yamaki, K.; Yanagisawa, R.; Takano, H.; Hayashi, H.; Mori, Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br. J. Pharmacol. 2003, 138, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.M.; Takamoto, M.; Sugane, K. Exposure to bisphenol a prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4(+)CD25(+) regulatory T cells. Environ. Health Perspect. 2008, 116, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.J.; Chen, C.Y.; Bornehag, C.G. Bisphenol A exposure may increase the risk of development of atopic disorders in children. Int. J. Hyg. Environ. Health 2016, 219, 311–316. [Google Scholar] [CrossRef]
- Liao, S.L.; Chen, L.C.; Tsai, M.H.; Hua, M.C.; Yao, T.C.; Su, K.W.; Yeh, K.W.; Chiu, C.Y.; Lai, S.H.; Huang, J.L. Prenatal exposure to bisphenol—A is associated with dysregulated perinatal innate cytokine response and elevated cord IgE level: A population-based birth cohort study. Environ. Res. 2020, 191, 110123. [Google Scholar] [CrossRef]
- Mendy, A.; Salo, P.M.; Wilkerson, J.; Feinstein, L.; Ferguson, K.K.; Fessler, M.B.; Thorne, P.S.; Zeldin, D.C. Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environ. Res. 2020, 183, 108944. [Google Scholar] [CrossRef]
- Quirós-Alcalá, L.; Hansel, N.N.; McCormack, M.; Calafat, A.M.; Ye, X.; Peng, R.D.; Matsui, E.C. Exposure to bisphenols and asthma morbidity among low-income urban children with asthma. J. Allergy Clin. Immunol. 2021, 147, 577–586.e7. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.V.; Kulkarni, H. Association of urinary bisphenol A concentration with allergic asthma: Results from the National Health and Nutrition Examination Survey 2005-2006. J. Asthma 2012, 49, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, J.; Pu, Y.; Ehrhardt, R.; Karthikraj, R.; Kannan, K.; Veiga-Lopez, A. Toxicokinetics of bisphenol A, bisphenol S, and bisphenol F in a pregnancy sheep model. Chemosphere 2019, 220, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Choi, J.W.; Ahn, Y.A.; Kim, S. Pharmacokinetics of bisphenol S in humans after single oral administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef]
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300. [Google Scholar] [CrossRef]
- Park, C.; Yu, S.D. Status and Prospects of the Korean National Environmental Health Survey (KoNEHS). J. Environ. Health Sci. Eng. 2014, 40, 1–9. [Google Scholar]
- Ha, M.; Kwon, H.-J.; Leem, J.-H.; Kim, H.-C.; Lee, K.J.; Park, I.; Lim, Y.-W.; Lee, J.-H.; Kim, Y.; Seo, J.-H. Korean Environmental Health Survey in Children and Adolescents (KorEHS-C): Survey design and pilot study results on selected exposure biomarkers. Int. J. Hyg. Environ. Health 2014, 217, 260–270. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Research. Manual for Analysis of Environmental Pollutants in Biological Samples (Organic Chemicals) (in Korean). 2018. Available online: https://ecolibrary.me.go.kr/nier/#/search/detail/5683881 (accessed on 28 October 2021).
- Moral, L.; Vizmanos, G.; Torres-Borrego, J.; Praena-Crespo, M.; Tortajada-Girbes, M.; Pellegrini, F.J.; Asensio, O. Asthma diagnosis in infants and preschool children: A systematic review of clinical guidelines. Allergol. Immunopathol. 2019, 47, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korean Academy of Asthma, Allergy and Clinical Immunology, Korean Academy of Allergy and Respiratory Disease. Korean Guideline for Asthma Summary (in Korean). 2021. Available online: https://www.allergy.or.kr/content/member/file/211028.pdf (accessed on 28 October 2021).
- Miller, J.E. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. Am. J. Public Health 2000, 90, 428–430. [Google Scholar]
- Ehrlich, R.; Kattan, M.; Godbold, J.; Saltzberg, D.S.; Grimm, K.T.; Landrigan, P.J.; Lilienfeld, D.E. Childhood Asthma and Passive Smoking-Urinary Cotinine as a Biomarker of Exposure. Am. J. Respir. Crit. Care. Med. 1992, 145, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.Y.; Wong, L.Y.; Kramer, J.; Zhou, X.L.; Jia, T.; Calafat, A.M. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of US Adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Deschildre, A.; Pin, I.; El Abd, K.; Belmin-Larrar, S.; El Mourad, S.; Thumerelle, C.; Le Roux, P.; Langlois, C.; de Blic, J. Asthma control assessment in a pediatric population: Comparison between GINA/NAEPP guidelines, Childhood Asthma Control Test (C-ACT), and physician’s rating. Allergy 2014, 69, 784–790. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.S.; Guo, T.L. Modulation of cytokine/chemokine production in human macrophages by bisphenol A: A comparison to analogues and interactions with genistein. J. Immunotoxicol. 2018, 15, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Yang, M.; Liu, S.; Lei, P.; Hu, L.; Chen, B.; Wu, M.; Wang, K.J. Toxic Effects of Bisphenol S Showing Immunomodulation in Fish Macrophages. Environ. Sci. Technol. 2018, 52, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bebo, B.F., Jr.; Fyfe-Johnson, A.; Adlard, K.; Beam, A.G.; Vandenbark, A.A.; Offner, H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 2001, 166, 2080–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, L.; Guilbert, L.J.; Russell, A.S.; Wegmann, T.G.; Mosmann, T.R.; Belosevic, M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J. Immunol. 1996, 156, 644–652. [Google Scholar]
- Cipelli, R.; Harries, L.; Okuda, K.; Yoshihara, S.; Melzer, D.; Galloway, T. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-alpha in T-cells. Reproduction 2014, 147, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, W.H.; Shao, H.Y.; Lei, P.H.; Zheng, C.M.; Qiu, C.X.; Yang, M.; Zheng, Y. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 2018, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gear, R.B.; Belcher, S.M. Impacts of Bisphenol A and Ethinyl Estradiol on Male and Female CD-1 Mouse Spleen. Sci. Rep. 2017, 7, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzold, S.; Averbeck, M.; Simon, J.C.; Lehmann, I.; Polte, T. Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model. PLoS ONE 2014, 9, e100468. [Google Scholar] [CrossRef] [PubMed]
- Midoro-Horiuti, T.; Tiwari, R.; Watson, C.S.; Goldblum, R.M. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ. Health Perspect 2010, 118, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N. Non-Monotonic Dose Responses in Studies of Endocrine Disrupting Chemicals: Bisphenol a as a Case Study. Dose-Response 2014, 12, 259–276. [Google Scholar] [CrossRef]
- Thayer, K.A.; Taylor, K.W.; Garantziotis, S.; Schurman, S.H.; Kissling, G.E.; Hunt, D.; Herbert, B.; Church, R.; Jankowich, R.; Churchwell, M.I. Bisphenol A, Bisphenol S, and 4-Hydro xyphenyl 4-Isopro oxyphenyl sulfone (BPSIP) in Urine and Blood of Cashiers. Environ. Health Perspect. 2016, 124, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; Welshons, W.V.; Swan, S.H. Bisphenol A Data in NHANES Suggest Longer than Expected Half-Life, Substantial Nonfood Exposure, or Both. Environ. Health Perspect. 2009, 117, 784–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rubio, S.; Vike-Jonas, K.; Gonzalez, S.V.; Ballesteros-Gomez, A.; Sonne, C.; Dietz, R.; Boertmann, D.; Rasmussen, L.M.; Jaspers, V.L.B.; Asimakopoulos, A.G. Bioaccumulation potential of bisphenols and benzophenone UV filters: A multiresidue approach in raptor tissues. Sci. Total. Environ. 2020, 741, 140330. [Google Scholar] [CrossRef]
- Carwile, J.L.; Luu, H.T.; Bassett, L.S.; Driscoll, D.A.; Yuan, C.; Chang, J.Y.; Ye, X.Y.; Calafat, A.M.; Michels, K.B. Polycarbonate Bottle Use and Urinary Bisphenol A Concentrations. Environ. Health Perspect. 2009, 117, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Sol, I.S.; Jang, H.; Noh, J.; Kim, S.Y.; Kim, M.J.; Kim, Y.H.; Kim, C.; Sohn, M.H.; Kim, K.W. Mortality and morbidity in children with asthma: A nationwide study in Korea. Respir. Med. 2021, 177, 106306. [Google Scholar] [CrossRef]
- Sol, I.S.; Kim, Y.H.; Kim, S.Y.; Choi, S.H.; Kim, J.D.; Kim, B.O.; Moon, J.E.; Kim, K.W.; Sohn, M.H. Prescription Patterns and Burden of Pediatric Asthma in Korea. Allergy Asthma Immunol. Res. 2019, 11, 280–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. All Hands In? Making Diversity Work for All. 2020. Available online: https://www.oecd.org/social/all-hands-in-making-diversity-work-for-all-efb14583-en.htm (accessed on 28 October 2021).
- Barnes, K.C.; Grant, A.V.; Hansel, N.N.; Gao, P.; Dunston, G.M. African Americans with asthma: Genetic insights. Proc. Am. Thorac. Soc. 2007, 4, 58–68. [Google Scholar] [CrossRef] [Green Version]
| Sex | Male (N = 478) | Female (N = 422) | ||||
|---|---|---|---|---|---|---|
| Asthma | Ever Diagnosed (N = 55) | Never Diagnosed (N = 423) | p-Value | Ever Diagnosed (N = 33) | Never Diagnosed (N = 389) | p-Value |
| Age (years) | 0.45 | 0.15 | ||||
| 12 | 4 (8.1%) | 70 (16.4%) | 3 (9.2%) | 64 (16.4%) | ||
| 13 | 7 (12.2%) | 69 (16.3%) | 4 (12.2%) | 53 (13.6%) | ||
| 14 | 7 (12.4%) | 61 (14.4%) | 3 (10.5%) | 63 (16.1%) | ||
| 15 | 10 (17.9%) | 76 (18%) | 14 (41.4%) | 74 (19.1%) | ||
| 16 | 14 (24.9%) | 76 (17.8%) | 3 (10.5%) | 77 (19.8%) | ||
| 17 | 13 (24.5%) | 72 (17.1%) | 5 (16.1%) | 58 (14.9%) | ||
| Family income (per month) | 0.25 | 0.81 | ||||
| <1 million won | 2 (3.1%) | 9 (2.2%) | 1 (2.5%) | 10 (2.5%) | ||
| 1–2 million won | 2 (4.3%) | 46 (10.8%) | 5 (15.2%) | 42 (10.8%) | ||
| 2–3 million won | 6 (10.1%) | 74 (17.4%) | 6 (19.6%) | 51 (13.2%) | ||
| 3–5 million won | 13 (23.1%) | 120 (28.3%) | 7 (22.5%) | 132 (33.9%) | ||
| 5–7 million won | 13 (23.9%) | 92 (21.8%) | 7 (20.7%) | 94 (24.2%) | ||
| ≥million won | 11 (19.6%) | 57 (13.5%) | 5 (16.1%) | 39 (9.9%) | ||
| Unknown | 9 (16.0%) | 26 (6.1%) | 1 (3.4%) | 21 (5.5%) | ||
| BMI (kg/m2) | 21.3 (19.7, 24.1) | 22.2 (19.8, 24.9) | 0.44 | 22.2 (20.5, 24.8) | 21.0 (19.5, 22.8) | 0.08 |
| Urinary BPA (μg/g creatinine) | 0.67 (0.37, 0.67) | 0.86 (0.46, 1.56) | 0.33 | 1.37 (0.57, 2.31) | 1.03 (0.55, 1.85) | 0.22 |
| Urinary BPS (μg/g creatinine) | 0.02 (0.01, 0.08) | 0.03 (0.01, 0.07) | 0.67 | 0.11(0.01, 0.20) | 0.03 (0.01, 0.08) | <0.01 |
| Urinary BPF (μg/g creatinine) | 0.04 (0.03, 0.11) | 0.04 (0.03, 0.11) | 0.65 | 0.08 (0.04, 0.17) | 0.05 (0.03, 0.15) | 0.40 |
| Urinary cotinine (μg/g creatinine) | 1.57 (1.05, 12.40) | 1.57 (1.05, 2.43) | 0.32 | 1.29 (0.85, 1.92) | 1.30 (0.82, 2.78) | 0.95 |
| Time of the first asthma diagnosis | ||||||
| ≤60 months | 29 (52.4%) | - | - | 17 (49.9%) | - | - |
| >60 months | 26 (47.6%) | - | - | 17 (50.1%) | - | - |
| Current Asthma Treatment | ||||||
| Yes | 3 (5.4%) | - | - | 1 (3.9%) | - | - |
| No | 52 (94.6%) | - | - | 32 (96.1%) | - | - |
| Serum IgE (IU/mL) | 249.6 (91.6, 539.2) | 98.8 (25.1, 213.2) | 0.06 | 152.7 (85.2, 544.0) | 127.3 (48.6, 330.0) | <0.001 |
| ln (Urinary BPS) | ln (Urinary BPA) | ln (Urinary BPF) | Age | ln (Urinary Cotinine) | BMI | ||
|---|---|---|---|---|---|---|---|
| ln (serum IgE) | Female | 0.01 | 0.00 | −0.01 | 0.05 | −0.01 | 0.11 * |
| Male | −0.01 | 0.02 | 0.02 | −0.05 | −0.07 | 0.12 * |
| Asthma (Never vs. Ever Diagnosed) * | Asthma (Never + Diagnosed ≤ 60 Months vs. Diagnosed > 60 Months) ** | Asthma (Never vs. Diagnosed ≤ 60 Months vs. Diagnosed after > 60 Months) *** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||||
| ln (urinary BPS) (μg/g creatinine) | Model1 | Asthma ever | 1.49 | (1.20–1.86) | <0.001 | Asthma (>60 mo) | 1.72 | (1.33–2.23) | <0.001 | Asthma (≤60 mo) | 1.26 | (0.77–2.07) | 0.36 |
| Asthma (>60 mo) | 1.75 | (1.37–2.22) | <0.001 | ||||||||||
| Model2 | Asthma ever | 1.37 | (1.09–1.73) | <0.05 | Asthma (>60 mo) | 1.51 | (1.26–1.81) | <0.001 | Asthma (≤60 mo) | 1.18 | (0.73–1.9) | 0.51 | |
| Asthma (>60 mo) | 1.53 | (1.29–1.81) | <0.001 | ||||||||||
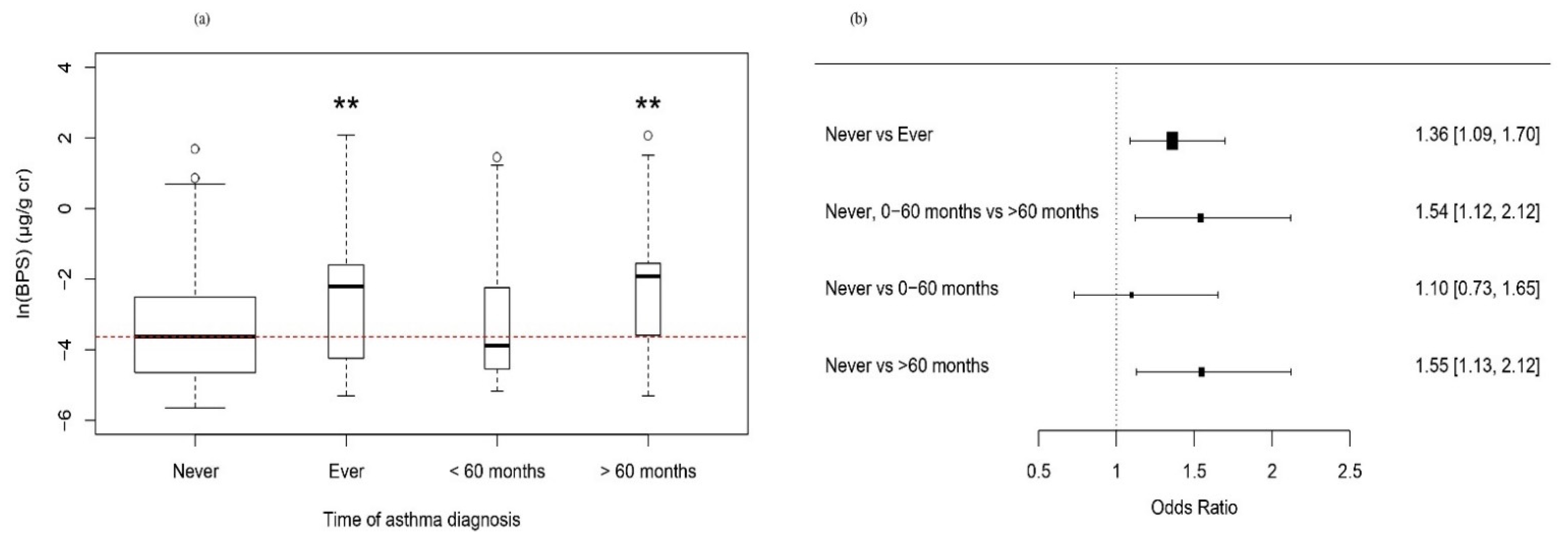
| Model3 | Asthma ever | 1.36 | (1.09–1.70) | <0.05 | Asthma (>60 mo) | 1.54 | (1.12–2.12) | <0.05 | Asthma (≤60 mo) | 1.10 | (0.70–1.58) | 0.69 | |
| Asthma (>60 mo) | 1.55 | (1.13–2.12) | <0.01 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, K.; Park, J.-T.; Kwak, K. Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey. Toxics 2021, 9, 291. https://doi.org/10.3390/toxics9110291
Baek K, Park J-T, Kwak K. Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey. Toxics. 2021; 9(11):291. https://doi.org/10.3390/toxics9110291
Chicago/Turabian StyleBaek, Kiook, Jong-Tae Park, and Kyeongmin Kwak. 2021. "Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey" Toxics 9, no. 11: 291. https://doi.org/10.3390/toxics9110291
APA StyleBaek, K., Park, J.-T., & Kwak, K. (2021). Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey. Toxics, 9(11), 291. https://doi.org/10.3390/toxics9110291






