Intergenerational Patterns of DNA Methylation in Procambarus clarkii Following Exposure to Genotoxicants: A Conjugation in Past Simple or Past Continuous?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Maintenance
2.3. Experimental Setup
2.4. DNA Extraction
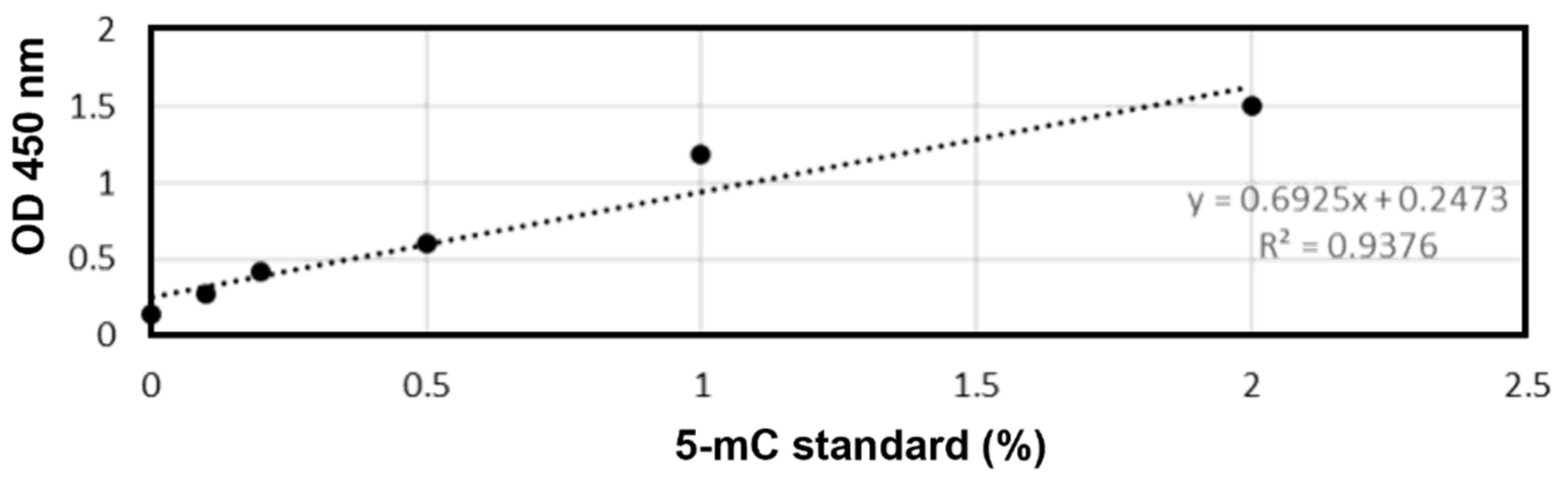
2.5. DNA Methylation Analysis
2.6. Statistical Analysis
3. Results
3.1. Sub-Trial 1: Direct Exposure of Adult Crayfish (F0)
3.2. Sub-Trial 2.1: Indirect Exposure of Juveniles and Adult Crayfish (F1)
3.3. Sub-Trial 2.2: Direct Exposure of Juvenile Crayfish (F1) under the (Indirect) Influence of F0 Exposure
4. Discussion
4.1. DNA Methylation in P. clarkii (F0) after Direct Genotoxic Exposure
4.2. DNA Methylation in Unexposed Crayfish Descendants (F1) from a Genotoxic-Exposed Generation (F0)
4.3. DNA Methylation in Juvenile Crayfish (F1) Submitted to a Current Exposure
4.3.1. Exposure to the Same Genotoxicant
4.3.2. Exposure to a Different Genotoxicant
4.4. The Legacy of a Parental Exposure: An Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, P.Y.; Foley, H.B.; Handschumacher, L.; Suzuki, A.; Karamanukyan, T.; Edmands, S. Acclimation and adaptation to common marine pollutants in the copepod Tigriopus californicus. Chemosphere 2014, 112, 465–471. [Google Scholar] [CrossRef]
- Meyer, J.N.; Di Giulio, R.T. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol. Appl. 2003, 13, 490–503. [Google Scholar] [CrossRef]
- Skinner, M.K. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008, 25, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandegehuchte, M.B.; Janssen, C.R. Epigenetics and its implications for ecotoxicology. Ecotoxicology 2011, 20, 607–624. [Google Scholar] [CrossRef]
- Head, J.A.; Dolinoy, D.C.; Basu, N. Epigenetics for ecotoxicologists. Environ. Toxicol. Chem. 2012, 31, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.D. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J. Biol. Chem. 1948, 175, 315–332. [Google Scholar] [CrossRef]
- Compere, S.J.; Palmiter, R.D. DNA methylation controls the inducibility of the mouse metallothionein-I gene in lymphoid cells. Cell 1981, 25, 233–240. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; Janssen, C.R. Epigenetics in an ecotoxicological context. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 764–765, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevik, K.; Lindström, L.; McKay, S.D.; Chen, Y.H. Transgenerational effects of insecticides—Implications for rapid pest evolution in agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Collotta, M.; Bertazzi, P.A.; Bollati, V. Epigenetics and pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef]
- Roberts, S.B.; Gavery, M.R. Is there a relationship between DNA methylation and phenotypic plasticity in invertebrates? Front. Physiol. 2012, 2, 116. [Google Scholar] [CrossRef] [Green Version]
- Stenz, L.; Schechter, D.S.; Serpa, S.R.; Paoloni-Giacobino, A. Intergenerational Transmission of DNA Methylation Signatures Associated with Early Life Stress. Curr. Genomics 2018, 19, 665–675. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, G.; Jilisa, M.; Sun, J. Influence of Cu, Zn, Pb, Cd and their heavy metalion mixture on the DNA methylation level of the fish (Carassius auratus). Zhongguo Huanjing Kexue/China Environ. Sci. 2001, 21, 549–552. [Google Scholar]
- Vandegehuchte, M.B.; Lemière, F.; Janssen, C.R. Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 343–348. [Google Scholar] [CrossRef]
- Sargsyan, A.; Simonyan, A.; Hovhannisyan, G.; Arakelyan, M.; Aroutiounian, R. Application of the comet assay, micronucleus test and global DNA methylation analysis in Darevskia lizards as a sentinel organism for genotoxic monitoring of soil pollution. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 117–124. [Google Scholar] [CrossRef]
- Head, J.A. Patterns of DNA methylation in animals: An ecotoxicological Perspective. Integr. Comp. Biol. 2014, 54, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, F.M.; Parrott, B.B.; Bowden, J.A.; Kassim, B.L.; Somerville, S.E.; Bryan, T.A.; Bryan, C.E.; Lange, T.R.; Delaney, J.P.; Brunell, A.M.; et al. Global DNA methylation loss associated with mercury contamination and aging in the American alligator (Alligator mississippiensis). Sci. Total Environ. 2016, 545, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anway, M.D.; Cupp, A.S.; Uzumcu, N.; Skinner, M.K. Toxicology: Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yauk, C.; Polyzos, A.; Rowan-Carroll, A.; Somers, C.M.; Godschalk, R.W.; Van Schooten, F.J.; Berndt, M.L.; Pogribny, I.P.; Koturbash, I.; Williams, A.; et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc. Natl. Acad. Sci. USA 2008, 105, 605–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard Pilsner, J.; Lazarus, A.L.; Nam, D.H.; Letcher, R.J.; Sonne, C.; Dietz, R.; Basu, N. Mercury-associated DNA hypomethylation in polar bear brains via the LUminometric Methylation Assay: A sensitive method to study epigenetics in wildlife. Mol. Ecol. 2010, 19, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Vandegehuchte, M.B.; Lemière, F.; Vanhaecke, L.; Vanden Berghe, W.; Janssen, C.R. Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavery, M.R.; Roberts, S.B. DNA methylation patterns provide insight into epigenetic regulation in the Pacific oyster (Crassostrea gigas). BMC Genomics 2010, 11, 483. [Google Scholar] [CrossRef] [Green Version]
- Vogt, G.; Falckenhayn, C.; Schrimpf, A.; Schmid, K.; Hanna, K.; Panteleit, J.; Helm, M.; Schulz, R.; Lyko, F. The marbled crayfish as a paradigm for saltational speciation by autopolyploidy and parthenogenesis in animals. Biol. Open 2015, 4, 1583–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ 2017, 5, e4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulk, C.; Dolinoy, D.C. Timing is everything: The when and how of environmentally induced changes in the epigenome of animals. Epigenetics 2011, 6, 791–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Pesticide Fact Sheet: Penoxsulam; USEPA Office of Prevention, Pesticides and Toxic Substances (7501C): Washington, DC, USA, 2004. [Google Scholar]
- European Commission. Review Report for the Active Substance Penoxsulam; European Commission Health & Consumer Protection Directorate-General: Brussels, Berlgium, 2010. [Google Scholar]
- Murussi, C.R.; Thorstenberg, M.L.; Leitemperger, J.; Costa, M.; Clasen, B.; Santi, A.; Menezes, C.; Engers, V.K.; Loro, V.L. Toxic effects of penoxsulam herbicide in two fish species reared in Southern Brazil. Bull. Environ. Contam. Toxicol. 2014, 92, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Patetsini, E.; Dimitriadis, V.K.; Kaloyianni, M. Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat. Toxicol. 2013, 126, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Pereira, J.L.; Santos, M.A.; Pacheco, M.; Guilherme, S. The role of contamination history and gender on the genotoxic responses of the crayfish Procambarus clarkii to a penoxsulam-based herbicide. Ecotoxicology 2018, 27, 908–918. [Google Scholar] [CrossRef]
- Marçal, R.; Pacheco, M.; Guilherme, S. DNA of crayfish spermatozoa as a target of waterborne pesticides—An ex vivo approach as a tool to short-term spermiotoxicity screening. J. Hazard. Mater. 2020, 400, 123300. [Google Scholar] [CrossRef]
- Sega, G.A. A review of the genetic effects of ethyl methanesulfonate. Mutat. Res. Genet. Toxicol. 1984, 134, 113–142. [Google Scholar] [CrossRef]
- Amini, M. Ethyl Methanesulfonate. In Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123864543. [Google Scholar]
- Dagnac, T.; García-Chao, M.; Fernández-Álvarez, M.; Castro-Insua, J.; García-Pomar, M.I.; Llompart, M. Study of the presence of priority pesticides in surface water of river basins located in two areas of intensive dairy farming in the NW Spain (Galicia). Int. J. Environ. Anal. Chem. 2012, 92, 995–1011. [Google Scholar] [CrossRef]
- Capela, R.; Raimundo, J.; Santos, M.M.; Caetano, M.; Micaelo, C.; Vale, C.; Guimarães, L.; Reis-Henriques, M.A. The use of biomarkers as integrative tools for transitional water bodies monitoring in the Water Framework Directive context—A holistic approach in Minho river transitional waters. Sci. Total Environ. 2016, 539, 85–96. [Google Scholar] [CrossRef]
- Cavas, T. In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem. Toxicol. 2011, 49, 1431–1435. [Google Scholar] [CrossRef]
- Zar, J. Multiple Comparisons. In Biostatistical Analysis; Prentice Hall International Inc.: Upper Saddle River, NJ, USA, 2010; pp. 226–248. [Google Scholar]
- Anastácio, P.M. Ciclo Biológico e Produção do Lagostim Vermelho da Louisiana na Região do Baixo Mondego. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 1993. [Google Scholar]
- Vogt, G.; Huber, M.; Thiemann, M.; Van Den Boogaart, G.; Schmitz, O.J.; Schubart, C.D. Production of different phenotypes from the same genotype in the same environment by developmental variation. J. Exp. Biol. 2008, 211, 510–523. [Google Scholar] [CrossRef] [Green Version]
- Geiger, W.; Alcorlo, P.; Baltanás, A.; Montes, C. Impact of an introduced Crustacean on the trophic webs of Mediterranean wetlands. Biol. Invasions 2005, 7, 49–73. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Vogt, G. The marbled crayfish: A new model organism for research on development, epigenetics and evolutionary biology. J. Zool. 2008, 276, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhang, J.; Chen, Y.; Zuo, Z. DNA hypomethylation induced by tributyltin, triphenyltin, and a mixture of these in Sebastiscus marmoratus liver. Aquat. Toxicol. 2009, 95, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Akcha, F.; Barranger, A.; Bachère, E. Genotoxic and epigenetic effects of diuron in the Pacific oyster: In vitro evidence of interaction between DNA damage and DNA methylation. Environ. Sci. Pollut. Res. 2021, 28, 8266–8280. [Google Scholar] [CrossRef]
- Griffiths, A.J.; Miller, J.H.; Suzuki, D.T.; Lewontin, R.C.; Gelbart, W.M. An Introduction to Genetic Analysis, 7th ed.; WH Freeman: New York, NY, USA, 2000. [Google Scholar]
- Hiendleder, S.; Wirtz, M.; Mund, C.; Klempt, M.; Reichenbach, H.D.; Stojkovic, M.; Weppert, M.; Wenigerkind, H.; Elmlinger, M.; Lyko, F.; et al. Tissue-specific effects of in vitro fertilization procedures on genomic cytosine methylation levels in overgrown and normal sized bovine fetuses. Biol. Reprod. 2006, 75, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.K.; Brisson, J.A.; Robertson, H.M.; Gordon, K.; Jaubert-Possamai, S.; Tagu, D.; Edwards, O.R. A functional DNA methylation system in the pea aphid, Acyrthosiphon pisum. Insect Mol. Biol. 2010, 19, 215–228. [Google Scholar] [CrossRef]
- Kvist, J.; Athanàsio, C.G.; Pfrender, M.E.; Brown, J.B.; Colbourne, J.K.; Mirbahai, L. A comprehensive epigenomic analysis of phenotypically distinguishable, genetically identical female and male Daphnia pulex. BMC Genomics 2020, 21, 17. [Google Scholar] [CrossRef]
- Gombeau, K.; Pereira, S.; Ravanat, J.L.; Camilleri, V.; Cavalie, I.; Bourdineaud, J.P.; Adam-Guillermin, C. Depleted uranium induces sex- and tissue-specific methylation patterns in adult zebrafish. J. Environ. Radioact. 2016, 154, 25–33. [Google Scholar] [CrossRef]
- Counts, J.L.; Goodman, J.I. Hypomethylation of DNA: A nongenotoxic mechanism involved in tumor promotion. Toxicol. Lett. 1995, 82, 663–672. [Google Scholar] [CrossRef]
- Oppold, A.; Kreß, A.; Vanden Bussche, J.; Diogo, J.B.; Kuch, U.; Oehlmann, J.; Vandegehuchte, M.B.; Müller, R. Epigenetic alterations and decreasing insecticide sensitivity of the Asian tiger mosquito Aedes albopictus. Ecotoxicol. Environ. Saf. 2015, 122, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Schübeler, D. Genomic patterns of DNA methylation: Targets and function of an epigenetic mark. Curr. Opin. Cell Biol. 2007, 19, 273–280. [Google Scholar] [CrossRef]
- Elango, N.; Hunt, B.G.; Goodisman, M.A.D.; Yi, S.V. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc. Natl. Acad. Sci. USA 2009, 106, 11206–11211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foret, S.; Kucharski, R.; Pittelkow, Y.; Lockett, G.A.; Maleszka, R. Epigenetic regulation of the honey bee transcriptome: Unravelling the nature of methylated genes. BMC Genomics 2009, 10, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, S.L.; Zhao, J.Z.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotechnol. 2005, 23, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Boothby, T.C. Mechanisms and evolution of resistance to environmental extremes in animals. EvoDevo 2019, 10, 30. [Google Scholar] [CrossRef]
- Donelson, J.M.; Salinas, S.; Munday, P.L.; Shama, L.N.S. Transgenerational plasticity and climate change experiments: Where do we go from here? Glob. Chang. Biol. 2018, 24, 13–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez-Ulloa, V.; Gonzalez-Romero, R.; Eirin-Lopez, J.M. Environmental epigenetics: A promising venue for developing next-generation pollution biomonitoring tools in marine invertebrates. Mar. Pollut. Bull. 2015, 98, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F. Crayfish as global invaders: Distribution, impact on ecosystem services and management options. Freshw. Crayfish 2013, 19, 177–187. [Google Scholar]
- Souty-Grosset, C.; Anastaácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
 ) and female (
) and female (  ) adults, from F0, to Px and EMS, in comparison with a control group (blue line). After reproduction of the F0 organisms (intragroup crosses), F1 offspring were divided into two sub-trials (2.1 and 2.2). Sub-trial 2.1: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water until adulthood (blue line); global DNA methylation was analyzed in both stages (juvenile and adult). Sub-trial 2.2: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water only until the juvenile stage and then exposed to Px and EMS for 7 days, and thereafter analyzed in comparison with a control group.
) adults, from F0, to Px and EMS, in comparison with a control group (blue line). After reproduction of the F0 organisms (intragroup crosses), F1 offspring were divided into two sub-trials (2.1 and 2.2). Sub-trial 2.1: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water until adulthood (blue line); global DNA methylation was analyzed in both stages (juvenile and adult). Sub-trial 2.2: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water only until the juvenile stage and then exposed to Px and EMS for 7 days, and thereafter analyzed in comparison with a control group.  represents the sampling moments.
represents the sampling moments.
 ) and female (
) and female (  ) adults, from F0, to Px and EMS, in comparison with a control group (blue line). After reproduction of the F0 organisms (intragroup crosses), F1 offspring were divided into two sub-trials (2.1 and 2.2). Sub-trial 2.1: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water until adulthood (blue line); global DNA methylation was analyzed in both stages (juvenile and adult). Sub-trial 2.2: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water only until the juvenile stage and then exposed to Px and EMS for 7 days, and thereafter analyzed in comparison with a control group.
) adults, from F0, to Px and EMS, in comparison with a control group (blue line). After reproduction of the F0 organisms (intragroup crosses), F1 offspring were divided into two sub-trials (2.1 and 2.2). Sub-trial 2.1: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water until adulthood (blue line); global DNA methylation was analyzed in both stages (juvenile and adult). Sub-trial 2.2: the progeny (F1) of each F0 group was allowed to grow in uncontaminated water only until the juvenile stage and then exposed to Px and EMS for 7 days, and thereafter analyzed in comparison with a control group.  represents the sampling moments.
represents the sampling moments.
 ) and females (
) and females (  ), following exposure to 23 µg·L−1 of penoxsulam (Px; green) or 5 mg·L−1 of ethyl methanesulfonate (EMS; red), in comparison with the corresponding control groups (C; blue). Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) between treatments, within the same gender; (
), following exposure to 23 µg·L−1 of penoxsulam (Px; green) or 5 mg·L−1 of ethyl methanesulfonate (EMS; red), in comparison with the corresponding control groups (C; blue). Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) between treatments, within the same gender; (  ) vs. male group, within the same treatment.
) vs. male group, within the same treatment.
 ) and females (
) and females (  ), following exposure to 23 µg·L−1 of penoxsulam (Px; green) or 5 mg·L−1 of ethyl methanesulfonate (EMS; red), in comparison with the corresponding control groups (C; blue). Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) between treatments, within the same gender; (
), following exposure to 23 µg·L−1 of penoxsulam (Px; green) or 5 mg·L−1 of ethyl methanesulfonate (EMS; red), in comparison with the corresponding control groups (C; blue). Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) between treatments, within the same gender; (  ) vs. male group, within the same treatment.
) vs. male group, within the same treatment.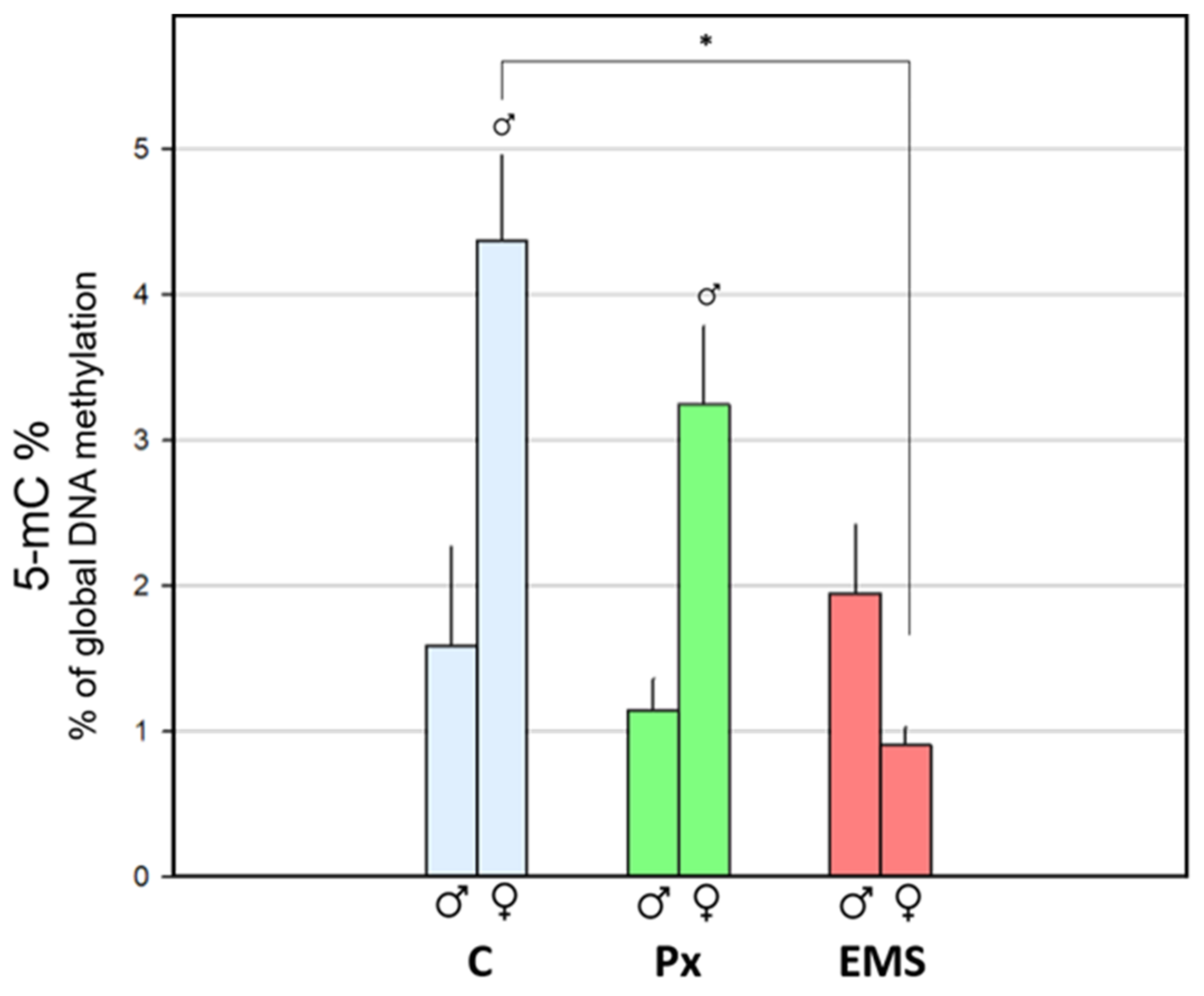
 ; females:
; females:  ), descendants from F0 unexposed (
), descendants from F0 unexposed (  ), penoxsulam-exposed (
), penoxsulam-exposed (  ), and ethyl methanesulfonate-exposed (
), and ethyl methanesulfonate-exposed (  ) groups. Dark blue dashed lines represent the 5-mC% mean values for the corresponding adult groups combining both genders. Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) vs. descendants from F0 unexposed group; (
) groups. Dark blue dashed lines represent the 5-mC% mean values for the corresponding adult groups combining both genders. Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) vs. descendants from F0 unexposed group; (  ) vs. male group, within the same exposure background.
) vs. male group, within the same exposure background.
 ; females:
; females:  ), descendants from F0 unexposed (
), descendants from F0 unexposed (  ), penoxsulam-exposed (
), penoxsulam-exposed (  ), and ethyl methanesulfonate-exposed (
), and ethyl methanesulfonate-exposed (  ) groups. Dark blue dashed lines represent the 5-mC% mean values for the corresponding adult groups combining both genders. Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) vs. descendants from F0 unexposed group; (
) groups. Dark blue dashed lines represent the 5-mC% mean values for the corresponding adult groups combining both genders. Bars represent the standard error. Statistically significant differences (p < 0.05) are: (*) vs. descendants from F0 unexposed group; (  ) vs. male group, within the same exposure background.
) vs. male group, within the same exposure background.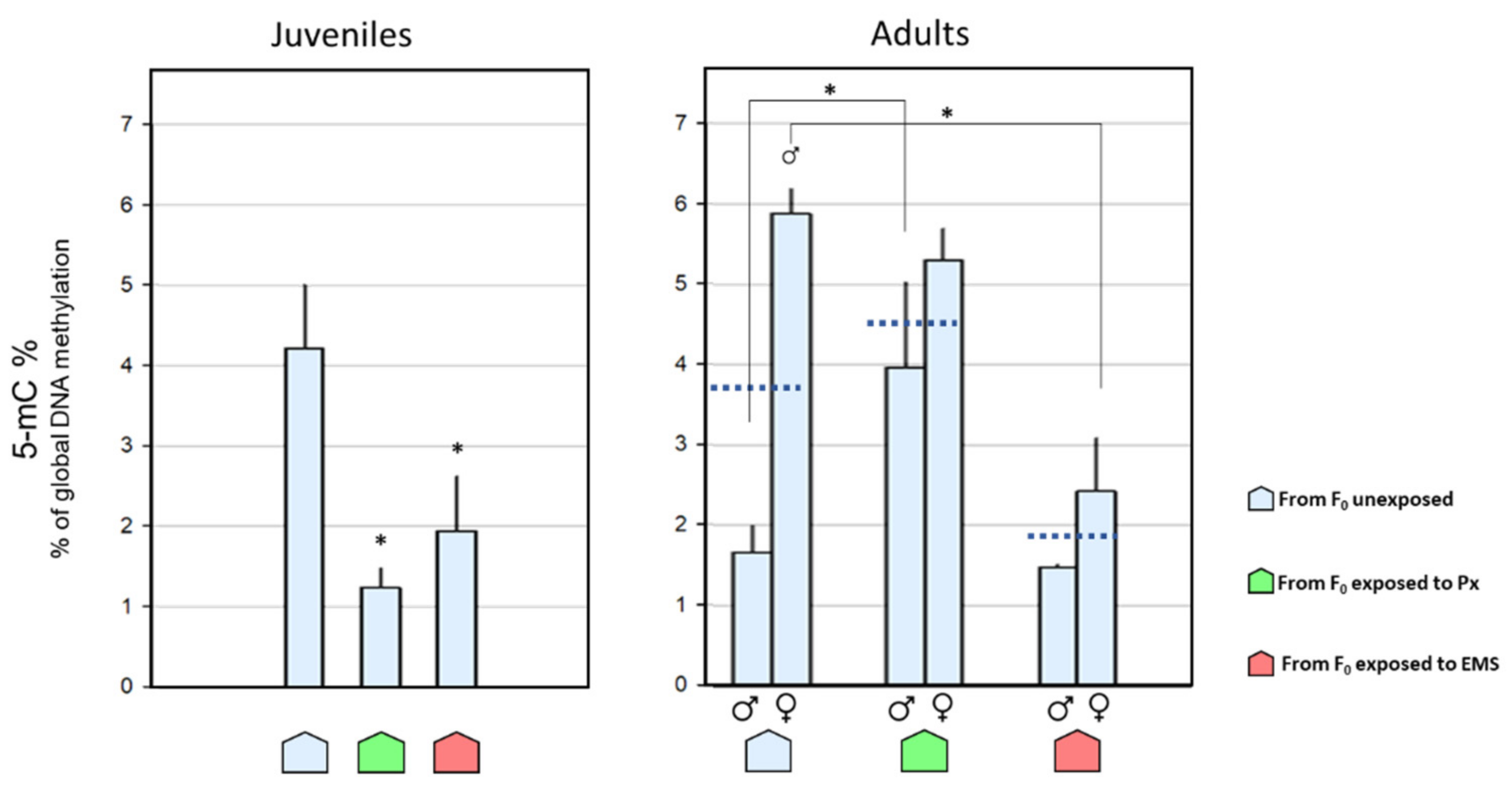
 ), penoxsulam-exposed (
), penoxsulam-exposed (  ), and ethyl methanesulfonate-exposed (
), and ethyl methanesulfonate-exposed (  ) groups were currently exposed to 23 µg·L−1 of penoxsulam (Px; green) or to 5 mg·L−1 of EMS (EMS; red) and compared with the control groups (C; light blue; it should be noted that these data are also represented in Figure 4). Bars represent the standard error. Statistically significant differences (p < 0.05) are (*) between different historical backgrounds, within the same current treatment.
) groups were currently exposed to 23 µg·L−1 of penoxsulam (Px; green) or to 5 mg·L−1 of EMS (EMS; red) and compared with the control groups (C; light blue; it should be noted that these data are also represented in Figure 4). Bars represent the standard error. Statistically significant differences (p < 0.05) are (*) between different historical backgrounds, within the same current treatment.
 ), penoxsulam-exposed (
), penoxsulam-exposed (  ), and ethyl methanesulfonate-exposed (
), and ethyl methanesulfonate-exposed (  ) groups were currently exposed to 23 µg·L−1 of penoxsulam (Px; green) or to 5 mg·L−1 of EMS (EMS; red) and compared with the control groups (C; light blue; it should be noted that these data are also represented in Figure 4). Bars represent the standard error. Statistically significant differences (p < 0.05) are (*) between different historical backgrounds, within the same current treatment.
) groups were currently exposed to 23 µg·L−1 of penoxsulam (Px; green) or to 5 mg·L−1 of EMS (EMS; red) and compared with the control groups (C; light blue; it should be noted that these data are also represented in Figure 4). Bars represent the standard error. Statistically significant differences (p < 0.05) are (*) between different historical backgrounds, within the same current treatment.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marçal, R.; Llorente, L.; Herrero, O.; Planelló, R.; Guilherme, S.; Pacheco, M. Intergenerational Patterns of DNA Methylation in Procambarus clarkii Following Exposure to Genotoxicants: A Conjugation in Past Simple or Past Continuous? Toxics 2021, 9, 271. https://doi.org/10.3390/toxics9110271
Marçal R, Llorente L, Herrero O, Planelló R, Guilherme S, Pacheco M. Intergenerational Patterns of DNA Methylation in Procambarus clarkii Following Exposure to Genotoxicants: A Conjugation in Past Simple or Past Continuous? Toxics. 2021; 9(11):271. https://doi.org/10.3390/toxics9110271
Chicago/Turabian StyleMarçal, Raquel, Lola Llorente, Oscar Herrero, Rosario Planelló, Sofia Guilherme, and Mário Pacheco. 2021. "Intergenerational Patterns of DNA Methylation in Procambarus clarkii Following Exposure to Genotoxicants: A Conjugation in Past Simple or Past Continuous?" Toxics 9, no. 11: 271. https://doi.org/10.3390/toxics9110271
APA StyleMarçal, R., Llorente, L., Herrero, O., Planelló, R., Guilherme, S., & Pacheco, M. (2021). Intergenerational Patterns of DNA Methylation in Procambarus clarkii Following Exposure to Genotoxicants: A Conjugation in Past Simple or Past Continuous? Toxics, 9(11), 271. https://doi.org/10.3390/toxics9110271






