Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Fatty Acid-BSA Conjunction Preparation
2.3. Cell Viability
2.4. Western-Blot
2.5. Oil Red-O Staining
2.6. Statistics
3. Results
3.1. Dose-Dependent Effect of DDE on Cell Viability
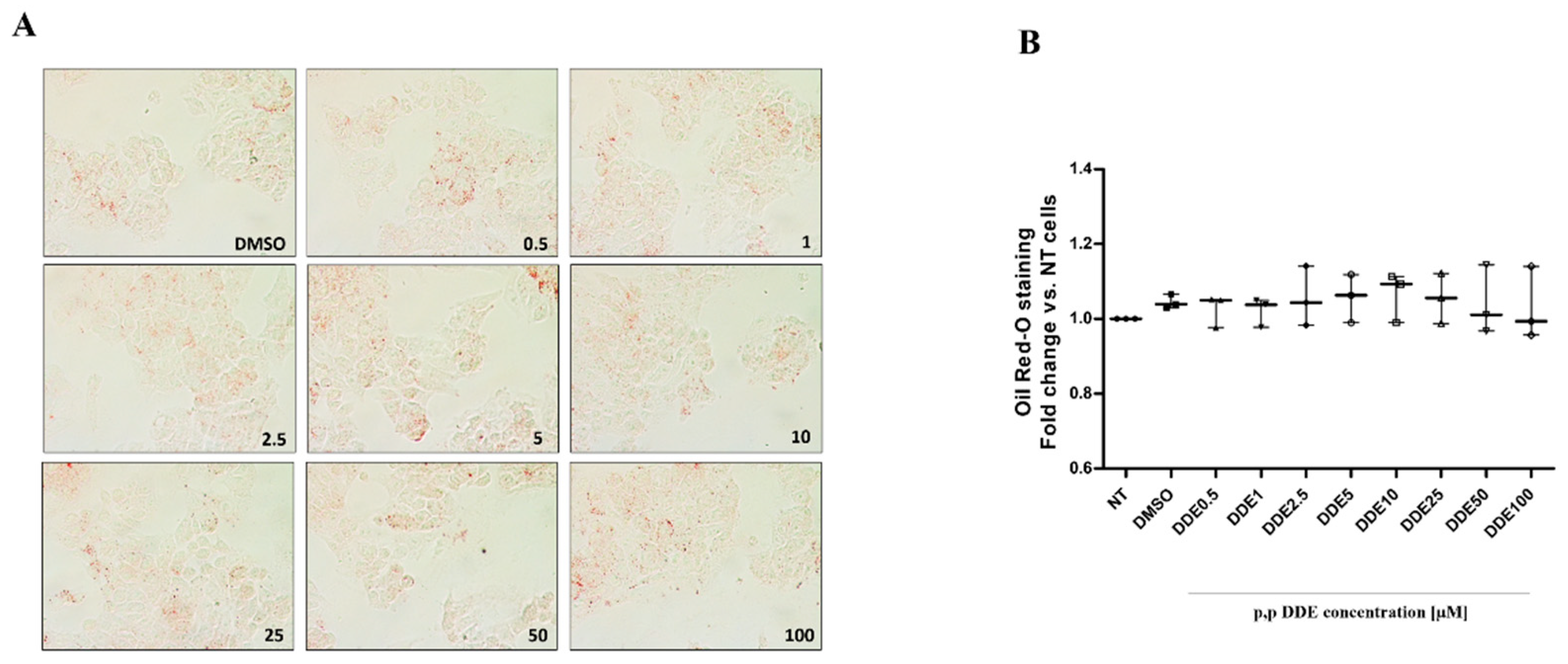
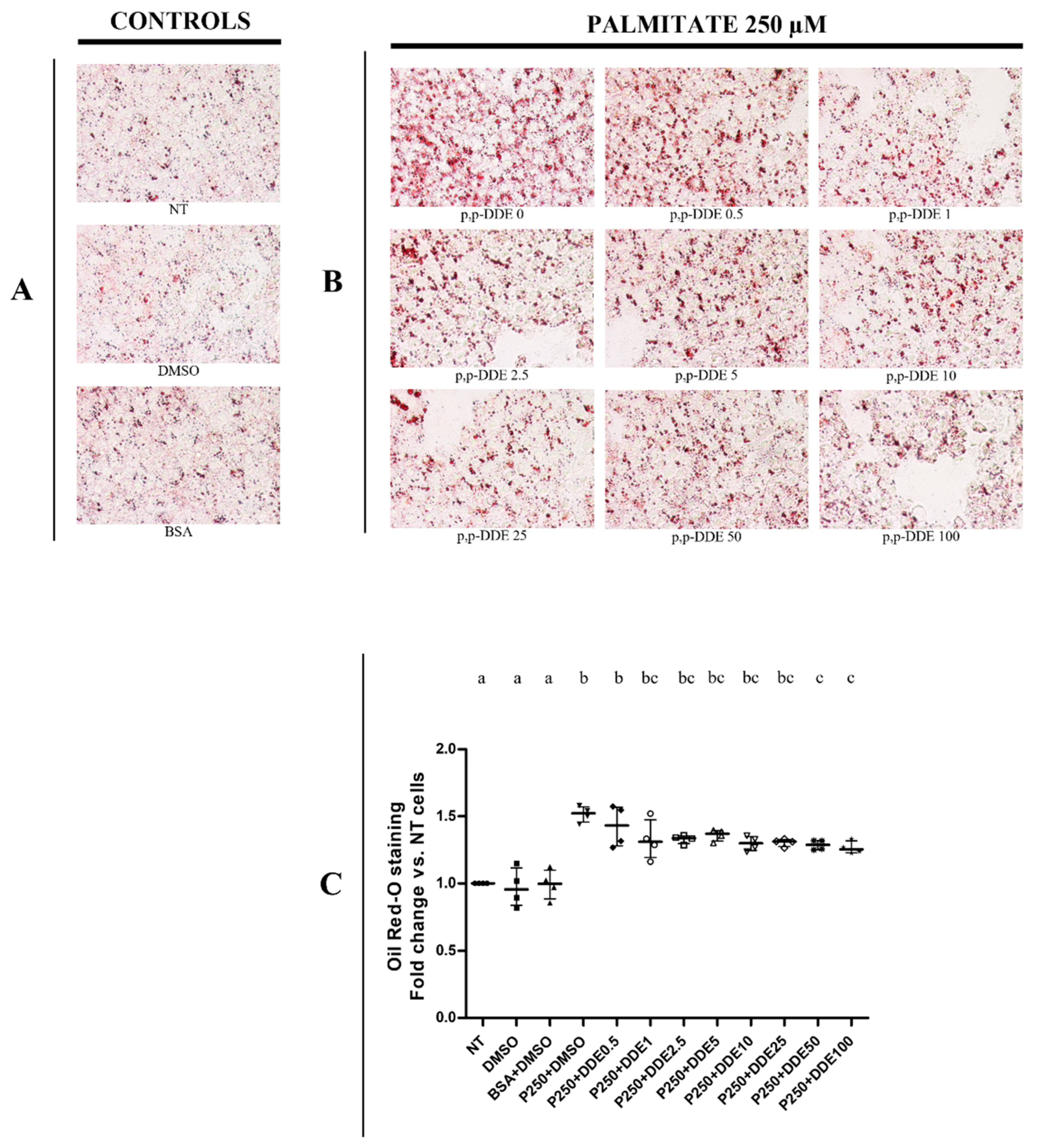
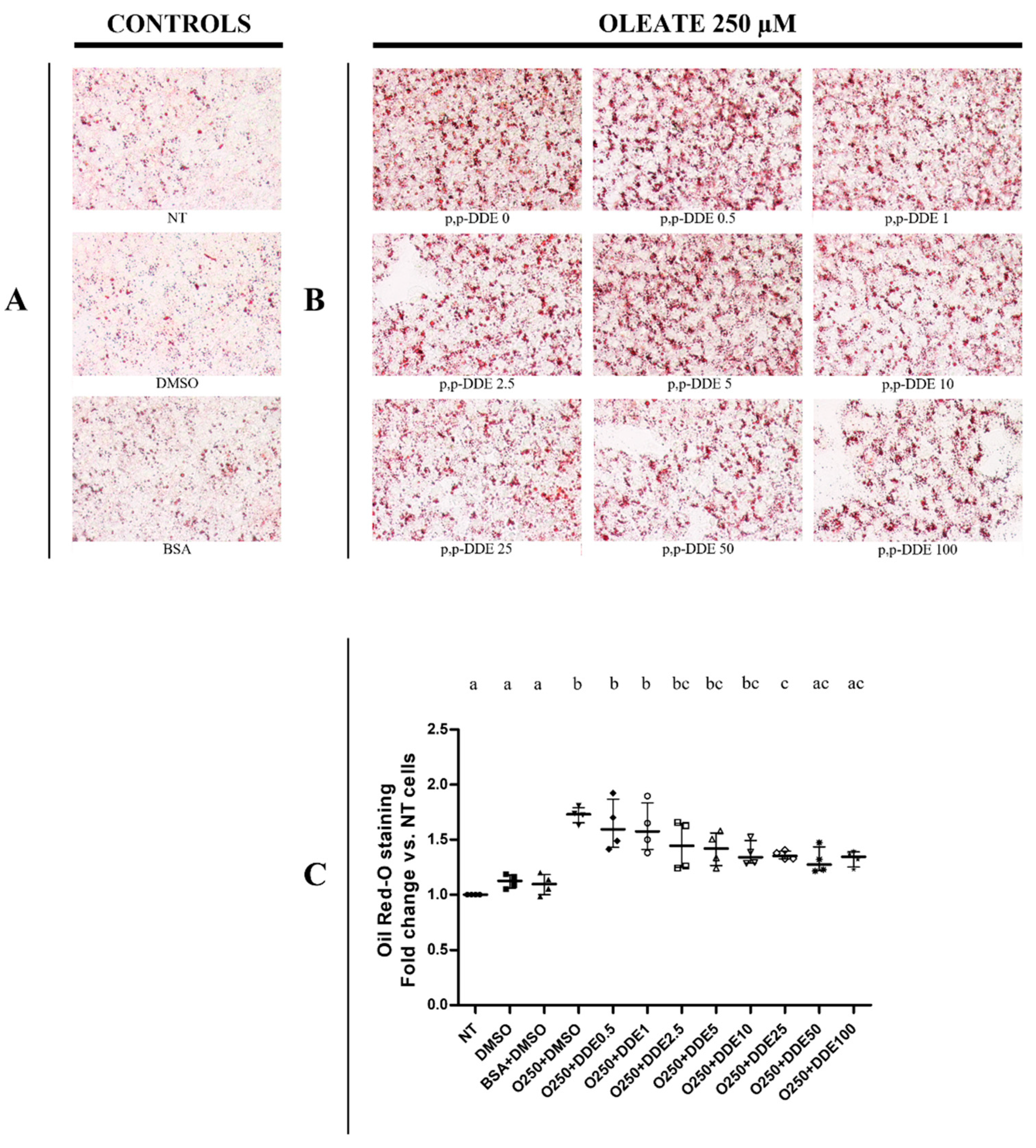
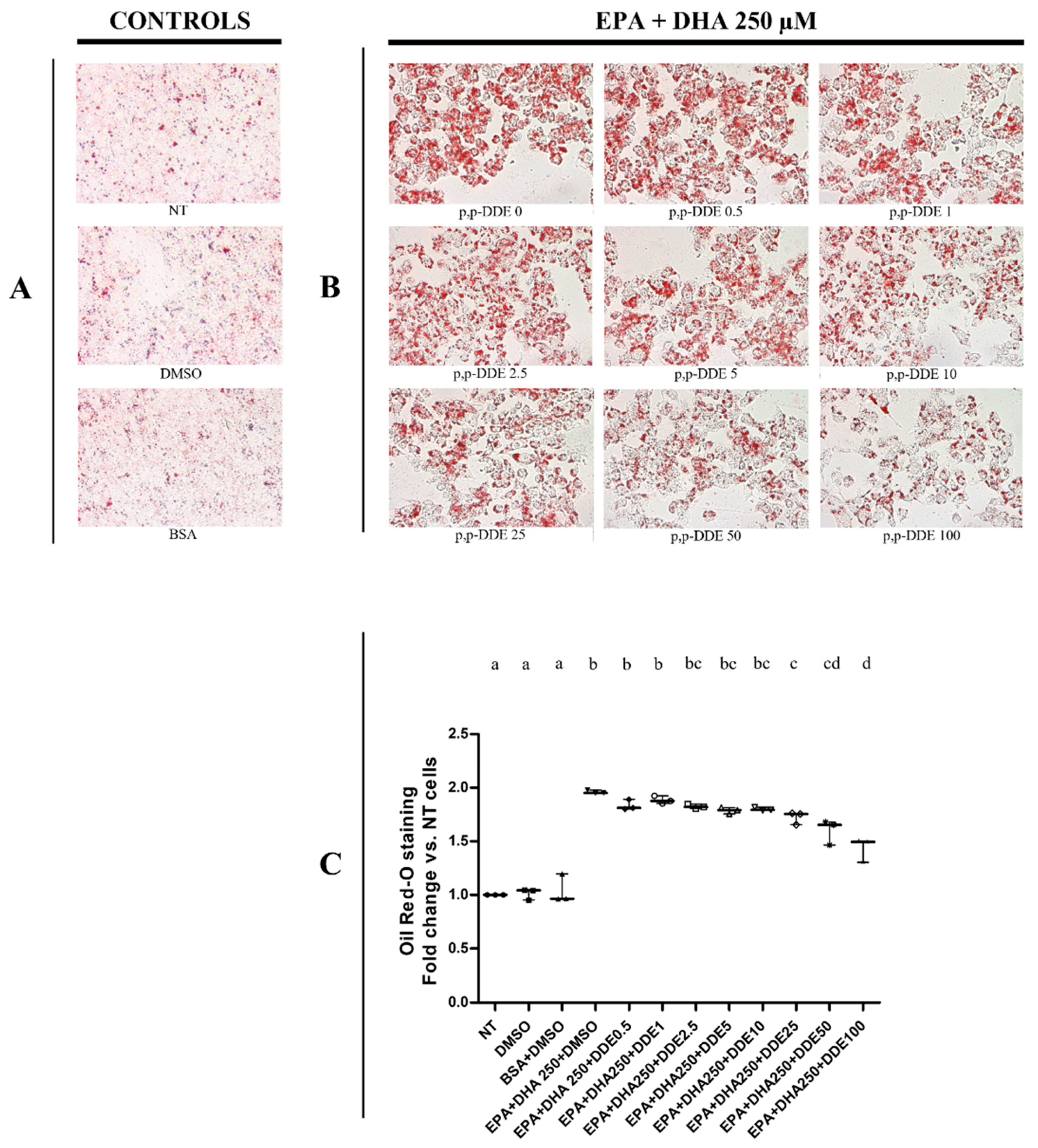
3.2. Dose-Dependent Effect of DDE on Lipid Depots in Cells Cotreated with Dietary Fatty Acids
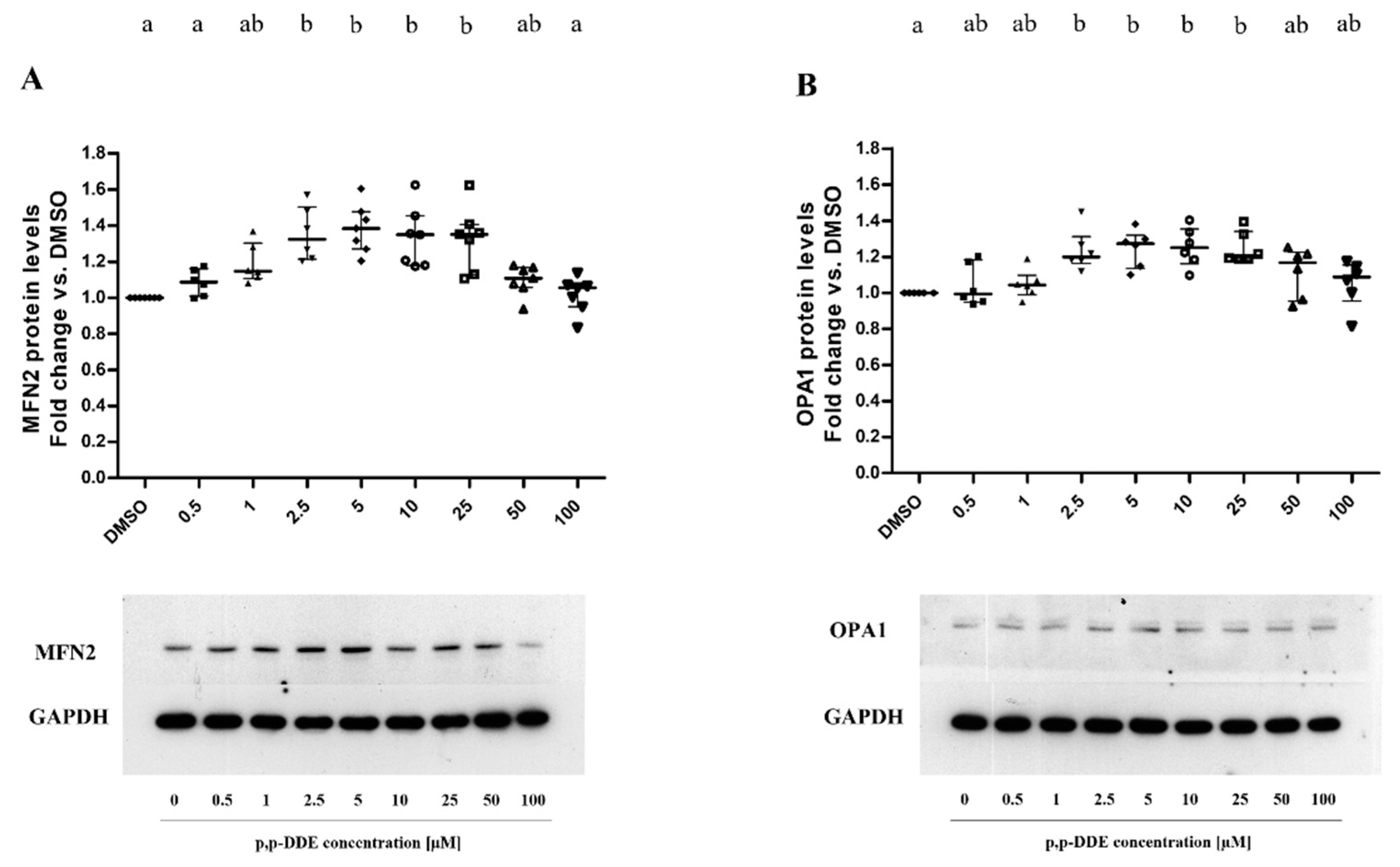
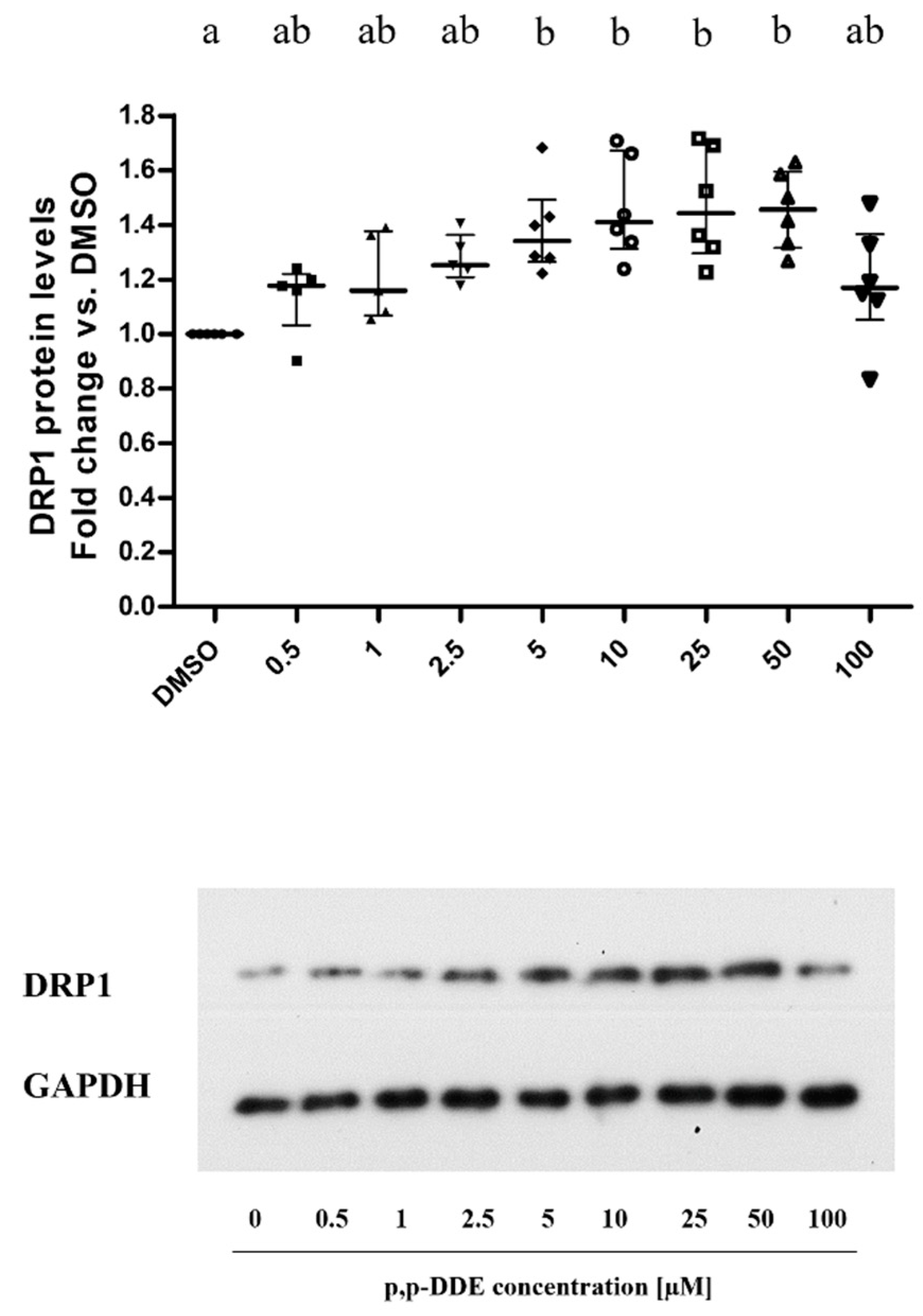
3.3. Dose-Dependent Dffect of DDE on Mitochondrial Content of Proteins Involved in Fusion and Fission Processes
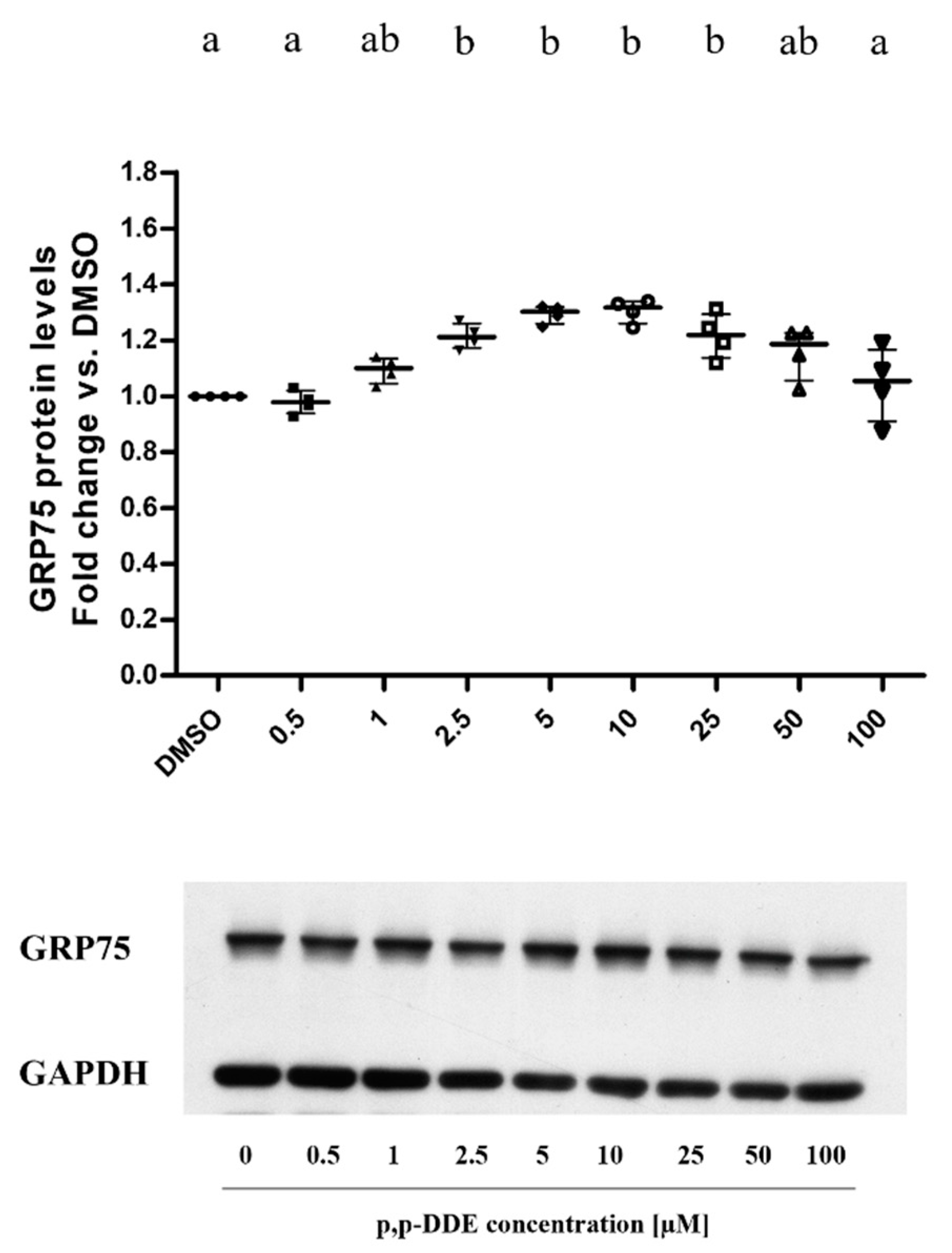
3.4. Dose-Dependent Effect of DDE on Mitochondrial Antioxidant Enzyme SOD2 and Mitochondria-ER Chaperone GRP75
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, J.N.; Leung, M.C.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial bioenergetic etiology of disease. J. Clin. Investig. 2013, 123, 1405–1412. [Google Scholar] [CrossRef]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Wallace, D.C.; Burelle, Y. The rise of mitochondria in medicine. Mitochondrion 2016, 30, 105–116. [Google Scholar] [CrossRef]
- Wallace, K.B. Mitochondrial toxicity. Toxicology 2017, 391, 1. [Google Scholar] [CrossRef]
- Shaughnessy, D.T.; Worth, L., Jr.; Lawler, C.P.; McAllister, K.A.; Longley, M.J.; Copeland, W.C. Meeting report: Identification of biomarkers for early detection of mitochondrial dysfunction. Mitochondrion 2010, 10, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Machado, N.G.; Bernardo, T.C.; Sardao, V.A.; Oliveira, P.J. Mitochondria as a biosensor for drug-induced toxicity-is it really relevant? In Biosensors for Health, Environment and Biosecurity; Serra, P.A., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2011; pp. 411–444. [Google Scholar]
- Naranmandura, H.; Xu, S.; Sawata, T.; Hao, W.H.; Liu, H.; Bu, N.; Ogra, Y.; Lou, Y.J.; Suzuki, N. Mitochondria are the main target organelle for trivalent monomethylarsonous acid (MMA(III))-induced cytotoxicity. Chem. Res Toxicol. 2010, 24, 1094–1103. [Google Scholar] [CrossRef]
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Waters, K.M.; Tanguay, R.L. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013, 271, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Bestman, J.E.; Stackley, K.D.; Rahn, J.J.; Williamson, T.J.; Chan, S.S. The cellular and molecular progression of mitochondrial dysfunction induced by 2,4-dinitrophenol in developing zebrafish embryos. Differentiation 2015, 89, 51–69. [Google Scholar] [CrossRef]
- Brunst, K.J.; Baccarelli, A.A.; Wright, R.J. Integrating mitochondriomics in children’s environmental health. J. Appl. Toxicol. 2015, 35, 976–991. [Google Scholar] [CrossRef] [PubMed]
- Caito, S.W.; Aschner, M. Mitochondrial redox dysfunction and environmental exposures. Antioxid. Redox Signal. 2015, 23, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Hamasaki, N. Maintenance of mitochondrial DNA integrity: Repair and degradation. Curr. Genet. 2002, 41, 311–322. [Google Scholar] [CrossRef]
- Venkatraman, A.; Landar, A.; Davis, A.J.; Chamlee, L.; Sanderson, T.; Kim, H.; Page, G.; Pompilius, M.; Ballinger, S.; Darley-Usmar, V.; et al. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J. Biol. Chem. 2004, 279, 22092–22101. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, V.; Sica, R.; Scudiero, R.; Simoniello, P.; Putti, R.; Lionetti, L. Physiological adaptation to simultaneous chronic exposure to high-fat diet and dichlorodipheniletylhene (DDE) in Wistar rat testis. Cells 2019, 8, 443. [Google Scholar] [CrossRef]
- Migliaccio, V.; Lionetti, L.; Putti, R.; Sica, R.; Scudiero, R. Combined effects of DDE and hyperlipidic diet on metallothionein expression and synthesis in rat tissues. Environ. Toxicol. 2019, 34, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, V.; Scudiero, R.; Sica, R.; Lionetti, L.; Putti, R. Oxidative stress and mitochondrial uncoupling protein 2 expression in hepatic steatosis induced by exposure to xenobiotic DDE and high fat diet in male Wistar rats. PLoS ONE 2019, 14, e0215955. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef]
- Elmore, S.E.; La Merrill, M.A. Oxidative phosphorylation impairment by DDT and DDE. Front. Endocrinol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Harley, K.G.; Marks, A.R.; Bradman, A.; Barr, D.B.; Eskenazi, B. DDT exposure, work in agriculture, and time to pregnancy among farmworkers in California. J. Occup. Environ. Med. 2008, 50, 1335–1342. [Google Scholar] [CrossRef]
- Malusá, E.; Tartanus, M.; Danelski, W.; Miszczak, A.; Szustakowska, E.; Kicińska, J.; Furmanczyk, E.M. Monitoring of DDT in agricultural soils under organic farming in Poland and the risk of crop contamination. Environ. Manag. 2020, 66, 916–929. [Google Scholar] [CrossRef]
- van den Berg, H.; Manuweera, G.; Konradsen, F. Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar. J. 2017, 16, 401. [Google Scholar] [CrossRef]
- Schecter, A.; Colacino, J.; Haffner, D.; Patel, K.; Opel, M.; Papke, O.; Birnbaum, L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Health Perspect. 2010, 118, 796–802. [Google Scholar] [CrossRef]
- Kidd, K.A.; Bootsma, H.A.; Hesslein, R.H.; Muir, D.C.; Hecky, R.E. Biomagnification of DDT through the benthic and pelagic food webs of Lake Malawi, East Africa: Importance of trophic level and carbon source. Environ. Sci. Technol. 2001, 35, 14–20. [Google Scholar] [CrossRef]
- Mota, P.C.; Cordeiro, M.; Pereira, S.P.; Oliveira, P.J.; Moreno, A.J.; Ramalho-Santos, J. Differential effects of p,p’-DDE on testis and liver mitochondria: Implications for reproductive toxicology. Reprod. Toxicol. 2011, 31, 80–85. [Google Scholar] [CrossRef]
- Arroyo-Salgado, B.; Olivero-Verbel, J.; Guerrero-Castilla, A. Direct effect of p,p’-DDT on mice liver. Braz. J. Pharm. Sci. 2016, 25, 287–298. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Q.; Xu, C.; Shao, W.; Zhang, C.; Liu, H.; Jiang, Z.; Gu, A. Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci. Rep. 2017, 7, 46339. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Health Effects Support Document for 1,1-Dichloro-2,2-bis(p-chlorophenyl)Ethylene (DDE); Health and Ecological Criteria Division; U.S. Environmental Protection Agency (USEPA): Washington, DC, USA, 2008; p. 128. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P1008J9N.PDF?Dockey=P1008J9N.PDF (accessed on 17 October 2021).
- Holloway, C.J. The biochemistry of hepatic detoxification. In Artificial Liver Support; Brunner, G., Schmidt, F.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 32–38. [Google Scholar]
- Angrish, M.M.; Kaiser, J.P.; McQueen, C.A.; Chorley, B.N. Tipping the balance: Hepatotoxicity and the four apical key events of hepatic steatosis. Toxicol. Sci. 2016, 150, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cribb, A.E.; Peyrou, M.; Muruganandan, S.; Schneider, L. The endoplasmic reticulum in xenobiotic toxicity. Drug Metab. Rev. 2005, 37, 405–442. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, J.; Jin, X.; Li, Z.; Zhao, M.; Liu, W. P, p′-Dichlorodiphenyldichloroethylene induces colorectal adenocarcinoma cell proliferation through oxidative stress. PLoS ONE 2014, 9, e112700. [Google Scholar] [CrossRef]
- Marouani, N.; Hallegue, D.; Sakly, M.; Benkhalifa, M.; Ben Rhouma, K.; Tebourbi, O. P,p’-DDT induces testicular oxidative stress-induced apoptosis in adult rats. Reprod. Biol. Endocrinol. 2017, 15, 40. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Wang, Y.P.; Song, Y.; Li, H.W.; Liu, C.J.; Wu, Z.G.; Yang, K.D. P,p’-DDE induces testicular apoptosis in prepubertal rats via the Fas/FasL pathway. Toxicol. Lett. 2010, 193, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liang, X.; Hu, Y.; Wang, Y.; Yu, H.; Yang, K. P,p’-DDE induces mitochondria-mediated apoptosis of cultured rat Sertoli cells. Toxicology 2008, 253, 53–61. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Yu, H.; Hu, Y.; Wang, Y.; Yang, K. P,p’-Dichlorodiphenoxydichloroethylene induced apoptosis of Sertoli cells through oxidative stress-mediated p38 MAPK and mitochondrial pathway. Toxicol. Lett. 2011, 202, 55–60. [Google Scholar] [CrossRef]
- Quan, C.; Shi, Y.; Wang, C.; Wang, C.; Yang, K. P,p’-DDE damages spermatogenesis via phospholipid hydroperoxide glutathione peroxidase depletion and mitochondria apoptosis pathway. Environ. Toxicol. 2016, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I. Mitochondrial dysfunction in obesity and diabetes. US Endocrinol. 2010, 6, 20–27. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.M.; Kim, S.G.; Lee, I.K.; Lee, H.J.; Kim, J.H.; Kim, J.; Moon, H.B.; Jacobs, D.R., Jr.; Lee, D.H. Associations of organochlorine pesticides and polychlorinated biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance. Chemosphere 2014, 94, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.; LeMieux, M.; Dufour, J.M.; Kaur, G.; Kalupahana, N.; Moustaid-Moussa, N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys Acta Mol. Basis Dis. 2017, 1863, 1106–1114. [Google Scholar] [CrossRef]
- Verma, S.K.; Garikipati, V.N.S.; Kishore, R. Mitochondrial dysfunction and its impact on diabetic heart. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1098–1105. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Palacios-Ramirez, R.; Nguyen Dinh Cat, A. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Cohen, A.; Paolella, G.; Lepretti, M.; Smith, Y.; Faggio, C.; Lionetti, L. Modulation of mitochondrial functions by xenobiotic-induced microRNA: From environmental sentinel organisms to mammals. Sci. Total Environ. 2018, 645, 79–88. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.H.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Villanueva-Paz, M.; de la Cruz-Ojeda, P.; de la Mata, M.; Cotán, D.; Oropesa-Ávila, M.; de Lavera, I.; Álvarez-Córdoba, M.; Luzón-Hidalgo, R.; Sánchez-Alcázar, J.A. Mitochondrial dynamics in mitochondrial diseases. Diseases 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Schmukler, E.; Solomon, S.; Simonovitch, S.; Solomon, S.; Simonovitch, S.; Goldshmit, Y.; Wolfson, E.; Michaelson, D.M.; Pinkas-Kramarski, R. Altered mitochondrial dynamics and function in APOE4-expressing astrocytes. Cell Death Dis. 2020, 11, 578. [Google Scholar] [CrossRef]
- Patten, D.A.; Wong, J.; Khacho, M.; Soubannier, V.; Mailloux, R.J.; Pilon-Larose, K.; MacLaurin, J.G.; Park, D.S.; McBride, H.M.; Trinkle-Mulcahy, L.; et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014, 33, 2676–2691. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef] [PubMed]
- Eisner, V.; Picard, M.; Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 2018, 20, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive oxygen species and mitochondrial dynamics: The Yin and Yang of mitochondrial dysfunction and cancer progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Madeira, V.M.; Moreno, A.J. Interactions of 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene with mitochondrial oxidative phosphorylation. Biochem. Pharmacol. 1997, 153, 299–308. [Google Scholar] [CrossRef]
- Knasmüller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef]
- Nikolaou, N.; Green, C.J.; Gunn, P.J.; Hodson, L.; Tomlinson, J.W. Optimizing human hepatocyte models for metabolic phenotype and function: Effects of treatment with dimethyl sulfoxide (DMSO). Physiol. Rep. 2016, 4, e12944. [Google Scholar] [CrossRef]
- Shabbir, A.; DiStasio, S.; Zhao, J.; Cardozo, C.P.; Wolff, M.S.; Caplan, A.J. Differential effects of the organochlorine pesticide DDT and its metabolite p,p’-DDE on p-glycoprotein activity and expression. Toxicol. Appl. Pharmacol. 2005, 203, 91–98. [Google Scholar] [CrossRef]
- Chu, S.; Covaci, A.; Schepens, P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 2003, 93, 167–176. [Google Scholar] [CrossRef]
- Chu, S.; Covaci, A.; Jacobs, W.; Haraguchi, K.; Schepens, P. Distribution of methyl sulfone metabolites of polychlorinated biphenyls and p,p’-DDE in human tissues. Environ. Health Perspect. 2003, 111, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ozbek, N.; Bali, E.B.; Karasu, C. Quercetin and hydroxytyrosol attenuates xanthine/xanthine oxidase-induced toxicity in H9c2 cardiomyocytes by regulation of oxidative stress and stress-sensitive signaling pathways. Gen Physiol. Biophys. 2015, 34, 407–414. [Google Scholar]
- Lepretti, M.; Paolella, G.; Giordano, D.; Marabotti, A.; Gay, F.; Capaldo, A.; Esposito, C.; Caputo, I. 4-Nonylphenol reduces cell viability and induces apoptosis and ER-stress in a human epithelial intestinal cell line. Toxicol. Vitr. 2015, 29, 1436–1444. [Google Scholar] [CrossRef]
- de Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar] [CrossRef]
- Dhami-Shah, H.; Vaidya, R.; Udipi, S.; Raghavan, S.; Abhijit, S.; Mohan, V.; Balasubramanyam, M.; Vaidya, A. Picroside II attenuates fatty acid accumulation in HepG2 cells via modulation of fatty acid uptake and synthesis. Clin. Mol. Hepatol. 2018, 24, 77–87. [Google Scholar] [CrossRef]
- de Sousa, I.F.; Migliaccio, V.; Lepretti, M.; Paolella, G.; Di Gregorio, I.; Caputo, I.; Ribeiro, E.B.; Lionetti, L. Dose- and Time-Dependent Effects of Oleate on Mitochondrial Fusion/Fission Proteins and Cell Viability in HepG2 Cells: Comparison with Palmitate Effects. Int. J. Mol. Sci. 2021, 22, 9812. [Google Scholar] [CrossRef]
- Torres-Avilés, N.A.; Albores-García, D.; Luna, A.L.; Moreno-Galván, M.; Salgado-Bustamante, M.; Portales-Pérez, D.P.; Calderón-Aranda, E.S. Exposure to p,p’-DDE induces morphological changes and activation of the PKCα-p38-C/EBPβ pathway in human promyelocytic HL-60 cells. Biomed. Res. Int. 2016, 2016, 1375606. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Li, H.W.; Wang, Y.P.; Liu, C.J.; Yang, K.D. P,p’-DDE induces apoptosis and mRNA expression of apoptosis-associated genes in testes of pubertal rats. Environ. Toxicol. 2013, 28, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Alhasawi, A.; Contavadoo, M.; Appanna, V.D. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front. Cell Dev. Biol. 2015, 3, 40. [Google Scholar] [CrossRef]
- Shi, Y.; Song, Y.; Wang, Y.; Liang, X.; Hu, Y.; Guan, X.; Cheng, J.; Yang, K. P,p’-DDE induces apoptosis of rat Sertoli cells via a FasL-dependent pathway. J. Biomed. Biotechnol. 2009, 2009, 181282. [Google Scholar] [CrossRef]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar]
- Svegliati-Baron, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferrer, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in non alcoholic fatty liver disease: Not all lipids are created equal. Expert. Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef]
- Migliaccio, V.; Sica, R.; Di Gregorio, I.; Putti, R.; Lionetti, L. High-fish oil and high-lard diets differently affect testicular antioxidant defence and mitochondrial fusion/fission balance in male Wistar rats: Potential protective effect of ω3 polyunsaturated fatty acids targeting mitochondria dynamics. Int. J. Mol. Sci. 2019, 20, 3110. [Google Scholar] [CrossRef]
- Migliaccio, V.; Di Gregorio, I.; Putti, R.; Lionetti, L. Mitochondrial involvement in the adaptive response to chronic exposure to environmental pollutants and high-fat feeding in rat liver and testis. Cells 2019, 8, 834. [Google Scholar] [CrossRef]
- Joaquim, M.; Escobar-Henriques, M. Role of Mitofusins and Mitophagy in Life or Death Decisions. Front. Cell Dev. Biol. 2020, 8, 1002. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, W.; Richardson, A.; Van Remmen, H.; Ikeno, Y.; Salmon, A.B. Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochem. Biophys. Res. Commun. 2013, 438, 78–83. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Greenamyre, J.T. Environment, mitochondria, and Parkinson’s disease. Neuroscientist 2002, 8, 192–197. [Google Scholar]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Koshiba, T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta 2013, 1833, 225–232. [Google Scholar] [CrossRef]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]
- Paul, A.; Belton, A.; Nag, S.; Martin, I.; Grotewiel, M.S.; Duttaroy, A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech. Ageing Dev. 2007, 128, 706–716. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]
- Bhaskar, S.; Sheshadri, P.; Joseph, J.P.; Potdar, C.; Prasanna, J.; Kumar, A. Mitochondrial superoxide dismutase specifies early neural commitment by modulating mitochondrial dynamics. iScience 2020, 23, 101564. [Google Scholar] [CrossRef]
- Honrath, B.; Metz, I.; Bendridi, N.; Rieusset, J.; Culmsee, C.; Dolga, A.M. Glucose-regulated protein 75 determines ER-mitochondrial coupling and sensitivity to oxidative stress in neuronal cells. Cell Death Discov. 2017, 3, 17076. [Google Scholar] [CrossRef]
- Filadi, R.; Theurey, P.; Pizzo, P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 2017, 62, 1–15. [Google Scholar] [CrossRef]
- Lee, S.; Min, K.T. The interface between ER and mitochondria: Molecular compositions and functions. Mol. Cells 2008, 41, 1000–1007. [Google Scholar]
- Heal, R.D.; McGivan, J.D. Induction of the stress protein Grp75 by amino acid deprivation in CHO cells does not involve an increase in Grp75 mRNA levels. Biochem. Soc. Trans. 1996, 24, 479S. [Google Scholar] [CrossRef]
- Ikesugi, K.; Mulhern, M.L.; Madson, C.J.; Hosoya, K.; Terasaki, T.; Kador, P.F.; Shinohara, T. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr. Eye Res. 2006, 31, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Song, X.D.; Zuo, J. Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol. Cell. Biochem. 2005, 268, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Qiukay, E.; Liu, X.; Liu, Y.; Liu, W.; Zuo, J. Over-expression of GRP75 inhibits liver injury induced by oxidative damage. Acta Biochim. Biophys. Sin. 2013, 45, 129–134. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos Aceves, M.A.; Migliaccio, V.; Lepretti, M.; Paolella, G.; Di Gregorio, I.; Penna, S.; Faggio, C.; Lionetti, L. Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins. Toxics 2021, 9, 270. https://doi.org/10.3390/toxics9110270
Burgos Aceves MA, Migliaccio V, Lepretti M, Paolella G, Di Gregorio I, Penna S, Faggio C, Lionetti L. Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins. Toxics. 2021; 9(11):270. https://doi.org/10.3390/toxics9110270
Chicago/Turabian StyleBurgos Aceves, Mario Alberto, Vincenzo Migliaccio, Marilena Lepretti, Gaetana Paolella, Ilaria Di Gregorio, Serena Penna, Caterina Faggio, and Lillà Lionetti. 2021. "Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins" Toxics 9, no. 11: 270. https://doi.org/10.3390/toxics9110270
APA StyleBurgos Aceves, M. A., Migliaccio, V., Lepretti, M., Paolella, G., Di Gregorio, I., Penna, S., Faggio, C., & Lionetti, L. (2021). Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins. Toxics, 9(11), 270. https://doi.org/10.3390/toxics9110270






