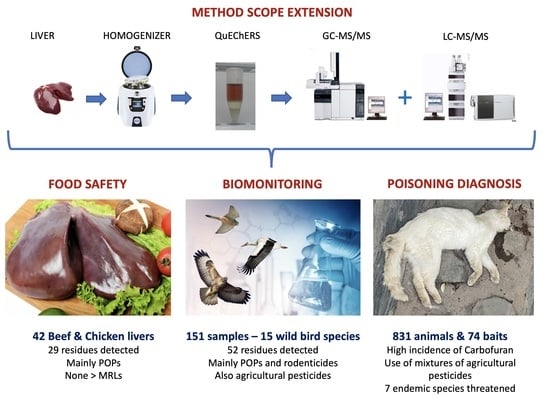
A Method Scope Extension for the Simultaneous Analysis of POPs, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Certified Standards and Reagents
2.2. Liver for Method Validation
2.3. Sample Preparation and Extraction
2.4. Instrumental Analysis
2.4.1. GC-MS/MS
2.4.2. LC-MS/MS
2.5. Validation Procedures
2.6. Samples for the Applicability of the Method
2.6.1. Sampling for the Food Safety Study
2.6.2. Sampling for the Biomonitoring Study
2.7. Statistical Analyses
3. Results and Discussion
3.1. Method Scope Extension Optimization
3.2. Validation Parameters
3.3. Application to Food Safety
3.4. Application to Biomonitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Compound | Technique | Retention Time (min) | Polarity | Quantification | Confirmation | Fragmentor Voltage (V) | ||
|---|---|---|---|---|---|---|---|---|---|
| MRM (m/z) | Collision energy (eV) | MRM transition (m/z) | Collision energy (eV) | ||||||
| 1 | 2-Phenylphenol | GC | 6.28 | positive | 169.0 → 115.0 | 30 | 169.0 → 141.0 | 15 | 70 |
| 2 | 4,4’-Dichlorobenzophenone (metabolite of dicofol) | GC | 9.99 | positive | 250.0 → 139.0 | 15 | 250.0 → 215.0 | 5 | 70 |
| 3 | Abamectine | LC | 10.99 | positive | 890.5 → 567.1 | 10 | 895.5 → 751.4 | 45 | 160 |
| 4 | Acenaphthene | GC | 6.15 | positive | 153.0 → 152.0 | 25 | 153.0 → 151.0 | 35 | 70 |
| 5 | Acenaphtylene | GC | 5.94 | positive | 152.0 → 151.0 | 25 | 152.0 → 126.0 | 30 | 70 |
| 6 | Acephate | LC | 1.64 | positive | 184.0 → 143.0 | 15 | 143.0 → 95.0 | 15 | 70 |
| 7 | Acetamiprid | LC | 4.43 | positive | 223.1 → 126.0 | 27 | 223.1 → 90.0 | 45 | 140 |
| 8 | Acrinathrin | LC | 10.70 | positive | 559.0 → 208.0 | 10 | 559.0 → 181.0 | 30 | 70 |
| 9 | Albendazole | LC | 7.14 | positive | 266.1 → 234.1 | 16 | 266.1 → 191.0 | 32 | 155 |
| 10 | Aldicarb | LC | 5.11 | positive | 208.0 → 116.0 | 10 | 116.0 → 89.1 | 4 | 100 |
| 11 | Aldicarb-sulfone | LC | 3.21 | positive | 240.1 → 76.0 | 16 | 223.1 → 86.1 | 13 | 75 |
| 12 | Aldicarb-sulfoxide | LC | 2.75 | positive | 207.1 → 131.9 | 10 | 207.1 → 89.1 | 10 | 86 |
| 13 | Aldrin | GC | 9.90 | positive | 255.0 → 220.0 | 25 | 263.0 → 228.0 | 10 | 70 |
| 14 | Anthracene | GC | 8.40 | positive | 178.0 → 176.0 | 35 | 178.0 → 152.0 | 30 | 70 |
| 15 | Atrazine | LC | 6.73 | positive | 216.0 → 173.9 | 15 | 216.0 → 103.8 | 30 | 130 |
| 16 | Azinphos-methyl | LC | 7.27 | positive | 318.0 → 132.1 | 8 | 340.0 → 160.0 | 10 | 60 |
| 17 | Azoxystrobin | LC | 7.59 | positive | 404.1 → 372.1 | 8 | 404.1 → 344.1 | 24 | 110 |
| 18 | BDE-28 | GC | 12.22 | positive | 406.0 → 246.0 | 20 | 406.0 → 167.0 | 25 | 70 |
| 19 | BDE-47 | GC | 14.31 | positive | 326.0 → 138.0 | 45 | 484.0 →324.0 | 25 | 70 |
| 20 | BDE-85 | GC | 17.08 | positive | 564.0 → 404.0 | 25 | 566.0 → 406.0 | 25 | 70 |
| 21 | BDE-99 | GC | 16.27 | positive | 566.0 → 406.0 | 25 | 564.0 → 404.0 | 30 | 70 |
| 22 | BDE-100 | GC | 15.85 | positive | 566.0 → 406.0 | 25 | 564.0 → 404.0 | 25 | 70 |
| 23 | BDE-153 | GC | 18.04 | positive | 644.0 → 484.0 | 25 | 486.0 → 377.0 | 30 | 70 |
| 24 | BDE-154 | GC | 17.47 | positive | 644.0 → 484.0 | 25 | 486.0 → 377.0 | 30 | 70 |
| 25 | BDE-183 | GC | 20.12 | positive | 561.6 → 454.7 | 40 | 563.6 → 454.7 | 40 | 70 |
| 26 | Benalaxyl | LC | 8.96 | positive | 326.2 → 148.0 | 20 | 326.2 → 208.0 | 12 | 90 |
| 27 | Bendiocarb | LC | 5.88 | positive | 224.1 → 166.9 | 8 | 224.2 → 108.9 | 15 | 120 |
| 28 | Bendiocarb metabolite (2,2-dimethylbenzo-1. 3-dioxol-4-ol) | GC | 4.84 | positive | 166.0 → 151.0 | 10 | 166.0 → 126.0 | 20 | 70 |
| 29 | Benfuracarb | LC | 9.73 | positive | 411.2 → 190.0 | 13 | 411.2 → 252.0 | 15 | 110 |
| 30 | Benzo[a]anthracene | GC | 13.95 | positive | 228.0 → 226.0 | 40 | 228.0 → 202.0 | 35 | 70 |
| 31 | Benzo[a]pyrene | GC | 16.89 | positive | 252.0 → 250.0 | 45 | 252.0 → 248.0 | 60 | 70 |
| 32 | Benzo[b]fluoranthene | GC | 16.30 | positive | 252.0 → 248.0 | 60 | 252.0 → 226.0 | 35 | 70 |
| 33 | Benzo[ghi]perylene | GC | 19.61 | positive | 276.0 → 274.0 | 50 | 276.0 → 272.0 | 60 | 70 |
| 34 | Benzo[k]fluoranthene | GC | 16.29 | positive | 252.0 → 250.0 | 45 | 252.0 → 224.0 | 40 | 70 |
| 35 | Bifenthrin | GC | 11.25 | positive | 440.0 → 181.0 | 5 | 440.0 → 165.0 | 60 | 94 |
| 36 | Bitertanol | LC | 9.23 | positive | 338.2 → 70.0 | 4 | 338.2 → 269.2 | 5 | 100 |
| 37 | Boscalid (formerly nicobifen) | GC | 7.84 | positive | 3434.0 → 272.0 | 30 | 343.0 → 140.0 | 45 | 100 |
| 38 | Brodifacoum | LC | 10.78 | negative | 521.3 → 79.0 | 50 | 523.3 → 135.0 | 45 | 220 |
| 39 | Bromadiolone | LC | 9.75 | negative | 525.3 → 250.0 | 40 | 527.3 → 250.0 | 40 | 200 |
| 40 | Bromopropylate | GC | 13.87 | positive | 341.0 → 183.0 | 15 | 341.0 → 157.0 | 45 | 70 |
| 41 | Bromuconazole (two isomers) | GC | 13.81/14.24 | positive | 295.0 → 173.0 | 10 | 295.0 → 175.0 | 10 | 70 |
| 42 | Bupirimate | LC | 11.78 | positive | 273.0 → 108.0 | 15 | 273.0 → 193.0 | 5 | 70 |
| 43 | Buprofezin | LC | 9.83 | positive | 306.1 → 201.0 | 12 | 306.1 → 116.0 | 12 | 140 |
| 44 | Cadusafos (ebufos) | LC | 9.39 | positive | 271.1 → 159.0 | 16 | 271.1 → 131.0 | 22 | 100 |
| 45 | Carbaryl | LC | 6.21 | positive | 202.1 → 145.1 | 4 | 202.1 → 127.1 | 28 | 95 |
| 46 | Carbendazim (azole) | LC | 2.90 | positive | 192.1 → 160.1 | 4 | 202.1 → 127.1 | 28 | 90 |
| 47 | Carbofuran | LC | 5.91 | positive | 222.1 → 123.1 | 20 | 222.1 → 165.1 | 30 | 80 |
| 48 | Carbofuran-3-hydroxy | LC | 4.27 | positive | 238.1 → 163.1 | 10 | 238.1 → 181.1 | 10 | 110 |
| 49 | Carbosulfan | LC | 11.03 | positive | 381.2 → 160.2 | 12 | 381.2 → 76.1 | 36 | 120 |
| 50 | Cefuroxima axetil (two isomers) | LC | 5.13 | positive | 533.0 → 447.0 | 15 | 533.0 → 386.0 | 20 | 160 |
| 51 | Chloramphenicol | LC | 4.63 | negative | 321.0 → 152.1 | 4 | 323.0 → 152.1 | 4 | 113 |
| 52 | Chlorantraniliprole | LC | 7.32 | positive | 483.9 → 452.9 | 16 | 483.9 → 285.9 | 8 | 105 |
| 53 | Chlorfenvinphos | LC | 9.09 | positive | 361.1 → 98.9 | 34 | 358.9 → 155.1 | 8 | 105 |
| 54 | Chlorobenzilate | GC | 12.14 | positive | 251.0 → 111.0 | 40 | 251.0 → 139.0 | 15 | 70 |
| 55 | Chlorophacinone | LC | 8.88 | negative | 373.2 → 201.0 | 20 | 375.2 → 203.0 | 20 | 160 |
| 56 | Chlorpropham | GC | 7.13 | positive | 213.0 → 127.0 | 15 | 153.0 → 90.0 | 25 | 70 |
| 57 | Chlorpyrifos | GC | 9.93 | positive | 314.0 → 258.0 | 15 | 314.0 → 286.0 | 5 | 70 |
| 58 | Chlorpyrifos methyl | GC | 9.12 | positive | 286.0 → 93.0 | 25 | 286.0 → 271.0 | 15 | 70 |
| 59 | Chlorthal dimethyl | GC | 10.02 | positive | 300.9 → 166.9 | 55 | 300.9 → 222.9 | 25 | 70 |
| 60 | Chrysene | GC | 13.86 | positive | 228.0 → 226.0 | 40 | 228.0 → 227.0 | 25 | 70 |
| 61 | Clindamycin | LC | 5.33 | positive | 425.2 → 126.1 | 20 | 425.2 → 377.2 | 20 | 150 |
| 62 | Clofentezine | LC | 9.19 | positive | 303.1 → 138.0 | 12 | 303.1 → 102.0 | 40 | 120 |
| 63 | Clothianidin | LC | 3.91 | positive | 250.0 → 169.0 | 8 | 250.0 → 131.9 | 8 | 100 |
| 64 | Cloxacillin | LC | 6.86 | positive | 436.1 → 160.0 | 8 | 436.1 → 277.0 | 12 | 126 |
| 65 | Coumachlor | LC | 8.63 | positive | 343.1 → 162.8 | 15 | 342.1 → 285.0 | 15 | 120 |
| 66 | Coumaphos | LC | 8.98 | positive | 363.0 → 227.0 | 30 | 363.0 → 306.9 | 15 | 120 |
| 67 | Coumatetralyl | LC | 8.31 | negative | 291.1 → 141.0 | 30 | 291.1 → 247.0 | 20 | 140 |
| 68 | Cyazofamid | LC | 8.49 | positive | 325.0 → 108.0 | 20 | 325.0 → 261.1 | 15 | 90 |
| 69 | Cyflufenamid | LC | 9.18 | positive | 413.1 → 223.1 | 33 | 413.1 → 295.1 | 23 | 70 |
| 70 | Cyfluthrin (sum of four isomers) | GC | 16.07/16.19/16.25/16.32 | positive | 226.0 → 206.0 | 25 | 198.9 → 170.1 | 25 | 70 |
| 71 | Cyhalothrin (lambda isomer) | GC | 10.49 | positive | 181.1 → 152.1 | 10 | 181.1 → 127.1 | 46 | 70 |
| 72 | Cymoxanil | LC | 4.67 | positive | 199.1 → 128.0 | 4 | 199.1 → 110.9 | 12 | 90 |
| 73 | Cypermethrin (sum of four isomers) | GC | 16.34/16.44/16.52/16.63 | positive | 163.0 → 109.0 | 20 | 163.0 → 127.0 | 5 | 70 |
| 74 | Cyproconazole (two isomers) | GC | 11.98 | positive | 222.0 → 125.0 | 20 | 222.0 → 82.0 | 10 | 70 |
| 75 | Cyprodinil | LC | 8.46 | positive | 226.0 → 93.0 | 33 | 226.0 → 108 | 25 | 100 |
| 76 | Cyromazine | LC | 1.23 | positive | 167.1 → 85.0 | 16 | 167.1 → 125.0 | 20 | 120 |
| 77 | Danofloxacin | LC | 4.04 | positive | 358.2 → 340.1 | 20 | 358.2 → 82.1 | 50 | 159 |
| 78 | Dazomet | GC | 7.80 | positive | 161.9 → 44.0 | 28 | 161.9 → 89.0 | 5 | 70 |
| 79 | Deltamethrin | LC | 10.65 | positive | 523.0 → 281.0 | 10 | 523.0 → 506.0 | 5 | 100 |
| 80 | Demeton-S-methyl | LC | 5.97 | positive | 230.9 → 88.9 | 5 | 230.9 → 61.0 | 30 | 50 |
| 81 | Demeton-S-methyl-sulfone (Dioxydemeton) | LC | 3.31 | positive | 263.0 → 169.0 | 24 | 263.0 → 109.0 | 12 | 120 |
| 82 | Dexamethasone | LC | 7.16 | positive | 393.2 → 373.2 | 2 | 393.2 → 355.2 | 6 | 103 |
| 83 | Diazinon | GC | 8.29 | positive | 137.1 → 54.0 | 20 | 304.0 → 179.0 | 15 | 70 |
| 84 | Dibenzo[a,h]anthracene | GC | 19.15 | positive | 278.0 → 276.0 | 40 | 278.0 → 250.0 | 60 | 70 |
| 85 | Dichlorodiphenyldichloroethane (p,p′ DDD) | GC | 12.31 | positive | 235.0 → 165.0 | 20 | 235.0 → 199.0 | 15 | 70 |
| 86 | Dichlorodiphenyldichloroethylene (p,p′ DDE) | GC | 11.58 | positive | 318.0 → 176.0 | 60 | 318.0 → 248.0 | 30 | 70 |
| 87 | Dichlorodiphenyltrichloroethane (p,p′ DDT) | GC | 12.84 | positive | 235.0 → 165.0 | 40 | 235.0 → 199.0 | 15 | 70 |
| 88 | Diclofenac | LC | 8.73 | positive | 296.0 → 215.1 | 16 | 296.0 → 214.1 | 48 | 103 |
| 89 | Dicloran | GC | 7.80 | positive | 206.0 → 176.0 | 10 | 206.0 → 148.0 | 25 | 70 |
| 90 | Diclorvos | GC | 4.74 | positive | 184.9 → 93.0 | 10 | 185.0 → 109.0 | 15 | 70 |
| 91 | Dicloxacillin | LC | 7.24 | positive | 470.0 → 160.0 | 8 | 470.0 → 310.8 | 10 | 106 |
| 92 | Dieldrin | GC | 11.66 | positive | 263.0 → 228.0 | 15 | 277.0 → 241.0 | 15 | 70 |
| 93 | Diethathyl ethyl | LC | 8.71 | positive | 312.2 → 238.1 | 15 | 312.2 → 162.0 | 30 | 120 |
| 94 | Diethofencarb | LC | 7.57 | positive | 268.2 → 226.1 | 5 | 268.2 → 152.0 | 20 | 110 |
| 95 | Difenacoum | LC | 10.38 | negative | 443.2 → 135.0 | 40 | 443.2 → 293.0 | 35 | 200 |
| 96 | Difenoconazole | LC | 9.41 | positive | 406.1 → 250.9 | 28 | 406.1 → 337.0 | 16 | 176 |
| 97 | Difethialone | LC | 10.93 | negative | 537.3 → 79.0 | 50 | 537.3 → 151.0 | 45 | 220 |
| 98 | Difloxacin | LC | 3.86 | positive | 400.2 → 382.1 | 20 | 400.2 → 356.1 | 16 | 149 |
| 99 | Diflubenzuron | LC | 8.63 | positive | 311.0 → 158.0 | 8 | 311.0 → 141.0 | 32 | 90 |
| 100 | Diflufenican | GC | 13.27 | positive | 394.0 → 266.0 | 10 | 266.0 → 246.0 | 10 | 70 |
| 101 | Dimethenamid-P (and its R-isomer) | LC | 7.68 | positive | 276.1 → 244.1 | 10 | 276.1 → 168.1 | 20 | 125 |
| 102 | Dimethoate | LC | 4.21 | positive | 230.0 → 125.0 | 16 | 230.0 → 198.8 | 20 | 70 |
| 103 | Dimethomorph (two isomers) | LC | 7.86 | positive | 388.1 → 301.1 | 20 | 388.1 → 165.1 | 32 | 180 |
| 104 | Dimethylphenylsulfamide (DMSA. metabolite of dichlofluanid) | LC | 5.21 | positive | 201.1 → 92.1 | 15 | 201.1 → 137.1 | 5 | 100 |
| 105 | Diniconazole-M | LC | 9.34 | positive | 326.1 → 70.0 | 28 | 328.1 → 70.0 | 28 | 110 |
| 106 | Dinocap | LC | 10.51 | negative | 295.4 → 208.9 | 30 | 295.4 → 193.0 | 35 | 150 |
| 107 | Diphacinone | LC | 8.60 | negative | 339.1 → 167.0 | 25 | 339.1 → 145.0 | 20 | 170 |
| 108 | Diphenylamine | GC | 6.98 | positive | 168.0 → 167.2 | 15 | 169.0 → 66.0 | 15 | 70 |
| 109 | Dodine | LC | 9.02 | positive | 228.3 → 43.0 | 40 | 228.3 → 57.0 | 25 | 150 |
| 110 | Doramectina | LC | 11.31 | positive | 921.5 → 777.4 | 55 | 899.5 → 145.1 | 30 | 220 |
| 111 | Endosulfan alfa | GC | 11.21 | positive | 241.0 → 206.0 | 15 | 195.0 → 160.0 | 10 | 70 |
| 112 | Endosulfan beta | GC | 12.21 | positive | 241.0 → 206.0 | 15 | 195.0 → 159.0 | 15 | 70 |
| 113 | Endosulfan sulfate | GC | 12.96 | positive | 270.0 → 235.0 | 15 | 387.0 → 289.0 | 5 | 70 |
| 114 | Endrin | GC | 12.05 | positive | 263.0 → 193.0 | 35 | 245.0 → 173.0 | 25 | 70 |
| 115 | Enrofloxacin | LC | 3.94 | positive | 360.2 → 316.1 | 16 | 360.2 → 245.1 | 28 | 144 |
| 116 | EPN | GC | 13.90 | positive | 157.0 → 63.0 | 10 | 157.0 → 110.0 | 15 | 70 |
| 117 | Epoxiconazole | LC | 8.47 | positive | 330.0 → 120.9 | 24 | 330.1 → 100.9 | 50 | 120 |
| 118 | Eprinomectin | LC | 10.84 | positive | 878.5 → 186.0 | 15 | 936.5 → 490.4 | 60 | 160 |
| 119 | Eritromicin | LC | 6.74 | positive | 734.5 → 158.1 | 32 | 734.5 → 576.3 | 16 | 172 |
| 120 | Esfenvalerate | GC | 17.56 | positive | 167.1 → 125.1 | 15 | 167.1 → 89.1 | 45 | 70 |
| 121 | Ethion (diethion) | LC | 10.03 | positive | 385.0 → 199.0 | 5 | 385.0 → 171.0 | 10 | 100 |
| 122 | Ethirimol | LC | 4.80 | positive | 210.2 → 140.1 | 20 | 210.2 → 98.1 | 28 | 160 |
| 123 | Ethofumesate | GC | 9.59 | positive | 286.0 → 207.0 | 5 | 286.0 → 161.0 | 20 | 70 |
| 124 | Ethoprophos | LC | 8.38 | positive | 243.1 → 97.0 | 30 | 243.1 → 130.9 | 15 | 90 |
| 125 | Etofenprox | GC | 16.75 | positive | 163.0 → 107.0 | 20 | 163.0 → 135.0 | 10 | 70 |
| 126 | Etoxazole | LC | 10.34 | positive | 360.1 → 141.0 | 26 | 360.1 → 304.0 | 16 | 160 |
| 127 | Famoxadone | LC | 9.07 | positive | 392.1 → 330.9 | 5 | 392.2 → 238.1 | 12 | 110 |
| 128 | Fenamidone | LC | 9.06 | positive | 392.1 → 330.9 | 5 | 392.1 → 238.1 | 12 | 110 |
| 129 | Fenamiphos | LC | 7.72 | positive | 304.1 → 217.1 | 20 | 304.1 → 202.0 | 36 | 120 |
| 130 | Fenamiphos sulfone | LC | 8.63 | positive | 336.1 → 188.0 | 31 | 336.1 → 266.0 | 23 | 120 |
| 131 | Fenamiphos sulfoxide | LC | 5.93 | positive | 320.1 → 233.0 | 20 | 320.1 → 108.1 | 44 | 120 |
| 132 | Fenarimol | GC | 15.03 | positive | 139.0 → 75.0 | 30 | 139.0 → 111.0 | 15 | 70 |
| 133 | Fenazaquin | LC | 10.73 | positive | 307.2 → 57.1 | 25 | 307.2 → 161.1 | 16 | 90 |
| 134 | Fenbendazole | LC | 8.04 | positive | 300.1 → 268.1 | 20 | 300.1 → 159.0 | 36 | 156 |
| 135 | Fenbuconazole | GC | 16.17 | positive | 198.0 → 102.0 | 30 | 198.0 → 78.0 | 30 | 70 |
| 136 | Fenhexamid | LC | 8.35 | positive | 302.1 → 97.1 | 20 | 302.1 → 55.1 | 40 | 130 |
| 137 | Fenitrothion | GC | 9.57 | positive | 277.0 → 109.0 | 15 | 277.0 → 125.0 | 15 | 70 |
| 138 | Fenoxycarb | LC | 8.69 | positive | 302.1 → 88.0 | 20 | 302.1 → 116.1 | 10 | 110 |
| 139 | Fenpropathrin | LC | 10.43 | positive | 367.2 → 125.0 | 16 | 350.1 → 125.0 | 16 | 72 |
| 140 | Fenpropidin | LC | 7.13 | positive | 274.3 → 147.0 | 30 | 274.3 → 86.0 | 25 | 170 |
| 141 | Fenpropimorph | LC | 7.37 | positive | 304.3 → 147.1 | 30 | 304.3 → 130.0 | 25 | 120 |
| 142 | Fenpyroximate | LC | 10.49 | positive | 422.2 → 366.2 | 12 | 422.2 → 135.0 | 36 | 160 |
| 143 | Fenthion | GC | 8.90 | positive | 278.0 → 109.0 | 15 | 278.0 → 125.0 | 20 | 70 |
| 144 | Fenthion oxon | LC | 7.31 | positive | 263.1 → 231.2 | 16 | 263.1 → 216.0 | 24 | 120 |
| 145 | Fenthion oxon sulfone | LC | 4.50 | positive | 295.0 → 217.0 | 15 | 295.0 → 104.2 | 24 | 110 |
| 146 | Fenthion oxon sulfoxide | LC | 4.26 | positive | 279.0 → 264.2 | 20 | 279.0 → 104.1 | 28 | 110 |
| 147 | Fenthion sulfone | LC | 6.39 | positive | 311.0 → 125.0 | 22 | 311.0 → 109.0 | 28 | 140 |
| 148 | Fenthion sulfoxide | LC | 6.16 | positive | 295.0 → 108.9 | 30 | 295.0 → 280.0 | 18 | 140 |
| 149 | Fenvalerate | GC | 17.36 | positive | 167.0 → 125.1 | 22 | 167.0 → 89.0 | 30 | 70 |
| 150 | Fipronil | LC | 8.68 | negative | 435.0 → 330.0 | 12 | 435.0 → 249.9 | 26 | 116 |
| 151 | Fipronil sulfide | GC | 10.49 | positive | 351.0 → 255.0 | 20 | 420.0 → 351.0 | 25 | 70 |
| 152 | Flocoumafen | LC | 10.44 | negative | 541.3 → 382.0 | 25 | 541.3 → 161.0 | 40 | 230 |
| 153 | Fluazinam | LC | 10.01 | negative | 462.9 → 416.0 | 10 | 462.9 → 398.0 | 9 | 140 |
| 154 | Flubendiamide | LC | 8.82 | positive | 408.0 → 274.0 | 15 | 408.0 → 256.0 | 30 | 120 |
| 155 | Flucythrinate (two isomers) | GC | 16.67/16.84 | positive | 156.9 → 107.1 | 15 | 199.1 → 107.1 | 25 | 70 |
| 156 | Fludioxonil | GC | 11.51 | positive | 248.0 → 127.0 | 30 | 248.1 → 182.1 | 10 | 70 |
| 157 | Flufenoxuron | LC | 10.37 | positive | 489.1 → 158.0 | 20 | 489.1 → 140.9 | 56 | 110 |
| 158 | Flumequine | LC | 6.12 | positive | 262.1 → 244.0 | 16 | 262.1 → 202.0 | 32 | 116 |
| 159 | Flunixin | LC | 8.09 | positive | 297.1 → 279.1 | 24 | 297.1 → 264.1 | 32 | 141 |
| 160 | Fluopyram | GC | 10.61 | positive | 173.0 → 95.0 | 35 | 223.0 → 196.0 | 40 | 70 |
| 161 | Fluoranthene | GC | 10.66 | positive | 202.0 → 201.0 | 27 | 202.0 → 152.0 | 42 | 70 |
| 162 | Fluorene | GC | 6.81 | positive | 165.0 → 163.0 | 40 | 165.0 → 139.0 | 30 | 70 |
| 163 | Fluquinconazole | GC | 15.81 | positive | 340.0 → 298.0 | 15 | 340.0 → 286.0 | 25 | 70 |
| 164 | Flusilazole | LC | 8.64 | positive | 316.1 → 247.1 | 15 | 316.1 → 165.0 | 20 | 160 |
| 165 | Flutolanil | LC | 7.93 | positive | 324.1 → 262.1 | 16 | 324.1 → 242.1 | 24 | 130 |
| 166 | Flutriafol | GC | 11.26 | positive | 219.0 → 95.0 | 35 | 219.0 → 123.0 | 15 | 70 |
| 167 | Fluvalinate tau | GC | 17.56 | positive | 250.1 → 55.1 | 30 | 252.0 → 200.0 | 20 | 70 |
| 168 | Fonofos | GC | 8.24 | positive | 246.0 → 109.0 | 15 | 246.0 → 237.0 | 5 | 70 |
| 169 | Formetanate | LC | 1.76 | positive | 222.1 → 165.1 | 12 | 222.1 → 46.2 | 28 | 105 |
| 170 | Fosthiazate | LC | 6.50 | positive | 284.0 → 104.0 | 20 | 284.0 → 227.8 | 8 | 90 |
| 171 | Heptachlor | GC | 9.31 | positive | 272.0 → 237.0 | 15 | 274.0 → 239.0 | 15 | 70 |
| 172 | Hexachlorobencene | GC | 7.77 | positive | 284.0 → 214.0 | 40 | 284.0 → 249.0 | 25 | 70 |
| 173 | Hexachlorocyclohexane (alpha) | GC | 7.64 | positive | 219.0 → 109.0 | 10 | 219.0 → 183.0 | 10 | 70 |
| 174 | Hexachlorocyclohexane (beta) | GC | 8.02 | positive | 219.0 → 109.0 | 40 | 219.0 → 183.0 | 5 | 70 |
| 175 | Hexachlorocyclohexane (delta) | GC | 8.50 | positive | 219.0 → 109.0 | 45 | 219.0 → 183.0 | 5 | 70 |
| 176 | Hexaclorocyclohexane (gamma. lindane) | GC | 8.13 | positive | 291.0 → 109.0 | 40 | 219.0 →183.0 | 10 | 70 |
| 177 | Hexaconazole (two isomers) | LC | 8.49 | positive | 314.1 → 70.1 | 20 | 316.0 → 70.1 | 20 | 95 |
| 178 | Hexaflumuron | LC | 9.58 | negative | 458.8 → 439.0 | 8 | 458.8 → 175.0 | 30 | 100 |
| 179 | Hexythiazox | LC | 10.18 | positive | 353.1 → 227.9 | 8 | 353.1 → 168.1 | 24 | 120 |
| 180 | Imazalil (enilconazole) | LC | 6.53 | positive | 297.1 → 159.0 | 20 | 297.1 → 69.1 | 18 | 100 |
| 181 | Imidacloprid | LC | 3.93 | positive | 256.0 → 175.0 | 12 | 256.0 → 209.0 | 12 | 110 |
| 182 | Indeno [1,2,3-cd] pyrene | GC | 19.08 | positive | 276.0 → 274.0 | 50 | 276.0 → 272.0 | 60 | 70 |
| 183 | Indoxacarb | LC | 9.49 | positive | 528.1 → 293.1 | 10 | 528.1 → 202.8 | 48 | 140 |
| 184 | Iprovalicarb | LC | 8.18 | positive | 321.2 → 119.0 | 15 | 321.2 → 202.9 | 20 | 110 |
| 185 | Isofenphos methyl | GC | 10.38 | positive | 199.0 → 121.0 | 10 | 241.0 → 121.0 | 25 | 70 |
| 186 | Isoprothiolane | LC | 7.94 | positive | 291.1 → 189.0 | 30 | 291.1 → 145.0 | 36 | 100 |
| 187 | Ivermectin B1a | LC | 11.52 | positive | 897.5 → 753.5 | 50 | 897.5 → 329.3 | 60 | 160 |
| 188 | Josamycin | LC | 7.40 | positive | 860.5 → 173.9 | 40 | 860.5 → 108.9 | 40 | 200 |
| 189 | Ketoprofen | LC | 7.34 | positive | 255.1 → 209.1 | 8 | 255.1 → 77.1 | 48 | 123 |
| 190 | Kresoxim methyl | GC | 11.78 | positive | 116.0 → 89.0 | 15 | 206.0 → 131.0 | 10 | 70 |
| 191 | Levamisole | LC | 3.12 | positive | 205.1 → 178.1 | 20 | 205.1 → 123.0 | 32 | 141 |
| 192 | Lincomycin | LC | 3.50 | positive | 407.2 → 126.1 | 24 | 407.2 → 359.2 | 16 | 150 |
| 193 | Linuron | LC | 7.54 | positive | 249.0 → 160.1 | 20 | 249.0 → 182.3 | 8 | 120 |
| 194 | Lufenuron | LC | 10.05 | negative | 509.0 → 339.0 | 5 | 509.0 → 326.1 | 15 | 90 |
| 195 | Mandipropamid | LC | 7.90 | positive | 412.1 → 328.1 | 8 | 412.1 → 356.1 | 4 | 130 |
| 196 | Mebendazole | LC | 6.68 | positive | 296.1 → 264.1 | 20 | 296.1 → 77.0 | 48 | 151 |
| 197 | Mefenamic acid | LC | 9.52 | positive | 242.1 → 209.1 | 28 | 242.1 → 180.1 | 0 | 108 |
| 198 | Mefenoxam (metalaxyl-M) | LC | 6.95 | positive | 280.0 → 220.0 | 10 | 280.0 → 192.0 | 15 | 110 |
| 199 | Meloxicam | LC | 7.17 | positive | 352.5 → 114.8 | 20 | 352.5 → 140.8 | 20 | 130 |
| 200 | Mepanipyrim | GC | 11.13 | positive | 222.0 → 221.0 | 15 | 222.0 → 207.0 | 15 | 70 |
| 201 | Mepiquat | LC | 0.64 | positive | 114.0 → 98.0 | 36 | 114.0 → 70.0 | 45 | 100 |
| 202 | Metaflumizone | LC | 9.94 | negative | 505.0 → 302.0 | 14 | 541.0 → 302.0 | 20 | 90 |
| 203 | Metalaxyl | GC | 9.31 | positive | 234.0 → 146.1 | 20 | 249.0 → 146.0 | 20 | 70 |
| 204 | Metaldehyde | LC | 3.87 | positive | 194.1 → 61.9 | 5 | 194.1 → 106.0 | 5 | 50 |
| 205 | Metconazole | LC | 9.17 | positive | 320.1 → 70.2 | 33 | 322.1 → 70.2 | 24 | 250 |
| 206 | Methamidophos (two isomers) | LC | 1.18 | positive | 142.0 → 94.0 | 12 | 142.0 → 125.0 | 12 | 85 |
| 207 | Methidathion | LC | 7.12 | positive | 320.1 → 144.8 | 8 | 320.1 → 85.0 | 30 | 84 |
| 208 | Methiocarb | LC | 7.67 | positive | 226.1 → 169.0 | 4 | 226.1 → 121.1 | 12 | 90 |
| 209 | Methiocarb-sufone | LC | 4.52 | positive | 258.1 → 201.1 | 8 | 258.1 → 122.1 | 22 | 100 |
| 210 | Methiocarb-sulfoxide | LC | 4.03 | positive | 242.0 → 185.0 | 22 | 242.0 → 122.0 | 28 | 90 |
| 211 | Methomyl | LC | 3.23 | positive | 163.1 → 88.0 | 5 | 163.0 → 106.0 | 8 | 80 |
| 212 | Methoxyfenozide | LC | 8.00 | positive | 369.2 → 149.0 | 10 | 369.2 → 313.1 | 15 | 85 |
| 213 | Metoxychlor | GC | 13.98 | positive | 227.0 → 141.0 | 20 | 227.0 → 169.0 | 15 | 70 |
| 214 | Metrafenone | LC | 9.27 | positive | 409.1 → 209.1 | 8 | 411.1 → 209.1 | 12 | 108 |
| 215 | Metronidazole | LC | 2.63 | positive | 172.1 → 128.0 | 12 | 172.1 → 82.1 | 24 | 98 |
| 216 | Mevinphos (phosdrin) | LC | 4.38 | positive | 225.0 → 193.1 | 15 | 225.0 → 127.0 | 12 | 65 |
| 217 | Mirex | GC | 5.66 | positive | 237.0 → 143.0 | 30 | 274.0 → 237.0 | 10 | 70 |
| 218 | Monocrotophos | LC | 3.31 | positive | 224.1 → 126.8 | 12 | 224.1 → 98.1 | 15 | 100 |
| 219 | Moxidectin | LC | 11.24 | positive | 641.4 → 529.2 | 5 | 641.4 → 499.2 | 5 | 100 |
| 220 | Myclobutanil | LC | 8.10 | positive | 289.1 → 70.1 | 16 | 289.1 → 125.1 | 32 | 110 |
| 221 | N-(2,4-dimethylphenyl)-N′-methylformamidine (DMPF, metabolite of amitraz) | LC | 3.35 | positive | 163.1 → 122.1 | 15 | 163.1 → 107.1 | 15 | 100 |
| 222 | N,N-Dimethyl-N′-p-tolylsulphamide (DMST, metabolite of tolyfluanid) | LC | 6.06 | positive | 215.1 → 106.1 | 10 | 215.1 → 151.1 | 4 | 90 |
| 223 | Nafcillin | LC | 7.33 | positive | 415.0 → 199.1 | 8 | 415.0 → 171.0 | 36 | 103 |
| 224 | Naphtalene | GC | 4.45 | positive | 128.0 → 127.0 | 15 | 128.0 → 102.0 | 25 | 70 |
| 225 | Naproxen | LC | 7.59 | positive | 231.0 → 185.0 | 10 | 231.1 → 169.9 | 21 | 120 |
| 226 | Nitenpyram | LC | 3.30 | positive | 271.1 → 56.1 | 36 | 271.1 → 224.9 | 12 | 100 |
| 227 | Novobiocin | LC | 9.69 | positive | 613.2 → 218.1 | 10 | 613.2 → 396.1 | 10 | 150 |
| 228 | Nuarimol | GC | 13.27 | positive | 235.0 → 139.0 | 15 | 235.0 → 111.0 | 40 | 70 |
| 229 | Ofurace | LC | 5.97 | positive | 282.0 → 159.9 | 20 | 282.0 → 147.9 | 30 | 100 |
| 230 | Omethoate | LC | 2.80 | positive | 214.1 → 124.8 | 22 | 214.1 → 183.0 | 5 | 100 |
| 231 | Oxadixyl | LC | 5.43 | positive | 279.1 → 219.2 | 5 | 279.1 → 132.2 | 32 | 110 |
| 232 | Oxamyl | LC | 2.87 | positive | 237.1 → 72.0 | 12 | 237.1 → 90.0 | 5 | 70 |
| 233 | Oxamyl-oxime | LC | 2.46 | positive | 163.3 → 115.2 | 10 | 163.3 → 72.1 | 10 | 70 |
| 234 | Oxfendazole | LC | 5.61 | positive | 316.1 → 159.0 | 32 | 316.1 → 191.1 | 16 | 166 |
| 235 | Oxolinic acid | LC | 5.04 | positive | 262.1 → 216.0 | 32 | 262.1 → 160.0 | 36 | 110 |
| 236 | Oxydemeton methyl | LC | 3.01 | positive | 247.0 → 169.0 | 12 | 247.0 → 109.0 | 24 | 100 |
| 237 | Oxyfluorfen | GC | 11.68 | positive | 252.0 → 146.0 | 40 | 300.0 → 223.0 | 15 | 70 |
| 238 | Paclobutrazol | LC | 7.89 | positive | 294.1 → 70.1 | 16 | 294.1 → 125.2 | 36 | 115 |
| 239 | Parathion methyl | GC | 9.12 | positive | 263.0 → 109.0 | 15 | 263.0 → 79.0 | 30 | 70 |
| 240 | PCB 28 | GC | 9.01 | positive | 256.0 → 186.0 | 25 | 256.0 → 151.0 | 50 | 70 |
| 241 | PCB 52 | GC | 9.58 | positive | 292.0 → 222.0 | 25 | 292.0 → 220.0 | 25 | 70 |
| 242 | PCB 77 | GC | 11.73 | positive | 292.0 → 220.0 | 25 | 292.0 → 222.0 | 25 | 70 |
| 243 | PCB 81 | GC | 11.56 | positive | 292.0 → 220.0 | 25 | 292.0 → 222.0 | 25 | 70 |
| 244 | PCB 101 | GC | 11.08 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 245 | PCB 105 | GC | 12.66 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 246 | PCB 114 | GC | 12.38 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 247 | PCB 118 | GC | 12.18 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 248 | PCB 123 | GC | 12.10 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 249 | PCB 126 | GC | 13.23 | positive | 326.0 → 256.0 | 30 | 328.0 → 256.0 | 30 | 70 |
| 250 | PCB 138 | GC | 13.07 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 251 | PCB 153 | GC | 12.57 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 252 | PCB 156 | GC | 13.96 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 253 | PCB 157 | GC | 14.07 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 254 | PCB 167 | GC | 13.55 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 255 | PCB 169 | GC | 14.61 | positive | 360.0 → 290.0 | 25 | 360.0 → 288.0 | 25 | 70 |
| 256 | PCB 180 | GC | 14.25 | positive | 394.0 → 324.0 | 30 | 394.0 → 322.0 | 30 | 70 |
| 257 | PCB 189 | GC | 15.25 | positive | 394.0 → 324.0 | 30 | 394.0 → 322.0 | 30 | 70 |
| 258 | Penconazole | GC | 10.52 | positive | 248.0 → 157.0 | 30 | 248.0 → 192.0 | 15 | 70 |
| 259 | Pencycuron | LC | 9.33 | positive | 329.1 → 125.1 | 24 | 329.1 → 217.9 | 12 | 160 |
| 260 | Pendimethalin | GC | 10.49 | positive | 252.0 → 162.0 | 10 | 252.0 → 191.0 | 5 | 70 |
| 261 | Penicillin V | LC | 6.47 | positive | 383.2 → 159.9 | 10 | 383.2 → 113.9 | 40 | 130 |
| 262 | Permethrin | GC | 15.69 | positive | 183.0 → 128.0 | 15 | 183.1 → 153.1 | 15 | 70 |
| 263 | Phenanthrene | GC | 8.40 | positive | 178.0 → 176.0 | 35 | 178.0 → 152.0 | 28 | 70 |
| 264 | Phenylbutazone | LC | 8.25 | positive | 309.2 → 160.2 | 20 | 309.2 → 77.1 | 55 | 140 |
| 265 | Phosalone | LC | 9.20 | positive | 385.1 → 182.0 | 20 | 385.1 → 110.9 | 55 | 80 |
| 266 | Phosmet | LC | 7.34 | positive | 318.0 → 159.9 | 16 | 318.0 → 133.0 | 40 | 90 |
| 267 | Pthalamide (Folpet deg) | GC | 5.94 | positive | 104.0 → 50.0 | 25 | 147.0 → 76.0 | 25 | 70 |
| 268 | Pirimicarb | LC | 5.11 | positive | 239.1 → 72.1 | 20 | 239.1 → 182.1 | 12 | 100 |
| 269 | Pirimicarb-desmethyl | LC | 3.71 | positive | 225.1 → 168.1 | 8 | 225.1 → 72.1 | 20 | 100 |
| 270 | Pirimiphos ethyl | GC | 10.26 | positive | 318.0 → 166.0 | 15 | 318.0 → 182.0 | 15 | 70 |
| 271 | Pirimiphos methyl | LC | 9.13 | positive | 306.1 → 164.0 | 20 | 306.1 → 108.1 | 32 | 100 |
| 272 | Prochloraz | LC | 9.08 | positive | 376.0 → 308.0 | 10 | 376.0 → 70.1 | 20 | 100 |
| 273 | Procymidone | GC | 10.80 | positive | 283.0 → 67.0 | 40 | 283.0 → 68.0 | 25 | 70 |
| 274 | Profenofos | LC | 9.75 | positive | 375.0 → 305.0 | 20 | 373.0 → 303.0 | 20 | 100 |
| 275 | Propamocarb | LC | 2.85 | positive | 189.2 → 102.0 | 12 | 189.2 → 144.0 | 8 | 110 |
| 276 | Propargite | LC | 10.37 | positive | 368.2 → 231.1 | 4 | 368.2 → 175.0 | 12 | 88 |
| 277 | Propiconazole | LC | 9.01 | positive | 342.0 → 69.0 | 21 | 342.0 → 159.0 | 39 | 90 |
| 278 | Propoxur | LC | 5.83 | positive | 210.1 → 168.1 | 35 | 210.1 → 65.1 | 40 | 70 |
| 279 | Propyzamide (pronamide) | LC | 7.92 | positive | 256.1 → 190.0 | 16 | 256.1 → 173.0 | 25 | 90 |
| 280 | Proquinazid | GC | 13.32 | positive | 288.0 → 245.0 | 15 | 288.0 → 217.0 | 30 | 70 |
| 281 | Prothioconazol | GC | 11.85 | positive | 186.0 → 49.0 | 20 | 186.0 → 53.0 | 25 | 70 |
| 282 | Prothiophos | GC | 11.45 | positive | 266.9 → 221.0 | 35 | 162.0 → 63.1 | 30 | 70 |
| 283 | Pymetrozine | LC | 2.74 | positive | 218.1 → 105.0 | 20 | 218.1 → 78.0 | 52 | 120 |
| 284 | Pyraclostrobin | LC | 9.15 | positive | 388.1 → 193.8 | 8 | 388.1 → 163.1 | 28 | 120 |
| 285 | Pyrazophos | LC | 9.22 | positive | 374.1 → 222.1 | 23 | 374.1 → 194.0 | 32 | 100 |
| 286 | Pyrene | GC | 11.13 | positive | 202.0 → 201.0 | 27 | 202.0 → 200.0 | 45 | 70 |
| 287 | Pyridaben | LC | 10.75 | positive | 365.2 → 309.0 | 8 | 309.1 → 147.0 | 16 | 168 |
| 288 | Pyridaphenthion | LC | 8.11 | positive | 341.0 → 189.0 | 22 | 341.0 → 205.0 | 34 | 100 |
| 289 | Pyrimethanil | GC | 8.27 | positive | 198.0 → 118.0 | 40 | 198.0 → 158.0 | 20 | 70 |
| 290 | Pyriproxifen | LC | 10.07 | positive | 322.2 → 96.0 | 12 | 322.2 → 184.9 | 24 | 80 |
| 291 | Quinalfos | LC | 8.72 | positive | 299.1 → 96.9 | 30 | 299.1 →147.1 | 20 | 130 |
| 292 | Quinoxyfen | LC | 10.13 | positive | 308.0 → 197.0 | 32 | 308.2 → 161.8 | 55 | 120 |
| 293 | Rifampicin | LC | 7.89 | positive | 823.5 → 791.4 | 15 | 823.5 → 399.1 | 25 | 160 |
| 294 | Rotenone | LC | 8.64 | positive | 395.1 → 213.1 | 20 | 395.1 → 192.1 | 25 | 150 |
| 295 | Roxithromycin | LC | 7.67 | positive | 838.5 → 158.1 | 40 | 838.5 → 116.1 | 55 | 200 |
| 296 | Sarafloxacin | LC | 4.16 | positive | 386.1 → 342.1 | 16 | 386.1 → 299.1 | 28 | 144 |
| 297 | Simazine | LC | 5.81 | positive | 202.4 → 68.1 | 30 | 202.4 → 68.1 | 20 | 120 |
| 298 | Spinosad (two isomers) | LC | 9.10/9.43 | positive | 732.4 → 142.0 | 22 | 732.4 → 98.0 | 60 | 130 |
| 299 | Spiramycin (two isomers) | LC | 4.58/4.90 | positive | 439.1 → 101.1 | 20 | 439.1 → 88.0 | 50 | 70 |
| 300 | Spirodiclofen | LC | 10.50 | positive | 411.1 → 71.2 | 15 | 411.1 → 313.0 | 5 | 110 |
| 301 | Spiromesifen | LC | 10.27 | positive | 388.0 → 273.0 | 25 | 273.0 → 187.0 | 15 | 110 |
| 302 | Spirotetramat | LC | 8.23 | positive | 374.2 → 302.2 | 12 | 374.2 → 216.1 | 36 | 150 |
| 303 | Spiroxamine | LC | 7.55 | positive | 298.3 → 144.1 | 16 | 298.3 → 100.1 | 32 | 120 |
| 304 | Strychnine | LC | 3.00/3.61 | positive | 335.1 → 184.0 | 45 | 335.1 → 156.0 | 40 | 105 |
| 305 | Sulfacetamide | LC | 2.13 | positive | 215.3 → 155.9 | 10 | 215.3 → 92.0 | 20 | 90 |
| 306 | Sulfachloropiridacine | LC | 3.77 | positive | 285.0 → 156.0 | 12 | 285.0 → 92.1 | 28 | 101 |
| 307 | Sulfadiacine | LC | 2.80 | positive | 251.0 → 92.0 | 28 | 251.0 → 156.0 | 12 | 111 |
| 308 | Sulfadimetoxine | LC | 4.81 | positive | 311.0 → 92.0 | 32 | 311.0 → 156.0 | 16 | 139 |
| 309 | Sulfadoxine | LC | 4.12 | positive | 311.1 → 92.0 | 32 | 311.1 → 156.0 | 16 | 126 |
| 310 | Sulfameracine | LC | 3.26 | positive | 265.0 → 92.0 | 28 | 265.0 → 156.0 | 12 | 126 |
| 311 | Sulfametacine | LC | 3.44 | positive | 279.1 → 186.0 | 12 | 279.1 → 92.0 | 32 | 134 |
| 312 | Sulfametizole | LC | 3.37 | positive | 271.0 → 92.0 | 28 | 271.0 → 155.9 | 8 | 103 |
| 313 | Sulfametoxazole | LC | 3.93 | positive | 254.0 → 92.0 | 28 | 254.0 → 156.0 | 12 | 111 |
| 314 | Sulfametoxipiridacine | LC | 3.45 | positive | 281.0 → 155.9 | 12 | 281.0 → 92.1 | 28 | 121 |
| 315 | Sulfamonomethoxine | LC | 4.11 | positive | 281.1 → 156.0 | 14 | 281.1 → 92.1 | 32 | 120 |
| 316 | Sulfapyridine | LC | 2.82 | positive | 250.0 → 156.0 | 12 | 250.0 → 92.0 | 28 | 126 |
| 317 | Sulfaquinoxaline | LC | 4.99 | positive | 301.0 → 156.0 | 12 | 301.0 → 92.1 | 32 | 159 |
| 318 | Sulfatiazole | LC | 2.98 | positive | 256.0 → 92.0 | 28 | 256.0 → 156.0 | 12 | 106 |
| 319 | Sulfisoxazole | LC | 4.12 | positive | 268.0 → 156.0 | 8 | 268.0 → 92.1 | 24 | 106 |
| 320 | Tebuconazole | LC | 8.92 | positive | 308.2 → 70.2 | 22 | 308.2 → 125.1 | 53 | 120 |
| 321 | Tebufenocide | LC | 8.66 | positive | 353.1 → 132.9 | 22 | 353.1 → 297.1 | 20 | 90 |
| 322 | Tebufenpyrad | LC | 9.88 | positive | 334.2 → 117.0 | 47 | 334.2 → 145.0 | 37 | 180 |
| 323 | Teflubenzuron | LC | 10.01 | negative | 379.0 → 339.0 | 15 | 379.0 → 196.0 | 25 | 100 |
| 324 | Tefluthrin | GC | 8.42 | positive | 177.0 → 127.0 | 15 | 177.0 → 87.0 | 15 | 70 |
| 325 | Telodrin (isobenzan) | GC | 10.14 | positive | 310.8 → 240.8 | 25 | 310.8 → 274.8 | 5 | 70 |
| 326 | Terbufos | GC | 8.15 | positive | 231.0 → 97.0 | 20 | 231.0 → 129.0 | 15 | 70 |
| 327 | Terbuthylazine | GC | 8.12 | positive | 214.0 → 104.0 | 20 | 214.0 → 132.0 | 10 | 70 |
| 328 | Tetrachlorvinphos | LC | 8.72 | positive | 367.0 → 127.0 | 16 | 365.0 → 127.0 | 16 | 110 |
| 329 | Tetraconazole | GC | 10.04 | positive | 336.0 → 204.0 | 35 | 336.0 → 218.0 | 20 | 70 |
| 330 | Tetradifon | GC | 14.36 | positive | 158.9 → 111.0 | 20 | 354.0 → 159.0 | 10 | 70 |
| 331 | Tetramethrin | GC | 13.87 | positive | 164.0 → 77.0 | 30 | 164.0 → 107.0 | 15 | 70 |
| 332 | Thiabendazole | GC | 5.94 | positive | 201.0 → 174.0 | 15 | 201.0 → 130.0 | 30 | 70 |
| 333 | Thiacloprid | LC | 4.80 | positive | 253.0 → 126.0 | 16 | 253.0 → 90.0 | 40 | 140 |
| 334 | Thiamethoxam | LC | 3.59 | positive | 292.0 → 211.1 | 8 | 292.0 → 132.0 | 22 | 80 |
| 335 | Thiophanate methyl | LC | 5.87 | positive | 343.0 → 151.0 | 20 | 343.0 → 93.0 | 46 | 90 |
| 336 | Tolclofos methyl | GC | 9.21 | positive | 265.0 → 93.0 | 30 | 265.0 → 220.0 | 25 | 70 |
| 337 | Tolfenamic acid | LC | 9.80 | negative | 260.0 → 216.1 | 8 | 260.0 → 35.1 | 20 | 108 |
| 338 | Triadimefon | LC | 8.03 | positive | 294.1 → 69.3 | 20 | 294.1 → 197.2 | 15 | 100 |
| 339 | Triadimenol | LC | 8.22 | positive | 296.1 → 70.0 | 10 | 298.1 → 70.0 | 10 | 80 |
| 340 | Triazophos (hostathion) | LC | 8.18 | positive | 314.1 → 162.0 | 19 | 314.1 → 118.9 | 35 | 100 |
| 341 | Trifloxystrobin | LC | 9.50 | positive | 409.1 → 186.0 | 12 | 409.1 → 145.0 | 52 | 110 |
| 342 | Triflumizole | LC | 9.53 | positive | 346.1 → 278.0 | 4 | 345.9 → 73.0 | 15 | 80 |
| 343 | Triflumuron | LC | 9.19 | positive | 359.0 → 156.0 | 8 | 359.0 → 139.0 | 32 | 120 |
| 344 | Trifluralin | GC | 7.27 | positive | 264.0 → 160.0 | 15 | 306.0 → 264.0 | 5 | 70 |
| 345 | Trimethoprim | LC | 3.45 | positive | 291.2 → 123.0 | 24 | 291.2 → 230.1 | 20 | 162 |
| 346 | Triticonazole | LC | 8.38 | positive | 318.1 → 70.1 | 33 | 320.1 → 70.1 | 16 | 110 |
| 347 | Tylmicosin | LC | 5.52 | positive | 869.6 → 174.1 | 48 | 869.6 → 696.4 | 44 | 294 |
| 348 | Tylosin | LC | 6.76 | positive | 916.5 → 174.1 | 40 | 916.5 → 772.4 | 28 | 210 |
| 349 | Vinclozolin | GC | 9.10 | positive | 212.0 → 145.0 | 25 | 212.0 → 109.0 | 50 | 70 |
| 350 | Warfarin | LC | 7.86 | negative | 307.1 → 161.1 | 20 | 307.1 → 250.1 | 20 | 140 |
| 351 | Zoxamide | LC | 9.03 | positive | 336.0 → 187.1 | 25 | 187.1 → 88.9 | 40 | 200 |
Appendix B
| 0.4 ng/mL | 1 ng/mL | 4 ng/mL | 20 ng/mL | 40 ng/mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precision (RSD. %) | Precision (RSD. %) | Precision (RSD. %) | Precision (RSD. %) | Precision (RSD. %) | |||||||||||||
| No. | Compound | LOQ (ng/mL) | Rec (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday |
| 1 | 2-Phenylphenol | 2 | 101.43 | 19.69 | 18.85 | 107.17 | 20.66 | 19.28 | 106.47 | 18.24 | 17.13 | 96.41 | 13.81 | 14.32 | |||
| 2 | 4,4’-Dichlorobenzophenone (metabolite of dicofol) | 2 | 91.36 | 13.23 | 14.48 | 86.99 | 13.79 | 15.85 | 116.77 | 11.76 | 10.07 | 98.37 | 15.59 | 15.85 | |||
| 3 | Abamectine | 4 | 106.43 | 14.53 | 13.65 | 93.89 | 5.21 | 5.55 | 99.43 | 14.50 | 14.58 | ||||||
| 4 | Acenaphthene | 1.2 | 110.88 | 19.39 | 19.29 | 94.91 | 6.78 | 7.14 | 96.45 | 12.08 | 12.52 | 95.23 | 4.52 | 4.75 | |||
| 5 | Acenaphtylene | 2 | 107.06 | 18.90 | 21.39 | 95.74 | 12.53 | 13.09 | 100.50 | 12.26 | 12.20 | 98.40 | 2.93 | 2.98 | |||
| 6 | Acephate | 8 | 90.75 | 20.15 | 22.20 | 93.24 | 14.96 | 16.04 | |||||||||
| 7 | Acetamiprid | 2 | 122.13 | 8.03 | 6.57 | 101.26 | 14.21 | 14.03 | 104.42 | 4.70 | 4.50 | 104.71 | 4.76 | 4.55 | |||
| 8 | Acrinathrin | 4 | 116.08 | 16.73 | 14.41 | 103.11 | 22.19 | 21.52 | 104.55 | 5.45 | 5.21 | ||||||
| 9 | Albendazole | 0.4 | 121.03 | 11.39 | 9.41 | 92.56 | 6.35 | 6.86 | 86.92 | 12.48 | 14.36 | 99.53 | 3.66 | 3.68 | 105.10 | 4.30 | 4.09 |
| 10 | Aldicarb | 0.8 | 119.20 | 13.25 | 10.26 | 97.22 | 7.43 | 7.64 | 89.92 | 14.70 | 16.35 | 100.15 | 4.24 | 4.23 | 107.89 | 5.24 | 4.86 |
| 11 | Aldicarb-sulfone | 2 | 121.84 | 18.51 | 15.19 | 94.84 | 16.90 | 19.91 | 85.77 | 12.91 | 15.05 | 97.26 | 10.13 | 10.42 | |||
| 12 | Aldicarb-sulfoxide | 4 | 104.82 | 18.42 | 17.11 | 81.91 | 5.97 | 7.29 | 105.26 | 12.19 | 11.58 | ||||||
| 13 | Aldrin | 2 | 123.66 | 19.11 | 15.45 | 91.59 | 15.25 | 16.65 | 106.54 | 5.58 | 5.24 | 97.33 | 12.12 | 12.45 | |||
| 14 | Anthracene | 1.6 | 99.67 | 15.35 | 15.40 | 91.47 | 15.47 | 16.91 | 105.34 | 7.32 | 6.95 | 94.68 | 6.50 | 6.87 | |||
| 15 | Atrazine | 0.8 | 124.53 | 4.21 | 3.38 | 93.04 | 7.01 | 7.53 | 87.60 | 13.23 | 15.10 | 102.49 | 5.46 | 5.33 | 110.16 | 6.62 | 6.01 |
| 16 | Azinphos-methyl | 2 | 126.85 | 18.74 | 14.77 | 103.98 | 13.75 | 13.22 | 105.19 | 3.50 | 3.33 | 102.89 | 5.44 | 5.29 | |||
| 17 | Azoxystrobin | 0.4 | 116.28 | 7.94 | 6.83 | 95.41 | 3.96 | 4.15 | 92.31 | 6.03 | 6.53 | 102.95 | 4.52 | 4.39 | 101.82 | 2.11 | 2.07 |
| 18 | BDE-28 | 1.2 | 100.53 | 14.19 | 14.12 | 81.65 | 9.78 | 11.98 | 104.45 | 6.20 | 5.94 | 96.62 | 11.51 | 11.91 | |||
| 19 | BDE-47 | 0.8 | 98.71 | 20.31 | 20.58 | 109.45 | 21.87 | 19.98 | 89.88 | 8.16 | 9.08 | 110.24 | 8.74 | 7.93 | 95.96 | 8.89 | 9.26 |
| 20 | BDE-85 | 0.8 | 100.77 | 15.79 | 15.59 | 103.10 | 16.65 | 16.15 | 85.61 | 12.99 | 15.17 | 108.23 | 16.09 | 14.87 | 98.51 | 17.91 | 13.96 |
| 21 | BDE-99 | 0.4 | 107.54 | 18.55 | 19.69 | 103.74 | 15.02 | 14.48 | 89.65 | 12.66 | 14.12 | 114.05 | 18.00 | 15.78 | 90.97 | 11.97 | 13.16 |
| 22 | BDE-100 | 0.8 | 98.15 | 21.94 | 22.35 | 106.72 | 17.54 | 21.81 | 93.71 | 10.60 | 11.31 | 109.98 | 12.80 | 11.64 | 94.05 | 19.80 | 21.05 |
| 23 | BDE-153 | 0.8 | 93.58 | 16.95 | 19.48 | 108.66 | 17.04 | 19.88 | 93.65 | 15.26 | 16.29 | 111.40 | 15.44 | 13.86 | 96.11 | 17.16 | 17.85 |
| 24 | BDE-154 | 0.4 | 96.19 | 15.79 | 16.42 | 113.57 | 19.84 | 17.47 | 92.43 | 10.71 | 11.59 | 118.15 | 16.34 | 13.83 | 93.42 | 13.93 | 15.62 |
| 25 | BDE-183 | 4 | 78.46 | 5.92 | 7.55 | 104.68 | 12.90 | 12.32 | 95.99 | 8.73 | 9.09 | ||||||
| 26 | Benalaxyl | 0.4 | 116.67 | 15.06 | 12.91 | 94.56 | 3.53 | 3.73 | 97.64 | 6.31 | 6.46 | 101.10 | 3.37 | 3.33 | 101.39 | 3.01 | 2.97 |
| 27 | Bendiocarb | 0.8 | 123.80 | 9.46 | 7.29 | 90.11 | 14.65 | 16.26 | 93.23 | 14.26 | 15.30 | 101.72 | 3.10 | 3.05 | 110.76 | 8.93 | 8.06 |
| 28 | Bendiocarb metabolite (2,2-dimethylbenzo-1. 3-dioxol-4-ol) | 4 | 86.86 | 16.14 | 21.61 | 102.82 | 16.72 | 16.26 | 95.65 | 3.05 | 3.19 | ||||||
| 29 | Benfuracarb | 0.8 | 121.43 | 6.42 | 5.24 | 88.09 | 12.05 | 13.68 | 85.50 | 9.48 | 11.09 | 99.93 | 4.45 | 4.45 | 102.76 | 5.96 | 5.80 |
| 30 | Benzo[a]anthracene | 0.8 | 98.90 | 19.38 | 19.71 | 97.89 | 8.00 | 8.17 | 85.04 | 4.75 | 5.59 | 109.73 | 14.44 | 13.16 | 101.08 | 11.10 | 10.98 |
| 31 | Benzo[a]pyrene | 0.8 | 118.01 | 19.78 | 20.66 | 94.10 | 10.74 | 11.41 | 90.35 | 9.53 | 10.55 | 106.01 | 11.75 | 11.08 | 98.16 | 14.17 | 14.44 |
| 32 | Benzo[b]fluoranthene | 1.2 | 101.28 | 11.89 | 11.61 | 91.62 | 10.11 | 11.03 | 105.11 | 8.02 | 7.63 | 93.83 | 8.80 | 9.38 | |||
| 33 | Benzo[ghi]perylene | 0.8 | 80.16 | 19.32 | 20.41 | 100.35 | 15.43 | 15.38 | 93.47 | 9.37 | 10.02 | 114.39 | 6.35 | 5.55 | 95.50 | 10.80 | 11.31 |
| 34 | Benzo[k]fluoranthene | 1.2 | 102.66 | 13.24 | 12.90 | 88.84 | 14.63 | 16.47 | 101.90 | 7.87 | 7.72 | 99.44 | 17.32 | 17.42 | |||
| 35 | Bifenthrin | 2 | 118.63 | 18.93 | 19.10 | 86.17 | 14.08 | 16.34 | 113.53 | 16.83 | 14.82 | 98.99 | 10.04 | 10.14 | |||
| 36 | Bitertanol | 0.4 | 121.41 | 23.34 | 18.61 | 84.35 | 14.31 | 16.97 | 80.69 | 10.74 | 13.31 | 99.71 | 4.76 | 4.77 | 105.11 | 2.05 | 1.95 |
| 37 | Boscalid (formerly nicobifen) | 0.8 | 74.96 | 22.43 | 22.81 | 85.76 | 5.16 | 6.02 | 85.67 | 9.78 | 11.42 | 112.15 | 6.66 | 5.94 | 89.33 | 8.33 | 9.32 |
| 38 | Brodifacoum | 0.4 | 102.44 | 23.57 | 23.01 | 88.99 | 13.67 | 15.36 | 85.84 | 12.99 | 15.13 | 90.41 | 5.61 | 6.21 | 107.51 | 6.87 | 6.39 |
| 39 | Bromadiolone | 0.4 | 118.13 | 11.13 | 19.82 | 97.11 | 20.55 | 19.19 | 89.46 | 13.13 | 14.68 | 97.66 | 8.85 | 9.06 | 101.30 | 8.87 | 8.76 |
| 40 | Bromopropylate | 0.4 | 110.83 | 21.15 | 22.86 | 96.37 | 19.85 | 20.60 | 93.29 | 8.21 | 8.80 | 107.25 | 10.02 | 9.34 | 96.53 | 10.67 | 11.05 |
| 41 | Bromuconazole (two isomers) | 1.6 | 106.70 | 16.63 | 14.96 | 89.48 | 17.19 | 19.21 | 112.75 | 13.08 | 11.60 | 94.54 | 8.05 | 8.51 | |||
| 42 | Bupirimate | 1.2 | 86.77 | 10.74 | 12.38 | 93.94 | 5.74 | 6.11 | 98.53 | 6.03 | 6.12 | 102.43 | 4.99 | 4.87 | |||
| 43 | Buprofezin | 0.4 | 115.89 | 11.18 | 9.65 | 93.22 | 1.92 | 2.06 | 88.57 | 10.64 | 12.01 | 101.63 | 4.50 | 4.43 | 102.68 | 3.54 | 3.45 |
| 44 | Cadusafos (ebufos) | 0.4 | 126.81 | 3.48 | 2.74 | 94.26 | 4.04 | 4.29 | 96.81 | 5.74 | 5.93 | 101.63 | 5.00 | 4.92 | 100.58 | 3.04 | 3.02 |
| 45 | Carbaryl | 0.8 | 118.84 | 13.56 | 10.52 | 96.23 | 6.27 | 6.52 | 87.81 | 11.26 | 12.82 | 100.43 | 4.37 | 4.35 | 108.80 | 4.34 | 3.99 |
| 46 | Carbendazim (azole) | 2 | 120.17 | 8.97 | 6.40 | 98.20 | 9.83 | 10.01 | 100.39 | 2.72 | 2.71 | 103.56 | 3.81 | 3.68 | |||
| 47 | Carbofuran | 0.4 | 123.88 | 10.74 | 8.67 | 94.30 | 6.42 | 6.81 | 88.14 | 13.54 | 15.36 | 104.41 | 6.50 | 6.23 | 105.57 | 3.33 | 3.15 |
| 48 | Carbofuran-3-hydroxy | 0.8 | 120.98 | 10.76 | 8.89 | 89.04 | 8.10 | 9.10 | 94.46 | 15.41 | 16.31 | 100.86 | 3.61 | 3.58 | 107.55 | 4.88 | 4.54 |
| 49 | Carbosulfan | 1.2 | 109.16 | 14.87 | 19.19 | 89.29 | 21.22 | 20.62 | 92.19 | 22.12 | 23.99 | 96.57 | 21.02 | 21.77 | |||
| 50 | Cefuroxima axetil (two isomers) | 4 | 132.33 | 13.51 | 10.21 | 119.80 | 8.73 | 7.29 | 127.08 | 13.98 | 11.00 | ||||||
| 51 | Chloramphenicol | 16 | 98.91 | 7.74 | 7.83 | ||||||||||||
| 52 | Chlorantraniliprole | 16 | 106.00 | 2.93 | 2.76 | ||||||||||||
| 53 | Chlorfenvinphos | 0.8 | 114.28 | 16.10 | 14.09 | 97.14 | 14.61 | 15.04 | 91.56 | 15.96 | 17.43 | 104.40 | 2.64 | 2.53 | 106.32 | 3.78 | 3.56 |
| 54 | Chlorobenzilate | 1.6 | 110.47 | 17.93 | 16.23 | 89.89 | 4.22 | 4.69 | 114.60 | 12.66 | 11.05 | 90.71 | 2.94 | 3.24 | |||
| 55 | Chlorophacinone | 8 | 98.40 | 21.84 | 22.36 | 99.28 | 8.31 | 8.37 | |||||||||
| 56 | Chlorpropham | 2 | 119.39 | 17.61 | 13.13 | 93.11 | 14.39 | 15.45 | 112.93 | 6.10 | 5.40 | 100.11 | 21.56 | 21.54 | |||
| 57 | Chlorpyrifos | 1.6 | 74.81 | 17.17 | 19.32 | 92.21 | 1.74 | 1.89 | 119.54 | 9.95 | 8.32 | 90.50 | 6.87 | 7.59 | |||
| 58 | Chlorpyrifos methyl | 2 | 115.24 | 17.63 | 14.98 | 88.08 | 15.56 | 19.02 | 90.85 | 9.97 | 10.97 | 106.30 | 6.98 | 6.57 | |||
| 59 | Chlorthal dimethyl | 0.8 | 104.38 | 17.25 | 19.43 | 96.72 | 14.55 | 15.04 | 91.37 | 6.53 | 7.15 | 113.90 | 9.82 | 8.62 | 90.34 | 3.66 | 4.05 |
| 60 | Chrysene | 1.6 | 109.91 | 12.42 | 11.30 | 91.83 | 7.03 | 7.66 | 106.10 | 17.06 | 16.08 | 100.25 | 11.34 | 11.31 | |||
| 61 | Clindamycin | 4 | 129.25 | 10.53 | 8.15 | 109.20 | 7.44 | 6.81 | 107.36 | 6.37 | 5.93 | ||||||
| 62 | Clofentezine | 0.8 | 116.80 | 20.59 | 17.63 | 94.99 | 7.99 | 8.41 | 83.66 | 11.18 | 13.36 | 96.74 | 7.45 | 7.70 | 105.68 | 4.56 | 4.31 |
| 63 | Clothianidin | 12 | 105.52 | 8.63 | 8.18 | 97.88 | 11.44 | 11.69 | |||||||||
| 64 | Cloxacillin | 8 | 99.33 | 18.27 | 18.39 | 107.79 | 10.04 | 9.31 | |||||||||
| 65 | Coumachlor | 0.8 | 120.85 | 16.85 | 20.84 | 99.90 | 12.14 | 12.15 | 85.48 | 16.06 | 18.79 | 101.57 | 4.20 | 4.14 | 102.89 | 2.20 | 2.14 |
| 66 | Coumaphos | 0.8 | 112.59 | 14.03 | 12.46 | 86.24 | 15.34 | 17.79 | 86.49 | 10.71 | 12.38 | 105.36 | 4.20 | 3.99 | 103.38 | 4.36 | 4.22 |
| 67 | Coumatetralyl | 1.6 | 116.12 | 22.62 | 19.48 | 97.65 | 9.98 | 10.22 | 96.87 | 4.29 | 4.43 | 102.71 | 4.39 | 4.27 | |||
| 68 | Cyazofamid | 2 | 112.26 | 13.66 | 12.17 | 100.29 | 12.06 | 12.03 | 103.98 | 7.08 | 6.81 | 97.87 | 5.04 | 5.15 | |||
| 69 | Cyflufenamid | 1.6 | 116.75 | 17.97 | 23.96 | 96.02 | 19.44 | 20.25 | 98.16 | 6.89 | 7.02 | 105.14 | 7.86 | 7.48 | |||
| 70 | Cyfluthrin (sum of four isomers) | 8 | 128.33 | 19.82 | 15.44 | 87.94 | 19.51 | 21.60 | |||||||||
| 71 | Cyhalothrin (lambda isomer) | 4 | 118.45 | 17.58 | 14.84 | 110.36 | 18.54 | 16.80 | 102.15 | 14.52 | 14.21 | ||||||
| 72 | Cymoxanil | 2 | 127.98 | 23.20 | 18.13 | 112.78 | 13.14 | 11.65 | 111.18 | 3.17 | 2.85 | 107.24 | 7.19 | 6.70 | |||
| 73 | Cypermethrin (sum of four isomers) | 20 | 125.42 | 15.64 | 12.47 | 74.31 | 10.18 | 13.70 | |||||||||
| 74 | Cyproconazole (two isomers) | 4 | 120.66 | 19.73 | 20.01 | 89.98 | 15.51 | 17.24 | 110.68 | 5.93 | 5.36 | 93.41 | 7.33 | 7.85 | |||
| 75 | Cyprodinil | 1.2 | 110.91 | 13.69 | 12.34 | 89.72 | 15.25 | 17.00 | 97.14 | 8.22 | 8.46 | 104.22 | 7.41 | 7.11 | |||
| 76 | Cyromazine | 8 | 94.58 | 11.29 | 11.94 | 96.74 | 11.32 | 11.70 | |||||||||
| 77 | Danofloxacin | 8 | 113.55 | 18.30 | 16.12 | 89.76 | 10.39 | 11.58 | |||||||||
| 78 | Dazomet | 4 | 71.53 | 17.55 | 21.50 | 83.03 | 13.84 | 16.67 | 90.82 | 16.95 | 18.66 | ||||||
| 79 | Deltamethrin | 4 | 95.89 | 17.74 | 19.36 | 107.28 | 18.61 | 17.24 | 100.61 | 12.28 | 12.21 | ||||||
| 80 | Demeton-S-methyl | 0.8 | 123.35 | 9.93 | 7.74 | 95.67 | 3.32 | 3.47 | 87.38 | 12.64 | 14.47 | 101.00 | 5.18 | 5.13 | 108.47 | 6.18 | 5.70 |
| 81 | Demeton-S-methyl-sulfone (Dioxydemeton) | 12 | 80.24 | 4.83 | 6.02 | 96.53 | 6.34 | 6.57 | |||||||||
| 82 | Dexamethasone | 2 | 123.79 | 17.67 | 12.29 | 104.61 | 13.93 | 13.32 | 106.53 | 14.48 | 13.59 | 102.96 | 4.32 | 4.20 | |||
| 83 | Diazinon | 1.2 | 101.78 | 19.05 | 18.68 | 97.76 | 8.04 | 8.22 | 110.63 | 15.03 | 13.59 | 92.16 | 7.39 | 8.02 | |||
| 84 | Dibenzo[a.h]anthracene | 0.8 | 70.39 | 18.68 | 16.54 | 112.50 | 16.62 | 14.77 | 93.06 | 11.73 | 12.60 | 111.29 | 4.83 | 4.34 | 84.41 | 6.54 | 7.75 |
| 85 | Dichlorodiphenyldichloroethane (p,p′ DDD) | 0.8 | 88.27 | 19.42 | 17.67 | 112.25 | 6.36 | 5.67 | 92.57 | 11.03 | 11.92 | 101.42 | 6.07 | 5.99 | 93.46 | 4.77 | 5.10 |
| 86 | Dichlorodiphenyldichloroethylene (p,p′ DDE) | 0.8 | 99.71 | 18.39 | 18.44 | 110.56 | 17.85 | 18.67 | 97.62 | 7.46 | 7.64 | 104.29 | 0.72 | 0.69 | 98.40 | 8.58 | 8.72 |
| 87 | Dichlorodiphenyltrichloroethane (p,p′ DDT) | 2 | 101.87 | 15.98 | 14.95 | 104.65 | 20.63 | 19.71 | 110.43 | 6.32 | 5.72 | 92.27 | 6.86 | 7.43 | |||
| 88 | Diclofenac | 4 | 111.25 | 21.71 | 19.51 | 91.03 | 12.48 | 13.71 | 94.44 | 12.42 | 13.15 | ||||||
| 89 | Dicloran | 4 | 95.68 | 14.38 | 15.03 | 113.17 | 15.89 | 14.04 | 92.28 | 5.09 | 5.52 | ||||||
| 90 | Diclorvos | 8 | 113.48 | 10.38 | 9.15 | 98.10 | 2.98 | 3.04 | |||||||||
| 91 | Dicloxacillin | 12 | 77.54 | 12.35 | 15.93 | 103.35 | 10.63 | 10.29 | |||||||||
| 92 | Dieldrin | 8 | 80.70 | 18.40 | 18.43 | 95.08 | 5.85 | 6.15 | |||||||||
| 93 | Diethathyl ethyl | 0.4 | 113.26 | 12.83 | 20.16 | 96.15 | 13.17 | 13.70 | 89.84 | 15.24 | 16.96 | 97.95 | 6.16 | 6.29 | 99.30 | 7.45 | 7.50 |
| 94 | Diethofencarb | 0.4 | 113.98 | 6.38 | 5.60 | 90.65 | 3.94 | 4.35 | 90.80 | 10.90 | 12.00 | 101.24 | 1.48 | 1.46 | 104.26 | 1.97 | 1.89 |
| 95 | Difenacoum | 0.8 | 117.66 | 13.84 | 21.51 | 88.27 | 10.36 | 11.74 | 85.40 | 7.72 | 9.04 | 92.19 | 6.14 | 6.66 | 104.37 | 3.77 | 3.61 |
| 96 | Difenoconazole | 0.8 | 119.78 | 17.98 | 13.85 | 94.45 | 8.83 | 9.35 | 84.08 | 7.72 | 9.18 | 97.08 | 6.45 | 6.64 | 106.47 | 4.12 | 3.87 |
| 97 | Difethialone | 1.6 | 112.44 | 10.79 | 9.60 | 88.10 | 18.22 | 20.68 | 95.86 | 6.45 | 6.73 | 103.22 | 8.66 | 8.39 | |||
| 98 | Difloxacin | 4 | 123.36 | 19.34 | 15.68 | 102.87 | 8.83 | 8.58 | 99.17 | 8.54 | 8.61 | ||||||
| 99 | Diflubenzuron | 1.6 | 121.03 | 16.61 | 18.98 | 99.73 | 16.54 | 16.58 | 102.86 | 17.01 | 16.54 | 105.63 | 4.13 | 3.91 | |||
| 100 | Diflufenican | 0.4 | 114.39 | 18.24 | 19.49 | 101.56 | 9.38 | 9.24 | 95.89 | 9.59 | 10.00 | 115.49 | 8.35 | 7.23 | 95.62 | 9.05 | 9.46 |
| 101 | Dimethenamid-P (and its R-isomer) | 0.4 | 112.73 | 15.63 | 12.74 | 87.82 | 4.74 | 5.40 | 90.21 | 8.85 | 9.81 | 103.64 | 3.95 | 3.81 | 102.49 | 5.02 | 4.90 |
| 102 | Dimethoate | 0.8 | 121.83 | 20.22 | 15.57 | 93.11 | 5.47 | 5.87 | 87.05 | 11.89 | 13.66 | 100.07 | 10.40 | 10.39 | 108.51 | 7.39 | 6.81 |
| 103 | Dimethomorph (two isomers) | 0.4 | 123.37 | 18.68 | 15.14 | 87.72 | 6.32 | 7.20 | 87.24 | 11.29 | 12.94 | 104.93 | 4.63 | 4.41 | 105.80 | 5.87 | 5.55 |
| 104 | Dimethylphenylsulfamide (DMSA, metabolite of dichlofluanid) | 4 | 114.05 | 11.55 | 10.13 | 109.55 | 9.65 | 8.81 | 102.37 | 4.33 | 4.23 | ||||||
| 105 | Diniconazole-M | 1.2 | 97.80 | 16.52 | 16.89 | 88.78 | 6.85 | 7.72 | 102.75 | 14.54 | 14.15 | 100.66 | 5.86 | 5.82 | |||
| 106 | Dinocap | 4 | 115.19 | 16.40 | 14.24 | 105.54 | 2.90 | 2.75 | 105.88 | 5.85 | 5.53 | ||||||
| 107 | Diphacinone | 8 | 96.74 | 18.11 | 18.72 | 103.66 | 19.59 | 18.90 | |||||||||
| 108 | Diphenylamine | 1.6 | 92.81 | 20.64 | 13.01 | 96.18 | 5.32 | 5.53 | 106.11 | 10.45 | 9.85 | 93.91 | 2.44 | 2.60 | |||
| 109 | Dodine | 0.8 | 107.19 | 12.63 | 11.78 | 97.64 | 8.18 | 8.38 | 87.26 | 10.47 | 12.00 | 102.27 | 5.32 | 5.20 | 102.46 | 4.26 | 4.16 |
| 110 | Doramectina | 8 | 101.04 | 19.63 | 19.43 | 99.13 | 9.03 | 9.11 | |||||||||
| 111 | Endosulfan alfa | 2 | 112.45 | 11.73 | 10.43 | 84.22 | 19.47 | 23.12 | 122.57 | 20.34 | 20.75 | 96.35 | 10.24 | 10.63 | |||
| 112 | Endosulfan beta | 4 | 85.36 | 23.81 | 19.45 | 115.99 | 3.80 | 3.28 | 88.35 | 9.64 | 10.91 | ||||||
| 113 | Endosulfan sulfate | 4 | 70.48 | 20.30 | 18.80 | 116.21 | 19.54 | 20.32 | 89.14 | 17.93 | 20.11 | ||||||
| 114 | Endrin | 4 | 88.96 | 17.68 | 22.54 | 107.57 | 12.66 | 11.77 | 92.69 | 12.73 | 13.73 | ||||||
| 115 | Enrofloxacin | 4 | 118.69 | 21.02 | 19.54 | 98.46 | 7.06 | 7.17 | 104.38 | 11.64 | 11.15 | ||||||
| 116 | EPN | 2 | 81.34 | 17.89 | 19.10 | 87.12 | 22.90 | 19.18 | 112.30 | 12.97 | 11.55 | 95.56 | 5.50 | 5.76 | |||
| 117 | Epoxiconazole | 0.8 | 122.98 | 16.59 | 13.49 | 89.44 | 11.43 | 13.96 | 97.21 | 19.34 | 17.51 | 106.20 | 12.49 | 11.76 | 106.31 | 5.68 | 5.34 |
| 118 | Eprinomectin | 1.2 | 100.03 | 21.36 | 21.35 | 89.96 | 19.07 | 21.20 | 95.57 | 10.18 | 10.65 | 102.43 | 8.65 | 8.44 | |||
| 119 | Eritromicin | 0.8 | 122.85 | 10.10 | 7.96 | 101.21 | 7.59 | 7.50 | 87.18 | 8.00 | 9.18 | 93.37 | 6.33 | 6.78 | 104.42 | 3.74 | 3.58 |
| 120 | Esfenvalerate | 2 | 127.54 | 21.58 | 16.92 | 105.91 | 19.49 | 17.83 | 101.51 | 4.20 | 4.14 | 90.24 | 7.45 | 8.26 | |||
| 121 | Ethion (diethion) | 0.8 | 109.21 | 7.32 | 6.70 | 92.19 | 7.99 | 8.67 | 89.52 | 8.27 | 9.24 | 98.50 | 2.63 | 2.67 | 100.66 | 3.50 | 3.48 |
| 122 | Ethirimol | 1.2 | 103.80 | 13.91 | 13.40 | 84.85 | 8.49 | 10.01 | 98.86 | 6.54 | 6.62 | 109.68 | 5.38 | 4.91 | |||
| 123 | Ethofumesate | 2 | 126.97 | 15.41 | 16.48 | 90.72 | 15.48 | 21.11 | 103.99 | 13.91 | 13.38 | 96.26 | 8.98 | 9.33 | |||
| 124 | Ethoprophos | 0.8 | 121.58 | 17.90 | 22.95 | 85.95 | 23.63 | 17.49 | 96.79 | 13.42 | 13.87 | 103.33 | 4.56 | 4.41 | 108.44 | 8.96 | 8.26 |
| 125 | Etofenprox | 1.2 | 103.05 | 20.35 | 19.75 | 88.00 | 12.88 | 14.64 | 87.54 | 4.70 | 5.37 | 107.43 | 8.42 | 7.84 | |||
| 126 | Etoxazole | 0.4 | 116.44 | 7.26 | 6.23 | 95.39 | 5.73 | 6.01 | 88.69 | 5.00 | 5.64 | 98.79 | 1.96 | 1.98 | 99.61 | 4.04 | 4.06 |
| 127 | Famoxadone | 1.2 | 105.50 | 21.91 | 19.80 | 84.93 | 8.09 | 9.53 | 100.06 | 8.50 | 8.49 | 105.60 | 8.62 | 8.16 | |||
| 128 | Fenamidone | 0.4 | 120.97 | 11.07 | 9.15 | 96.95 | 7.86 | 8.11 | 90.00 | 12.52 | 13.91 | 102.36 | 3.51 | 3.43 | 105.44 | 5.42 | 5.14 |
| 129 | Fenamiphos | 0.4 | 108.30 | 19.35 | 26.33 | 96.23 | 10.37 | 10.78 | 91.06 | 5.88 | 6.46 | 102.96 | 6.58 | 6.39 | 104.25 | 4.70 | 4.51 |
| 130 | Fenamiphos sulfone | 1.2 | 94.25 | 18.82 | 19.97 | 93.80 | 9.16 | 9.77 | 102.18 | 10.58 | 10.35 | 112.34 | 3.88 | 3.45 | |||
| 131 | Fenamiphos sulfoxide | 1.2 | 89.34 | 10.67 | 11.94 | 98.26 | 10.61 | 10.80 | 99.74 | 5.76 | 5.78 | 112.66 | 2.19 | 1.94 | |||
| 132 | Fenarimol | 1.6 | 100.34 | 12.81 | 12.77 | 99.76 | 7.71 | 7.73 | 109.44 | 6.21 | 5.67 | 94.11 | 1.40 | 1.49 | |||
| 133 | Fenazaquin | 0.4 | 120.20 | 17.57 | 14.62 | 91.49 | 3.55 | 3.88 | 82.11 | 9.35 | 11.39 | 100.13 | 2.89 | 2.89 | 101.08 | 3.40 | 3.36 |
| 134 | Fenbendazole | 0.4 | 120.77 | 22.32 | 17.07 | 98.07 | 9.36 | 9.54 | 84.88 | 12.32 | 14.51 | 99.37 | 8.88 | 8.94 | 105.25 | 5.15 | 4.89 |
| 135 | Fenbuconazole | 0.8 | 117.33 | 16.47 | 17.97 | 80.70 | 15.65 | 19.39 | 92.40 | 23.56 | 15.50 | 101.06 | 9.40 | 9.30 | 104.91 | 5.73 | 5.46 |
| 136 | Fenhexamid | 4 | 113.78 | 16.34 | 17.33 | 102.52 | 7.16 | 6.98 | 96.72 | 9.77 | 10.10 | ||||||
| 137 | Fenitrothion | 4 | 92.58 | 17.86 | 21.77 | 91.31 | 11.66 | 12.77 | 97.21 | 6.00 | 6.17 | ||||||
| 138 | Fenoxycarb | 0.4 | 105.61 | 17.40 | 16.48 | 94.00 | 12.18 | 12.96 | 87.03 | 6.88 | 7.91 | 101.05 | 3.54 | 3.50 | 99.09 | 6.54 | 6.60 |
| 139 | Fenpropathrin | 0.8 | 121.06 | 18.95 | 23.91 | 81.90 | 11.15 | 13.61 | 85.72 | 10.16 | 11.85 | 96.22 | 3.83 | 3.98 | 103.49 | 5.20 | 5.02 |
| 140 | Fenpropidin | 0.4 | 118.57 | 11.79 | 9.94 | 88.81 | 8.30 | 9.35 | 90.98 | 3.24 | 3.56 | 100.27 | 6.86 | 6.84 | 102.94 | 2.05 | 1.99 |
| 141 | Fenpropimorph | 0.8 | 118.79 | 13.41 | 11.29 | 85.42 | 10.61 | 12.42 | 89.45 | 8.55 | 9.56 | 101.39 | 8.15 | 8.04 | 103.06 | 4.18 | 4.06 |
| 142 | Fenpyroximate | 0.4 | 112.81 | 6.87 | 6.09 | 94.83 | 3.34 | 3.52 | 91.94 | 4.15 | 4.51 | 97.90 | 1.86 | 1.90 | 102.42 | 5.37 | 5.24 |
| 143 | Fenthion | 0.8 | 97.17 | 14.25 | 26.12 | 109.28 | 12.00 | 10.98 | 90.09 | 5.65 | 6.27 | 114.72 | 15.88 | 13.84 | 87.33 | 4.28 | 4.90 |
| 144 | Fenthion oxon | 0.4 | 112.27 | 10.39 | 9.25 | 93.67 | 4.79 | 5.11 | 87.28 | 8.70 | 9.97 | 102.19 | 5.74 | 5.62 | 105.28 | 3.24 | 3.08 |
| 145 | Fenthion oxon sulfone | 8 | 119.94 | 1.82 | 1.52 | 105.66 | 6.01 | 5.69 | |||||||||
| 146 | Fenthion oxon sulfoxide | 1.2 | 104.05 | 10.41 | 10.00 | 88.97 | 11.85 | 13.32 | 101.42 | 5.20 | 5.13 | 107.05 | 4.90 | 4.58 | |||
| 147 | Fenthion sulfone | 1.6 | 111.17 | 8.93 | 8.03 | 95.30 | 19.79 | 20.77 | 105.00 | 10.11 | 9.63 | 105.57 | 6.32 | 5.99 | |||
| 148 | Fenthion sulfoxide | 1.6 | 115.59 | 18.20 | 15.75 | 93.36 | 12.69 | 13.59 | 104.11 | 6.72 | 6.45 | 105.76 | 5.54 | 5.24 | |||
| 149 | Fenvalerate | 2 | 107.17 | 18.70 | 19.85 | 88.71 | 2.41 | 2.72 | 133.74 | 8.81 | 6.59 | 89.16 | 9.12 | 10.23 | |||
| 150 | Fipronil | 0.8 | 102.09 | 11.72 | 11.48 | 94.23 | 19.32 | 19.56 | 93.24 | 8.74 | 9.37 | 97.93 | 6.41 | 6.55 | 101.30 | 2.10 | 2.07 |
| 151 | Fipronil sulfide | 8 | 99.25 | 16.95 | 17.08 | 101.31 | 10.48 | 10.34 | |||||||||
| 152 | Flocoumafen | 0.4 | 106.82 | 17.50 | 16.38 | 94.93 | 7.63 | 8.04 | 84.00 | 8.55 | 10.18 | 101.02 | 3.52 | 3.48 | 102.48 | 3.21 | 3.13 |
| 153 | Fluazinam | 2 | 80.64 | 18.31 | 22.36 | 131.43 | 19.43 | 14.55 | 125.26 | 5.60 | 14.71 | 84.12 | 14.12 | 16.79 | |||
| 154 | Flubendiamide | 1.6 | 114.35 | 15.21 | 13.30 | 90.41 | 15.13 | 16.73 | 100.94 | 10.26 | 10.16 | 97.91 | 8.97 | 9.16 | |||
| 155 | Flucythrinate (two isomers) | 2 | 94.89 | 14.70 | 15.49 | 103.04 | 12.90 | 19.51 | 126.99 | 10.32 | 8.13 | 89.10 | 5.70 | 6.40 | |||
| 156 | Fludioxonil | 2 | 108.94 | 22.52 | 19.85 | 88.57 | 9.19 | 10.38 | 112.87 | 6.35 | 5.63 | 98.26 | 7.70 | 7.84 | |||
| 157 | Flufenoxuron | 0.8 | 118.74 | 20.77 | 17.49 | 90.49 | 6.24 | 6.90 | 83.45 | 5.81 | 6.96 | 100.22 | 5.17 | 5.16 | 102.52 | 5.23 | 5.10 |
| 158 | Flumequine | 0.8 | 127.04 | 7.72 | 5.63 | 91.69 | 7.33 | 7.99 | 90.22 | 9.12 | 10.11 | 95.00 | 6.52 | 6.86 | 104.99 | 4.54 | 4.32 |
| 159 | Flunixin | 0.8 | 123.19 | 8.48 | 6.88 | 99.80 | 18.40 | 18.44 | 86.25 | 12.61 | 14.62 | 93.81 | 5.62 | 5.99 | 103.21 | 8.75 | 8.48 |
| 160 | Fluopyram | 0.8 | 123.13 | 22.83 | 18.54 | 93.37 | 13.69 | 15.37 | 83.31 | 12.59 | 15.11 | 101.20 | 4.18 | 4.13 | 105.16 | 5.27 | 5.01 |
| 161 | Fluoranthene | 2 | 119.58 | 15.24 | 17.08 | 98.38 | 12.55 | 12.76 | 101.89 | 4.52 | 4.44 | 104.26 | 16.84 | 16.15 | |||
| 162 | Fluorene | 1.2 | 118.54 | 18.98 | 21.07 | 88.32 | 4.32 | 4.89 | 98.39 | 4.29 | 4.36 | 96.27 | 2.06 | 2.14 | |||
| 163 | Fluquinconazole | 1.2 | 110.55 | 16.66 | 18.74 | 86.38 | 6.85 | 7.93 | 100.01 | 12.08 | 12.08 | 81.40 | 7.90 | 9.71 | |||
| 164 | Flusilazole | 0.8 | 108.78 | 10.98 | 10.09 | 99.53 | 10.49 | 10.54 | 88.08 | 8.54 | 9.70 | 104.22 | 12.29 | 11.79 | 102.06 | 4.15 | 4.07 |
| 165 | Flutolanil | 0.8 | 117.13 | 16.40 | 14.00 | 99.03 | 10.07 | 10.17 | 90.65 | 12.51 | 13.80 | 100.14 | 3.12 | 3.12 | 102.55 | 7.51 | 7.32 |
| 166 | Flutriafol | 1.2 | 102.18 | 9.65 | 9.44 | 91.70 | 13.15 | 14.34 | 103.28 | 8.34 | 8.08 | 106.17 | 4.42 | 4.16 | |||
| 167 | Fluvalinate tau | 4 | 78.58 | 18.54 | 23.59 | 99.65 | 18.40 | 18.46 | 105.68 | 17.54 | 16.60 | ||||||
| 168 | Fonofos | 1.6 | 96.73 | 20.46 | 22.45 | 82.54 | 10.97 | 13.29 | 115.98 | 10.45 | 9.01 | 90.58 | 4.37 | 4.82 | |||
| 169 | Formetanate | 1.2 | 105.80 | 11.33 | 10.71 | 91.02 | 5.72 | 6.28 | 90.92 | 7.25 | 7.97 | 102.25 | 6.83 | 6.68 | |||
| 170 | Fosthiazate | 0.4 | 117.53 | 12.76 | 10.86 | 91.81 | 5.80 | 6.32 | 89.96 | 11.00 | 12.23 | 101.32 | 5.03 | 4.96 | 103.76 | 2.06 | 1.99 |
| 171 | Heptachlor | 1.2 | 105.63 | 13.94 | 13.20 | 95.68 | 11.66 | 12.19 | 109.43 | 7.97 | 7.28 | 91.44 | 3.65 | 3.99 | |||
| 172 | Hexachlorobencene | 0.8 | 98.45 | 17.43 | 18.64 | 107.10 | 20.87 | 20.35 | 87.60 | 4.82 | 5.50 | 101.47 | 9.94 | 9.80 | 90.60 | 2.38 | 2.63 |
| 173 | Hexachlorocyclohexane (alpha) | 2 | 117.54 | 12.12 | 8.21 | 88.98 | 5.41 | 6.08 | 103.02 | 9.89 | 9.60 | 92.16 | 8.25 | 8.95 | |||
| 174 | Hexachlorocyclohexane (beta) | 2 | 126.04 | 17.47 | 13.86 | 97.43 | 3.53 | 3.62 | 108.18 | 8.42 | 7.78 | 93.18 | 8.51 | 9.13 | |||
| 175 | Hexachlorocyclohexane (delta) | 4 | 90.15 | 19.06 | 20.45 | 104.74 | 16.22 | 15.49 | 95.22 | 11.94 | 12.54 | ||||||
| 176 | Hexaclorocyclohexane (gamma, lindane) | 4 | 93.75 | 14.15 | 23.24 | 107.48 | 6.80 | 6.33 | 95.82 | 12.95 | 13.51 | ||||||
| 177 | Hexaconazole (two isomers) | 1.6 | 95.19 | 15.08 | 15.84 | 91.80 | 7.62 | 8.30 | 102.65 | 5.70 | 5.55 | 103.19 | 2.94 | 2.85 | |||
| 178 | Hexaflumuron | 1.2 | 99.41 | 17.91 | 18.02 | 80.35 | 5.75 | 7.16 | 98.14 | 4.07 | 4.15 | 98.75 | 11.10 | 11.24 | |||
| 179 | Hexythiazox | 0.4 | 118.55 | 17.88 | 18.46 | 82.22 | 8.53 | 10.37 | 87.62 | 9.39 | 10.72 | 98.62 | 2.88 | 2.92 | 104.62 | 8.24 | 7.88 |
| 180 | Imazalil (enilconazole) | 0.8 | 130.92 | 15.49 | 17.18 | 95.75 | 7.74 | 8.08 | 89.57 | 9.74 | 10.87 | 100.82 | 4.01 | 3.98 | 104.51 | 5.19 | 4.97 |
| 181 | Imidacloprid | 4 | 117.55 | 5.49 | 4.67 | 107.18 | 8.37 | 7.81 | 102.44 | 8.24 | 8.04 | ||||||
| 182 | Indeno [1,2,3-cd] pyrene | 1.6 | 117.89 | 15.48 | 13.13 | 116.35 | 14.87 | 12.78 | 116.45 | 17.25 | 14.81 | 100.18 | 19.54 | 19.50 | |||
| 183 | Indoxacarb | 0.8 | 124.61 | 14.87 | 18.70 | 99.01 | 21.65 | 21.87 | 88.98 | 17.78 | 19.98 | 93.09 | 6.87 | 7.38 | 109.78 | 5.44 | 4.96 |
| 184 | Iprovalicarb | 0.8 | 115.20 | 10.97 | 9.52 | 93.14 | 7.55 | 8.11 | 89.11 | 6.56 | 7.36 | 103.80 | 5.49 | 5.29 | 102.29 | 3.92 | 3.83 |
| 185 | Isofenphos methyl | 2 | 126.11 | 13.75 | 10.90 | 96.55 | 9.27 | 9.60 | 112.12 | 5.55 | 4.95 | 96.12 | 9.68 | 10.07 | |||
| 186 | Isoprothiolane | 0.4 | 106.89 | 3.33 | 3.12 | 96.32 | 18.05 | 18.74 | 92.86 | 12.46 | 13.42 | 101.23 | 2.17 | 2.14 | 102.11 | 4.86 | 4.76 |
| 187 | Ivermectin B1a | 1.6 | 113.41 | 16.89 | 14.89 | 98.11 | 8.63 | 8.80 | 91.74 | 11.91 | 12.98 | 101.63 | 10.59 | 10.42 | |||
| 188 | Josamycin | 1.6 | 127.21 | 18.85 | 14.82 | 103.07 | 5.89 | 5.71 | 94.94 | 7.07 | 7.45 | 108.51 | 2.46 | 2.27 | |||
| 189 | Ketoprofen | 1.6 | 93.43 | 17.84 | 19.09 | 106.39 | 15.59 | 14.05 | 95.64 | 10.20 | 10.66 | 102.80 | 3.50 | 3.40 | |||
| 190 | Kresoxim methyl | 2 | 119.34 | 19.83 | 18.72 | 98.02 | 18.09 | 18.66 | 110.88 | 14.03 | 12.65 | 96.62 | 12.85 | 13.30 | |||
| 191 | Levamisole | 1.6 | 114.26 | 22.07 | 19.32 | 92.62 | 8.40 | 9.07 | 86.67 | 7.08 | 8.17 | 101.93 | 6.16 | 6.04 | |||
| 192 | Lincomycin | 4 | 120.69 | 19.64 | 16.27 | 112.23 | 7.04 | 6.27 | 96.55 | 6.39 | 6.62 | ||||||
| 193 | Linuron | 1.6 | 125.64 | 15.59 | 18.33 | 89.87 | 13.77 | 15.32 | 98.09 | 5.13 | 5.23 | 107.80 | 5.05 | 4.68 | |||
| 194 | Lufenuron | 0.8 | 112.88 | 16.42 | 14.55 | 97.32 | 13.82 | 14.48 | 78.06 | 11.50 | 14.73 | 102.81 | 10.91 | 10.61 | 102.87 | 4.15 | 4.03 |
| 195 | Mandipropamid | 0.4 | 110.19 | 14.68 | 13.32 | 91.62 | 7.61 | 8.31 | 91.88 | 8.21 | 8.94 | 103.64 | 4.69 | 4.53 | 104.43 | 3.29 | 3.15 |
| 196 | Mebendazole | 0.4 | 128.41 | 10.28 | 8.01 | 92.65 | 6.20 | 6.69 | 86.90 | 10.23 | 11.77 | 95.39 | 7.83 | 8.21 | 104.16 | 2.75 | 2.64 |
| 197 | Mefenamic acid | 1.6 | 123.97 | 17.95 | 14.48 | 102.51 | 18.77 | 18.31 | 92.91 | 13.78 | 14.83 | 102.92 | 3.83 | 3.72 | |||
| 198 | Mefenoxam (metalaxyl-M) | 0.4 | 119.22 | 10.17 | 8.53 | 93.63 | 6.26 | 6.69 | 91.63 | 10.08 | 11.00 | 101.70 | 6.18 | 6.08 | 103.38 | 2.08 | 2.01 |
| 199 | Meloxicam | 1.2 | 94.27 | 17.55 | 18.66 | 89.51 | 17.89 | 16.54 | 90.45 | 7.81 | 8.63 | 106.55 | 10.65 | 10.00 | |||
| 200 | Mepanipyrim | 2 | 114.71 | 20.02 | 17.45 | 92.36 | 6.47 | 7.01 | 105.72 | 8.41 | 7.95 | 102.76 | 2.90 | 2.82 | |||
| 201 | Mepiquat | 0.4 | 88.75 | 15.72 | 18.98 | 96.69 | 19.96 | 20.64 | 84.95 | 17.75 | 20.89 | 92.24 | 7.48 | 8.11 | 101.87 | 2.68 | 2.63 |
| 202 | Metaflumizone | 0.4 | 120.69 | 6.98 | 5.78 | 90.72 | 10.11 | 11.14 | 87.97 | 8.18 | 9.30 | 100.90 | 6.18 | 6.12 | 101.69 | 3.74 | 3.68 |
| 203 | Metalaxyl | 1.6 | 102.24 | 8.98 | 8.78 | 87.83 | 6.49 | 7.39 | 114.56 | 16.92 | 14.77 | 88.61 | 5.54 | 6.25 | |||
| 204 | Metaldehyde | 4 | 117.18 | 16.45 | 16.52 | 82.85 | 10.48 | 12.65 | 100.36 | 8.69 | 8.66 | ||||||
| 205 | Metconazole | 0.8 | 126.92 | 8.05 | 6.34 | 91.62 | 13.26 | 14.47 | 85.87 | 13.93 | 16.22 | 98.86 | 2.29 | 2.32 | 104.83 | 4.73 | 4.51 |
| 206 | Methamidophos (two isomers) | 8 | 90.08 | 12.33 | 13.69 | 95.62 | 7.09 | 7.41 | |||||||||
| 207 | Methidathion | 0.4 | 121.63 | 11.90 | 9.78 | 94.81 | 6.86 | 7.24 | 92.10 | 11.60 | 12.60 | 101.70 | 2.15 | 2.11 | 105.46 | 7.13 | 6.76 |
| 208 | Methiocarb | 0.4 | 126.57 | 12.25 | 9.68 | 82.56 | 7.76 | 9.40 | 96.74 | 10.42 | 10.77 | 106.92 | 5.78 | 5.41 | 109.28 | 7.12 | 6.52 |
| 209 | Methiocarb-sufone | 2 | 124.44 | 19.05 | 20.07 | 110.41 | 9.03 | 8.18 | 106.55 | 7.79 | 7.31 | 105.17 | 9.43 | 8.97 | |||
| 210 | Methiocarb-sulfoxide | 1.2 | 97.90 | 11.90 | 12.16 | 97.45 | 19.13 | 19.63 | 102.57 | 5.80 | 5.65 | 107.53 | 5.47 | 5.09 | |||
| 211 | Methomyl | 1.2 | 105.76 | 12.28 | 11.61 | 116.29 | 18.70 | 16.08 | 114.17 | 3.66 | 3.21 | 106.03 | 7.48 | 7.05 | |||
| 212 | Methoxyfenozide | 0.4 | 86.63 | 21.88 | 23.34 | 102.29 | 15.53 | 15.18 | 97.40 | 9.54 | 9.79 | ||||||
| 213 | Metoxychlor | 4 | 116.96 | 8.59 | 7.34 | 88.75 | 7.33 | 8.26 | 90.85 | 3.70 | 4.07 | 102.21 | 3.91 | 3.83 | 102.30 | 3.36 | 3.28 |
| 214 | Metrafenone | 0.4 | 126.22 | 20.21 | 16.01 | 98.22 | 13.26 | 13.50 | 85.59 | 7.19 | 8.40 | 95.31 | 6.55 | 6.87 | 107.70 | 5.47 | 5.08 |
| 215 | Metronidazole | 12 | 77.85 | 19.32 | 22.63 | 94.46 | 8.54 | 9.04 | |||||||||
| 216 | Mevinphos (phosdrin) | 1.2 | 107.14 | 19.43 | 18.14 | 87.21 | 9.70 | 11.12 | 99.51 | 6.93 | 6.96 | 112.13 | 4.49 | 4.00 | |||
| 217 | Mirex | 4 | 87.30 | 15.87 | 16.63 | 99.75 | 22.33 | 22.39 | 104.43 | 12.55 | 12.02 | ||||||
| 218 | Monocrotophos | 4 | 113.28 | 8.74 | 7.72 | 99.08 | 3.05 | 3.08 | 103.67 | 4.46 | 4.30 | ||||||
| 219 | Moxidectin | 4 | 97.05 | 17.46 | 19.81 | 97.15 | 19.05 | 19.61 | 101.68 | 7.42 | 7.30 | ||||||
| 220 | Myclobutanil | 0.8 | 104.83 | 19.53 | 19.31 | 99.69 | 13.23 | 13.27 | 87.82 | 10.56 | 12.02 | 102.16 | 9.43 | 9.23 | 104.53 | 3.52 | 3.37 |
| 221 | N-(2.4-dimethylphenyl)-N′-methylformamidine (DMPF, metabolite of amitraz) | 4 | 112.03 | 15.22 | 13.59 | 98.34 | 7.94 | 8.07 | 102.65 | 5.61 | 5.47 | ||||||
| 222 | N.N-Dimethyl-N’-p-tolylsulphamide (DMST, metabolite of tolyfluanid) | 4 | 115.30 | 9.04 | 7.84 | 106.19 | 4.77 | 4.49 | 106.16 | 3.58 | 3.37 | ||||||
| 223 | Nafcillin | 4 | 106.61 | 19.05 | 17.87 | 95.48 | 11.52 | 12.07 | 98.70 | 4.02 | 4.07 | ||||||
| 224 | Naphtalene | 1.6 | 82.87 | 15.67 | 17.47 | 114.46 | 17.98 | 14.45 | 106.51 | 18.61 | 17.47 | 96.49 | 6.52 | 6.76 | |||
| 225 | Naproxen | 2 | 128.28 | 18.41 | 16.21 | 112.22 | 2.15 | 18.76 | 113.75 | 19.60 | 17.23 | 102.62 | 6.86 | 6.68 | |||
| 226 | Nitenpyram | 8 | 109.62 | 19.55 | 17.83 | 100.70 | 5.77 | 5.73 | |||||||||
| 227 | Novobiocin | 1.2 | 96.87 | 20.40 | 19.19 | 85.98 | 18.66 | 21.70 | 89.43 | 13.67 | 15.29 | 95.39 | 7.93 | 8.31 | |||
| 228 | Nuarimol | 1.2 | 106.98 | 17.30 | 15.52 | 92.46 | 7.78 | 8.41 | 122.94 | 10.78 | 8.77 | 89.25 | 5.47 | 6.13 | |||
| 229 | Ofurace | 0.8 | 118.87 | 20.45 | 6.62 | 93.50 | 18.17 | 19.43 | 88.50 | 9.60 | 10.85 | 94.99 | 4.06 | 4.27 | 110.23 | 4.44 | 4.03 |
| 230 | Omethoate | 2 | 106.78 | 14.85 | 13.91 | 97.86 | 17.25 | 17.63 | 87.65 | 6.69 | 7.63 | 105.07 | 13.17 | 12.53 | |||
| 231 | Oxadixyl | 0.8 | 123.61 | 18.91 | 15.30 | 97.19 | 4.29 | 4.41 | 85.77 | 7.12 | 8.30 | 98.57 | 5.69 | 5.77 | 106.26 | 2.50 | 2.35 |
| 232 | Oxamyl | 8 | 107.50 | 18.14 | 16.87 | 110.16 | 21.87 | 19.85 | |||||||||
| 233 | Oxamyl-oxime | 8 | 99.19 | 10.92 | 11.01 | 80.81 | 9.57 | 11.84 | 93.86 | 10.65 | 11.35 | ||||||
| 234 | Oxfendazole | 0.8 | 132.57 | 17.25 | 13.01 | 100.86 | 3.40 | 3.37 | 90.01 | 13.08 | 14.53 | 95.72 | 9.30 | 9.72 | 105.18 | 2.02 | 1.92 |
| 235 | Oxolinic acid | 0.8 | 110.62 | 10.56 | 9.55 | 93.32 | 6.53 | 7.00 | 101.82 | 5.02 | 4.93 | ||||||
| 236 | Oxydemeton methyl | 4 | 125.26 | 11.57 | 8.95 | 103.93 | 6.92 | 6.66 | 90.21 | 15.13 | 16.77 | 94.20 | 6.15 | 6.53 | 103.58 | 4.77 | 4.61 |
| 237 | Oxyfluorfen | 4 | 87.69 | 18.03 | 21.96 | 104.71 | 11.43 | 10.92 | 97.01 | 14.18 | 14.62 | ||||||
| 238 | Paclobutrazol | 1.6 | 115.25 | 5.87 | 5.09 | 97.98 | 10.85 | 11.07 | 103.31 | 5.22 | 5.05 | 103.50 | 2.43 | 2.35 | |||
| 239 | Parathion methyl | 8 | 109.49 | 18.40 | 16.81 | 87.75 | 3.95 | 4.50 | |||||||||
| 240 | PCB 28 | 0.4 | 109.04 | 17.35 | 21.35 | 109.79 | 10.36 | 9.44 | 89.67 | 5.77 | 6.43 | 103.24 | 6.02 | 5.83 | 94.17 | 7.16 | 7.60 |
| 241 | PCB 52 | 0.8 | 109.29 | 10.86 | 9.94 | 108.98 | 14.57 | 22.55 | 83.40 | 13.14 | 15.76 | 104.75 | 10.45 | 9.98 | 100.99 | 8.14 | 8.06 |
| 242 | PCB 77 | 0.8 | 93.46 | 20.09 | 15.79 | 99.72 | 20.85 | 20.91 | 99.96 | 10.57 | 10.57 | 103.80 | 7.39 | 7.12 | 96.95 | 10.24 | 10.56 |
| 243 | PCB 81 | 0.8 | 89.48 | 15.17 | 16.95 | 106.42 | 16.55 | 16.19 | 87.13 | 11.29 | 12.96 | 104.54 | 2.16 | 2.07 | 99.16 | 8.64 | 8.71 |
| 244 | PCB 101 | 0.4 | 111.65 | 12.54 | 19.14 | 117.34 | 14.18 | 12.08 | 95.12 | 2.94 | 3.09 | 101.23 | 5.71 | 5.64 | 101.01 | 10.12 | 10.02 |
| 245 | PCB 105 | 0.4 | 102.23 | 14.66 | 13.90 | 126.39 | 14.98 | 19.76 | 88.39 | 7.67 | 8.68 | 105.83 | 8.19 | 7.74 | 93.21 | 5.05 | 5.42 |
| 246 | PCB 114 | 0.8 | 96.49 | 20.69 | 19.42 | 103.46 | 13.87 | 13.41 | 94.88 | 11.04 | 11.64 | 104.47 | 5.35 | 5.12 | 98.79 | 7.12 | 7.21 |
| 247 | PCB 118 | 1.2 | 102.19 | 11.53 | 11.28 | 94.03 | 8.10 | 8.61 | 98.74 | 4.78 | 4.84 | 98.20 | 8.76 | 8.92 | |||
| 248 | PCB 123 | 1.2 | 113.43 | 22.05 | 19.44 | 90.08 | 7.66 | 8.50 | 102.24 | 8.39 | 8.21 | 96.45 | 5.29 | 5.48 | |||
| 249 | PCB 126 | 0.8 | 87.42 | 18.29 | 19.43 | 111.04 | 14.57 | 13.12 | 91.66 | 7.70 | 8.40 | 107.90 | 9.85 | 9.13 | 96.39 | 7.94 | 8.24 |
| 250 | PCB 138 | 0.4 | 118.82 | 20.45 | 18.79 | 105.03 | 11.58 | 11.03 | 102.64 | 10.09 | 9.83 | 105.83 | 6.52 | 6.16 | 96.84 | 6.40 | 6.61 |
| 251 | PCB 153 | 0.8 | 105.67 | 17.51 | 20.11 | 110.87 | 8.22 | 7.41 | 96.92 | 7.41 | 7.65 | 101.59 | 4.87 | 4.79 | 97.13 | 3.45 | 3.55 |
| 252 | PCB 156 | 0.8 | 89.94 | 17.49 | 19.45 | 115.25 | 23.33 | 20.24 | 90.96 | 7.99 | 8.78 | 106.25 | 7.73 | 7.28 | 98.31 | 9.18 | 9.34 |
| 253 | PCB 157 | 0.8 | 82.22 | 19.43 | 17.63 | 116.24 | 20.04 | 17.24 | 88.00 | 7.66 | 8.70 | 107.18 | 9.20 | 8.58 | 101.20 | 10.33 | 10.21 |
| 254 | PCB 167 | 0.8 | 87.27 | 18.74 | 21.47 | 111.11 | 16.53 | 23.88 | 95.41 | 9.50 | 9.96 | 105.12 | 7.64 | 7.27 | 97.88 | 10.59 | 10.82 |
| 255 | PCB 169 | 1.2 | 99.05 | 18.89 | 17.17 | 98.13 | 9.84 | 10.03 | 103.47 | 10.12 | 9.78 | 96.44 | 8.72 | 9.04 | |||
| 256 | PCB 180 | 0.4 | 106.04 | 19.00 | 20.25 | 108.14 | 15.01 | 12.37 | 94.39 | 9.82 | 10.40 | 109.04 | 9.09 | 8.34 | 97.26 | 7.54 | 7.75 |
| 257 | PCB 189 | 0.8 | 79.22 | 18.60 | 10.45 | 97.14 | 19.02 | 19.58 | 86.39 | 10.81 | 12.51 | 108.00 | 7.22 | 6.69 | 92.10 | 4.18 | 4.54 |
| 258 | Penconazole | 0.8 | 119.47 | 10.66 | 18.97 | 96.80 | 18.50 | 19.11 | 81.78 | 13.65 | 16.69 | 94.22 | 7.10 | 7.54 | 103.41 | 8.49 | 8.21 |
| 259 | Pencycuron | 0.4 | 110.82 | 13.26 | 11.97 | 90.36 | 11.63 | 12.87 | 86.17 | 10.68 | 12.39 | 98.98 | 3.84 | 3.88 | 101.16 | 6.82 | 6.74 |
| 260 | Pendimethalin | 2 | 102.88 | 19.86 | 19.30 | 75.06 | 16.21 | 21.60 | 104.56 | 5.38 | 5.15 | 94.40 | 11.99 | 12.70 | |||
| 261 | Penicillin V | 8 | 105.22 | 18.10 | 17.20 | 108.53 | 6.81 | 6.27 | |||||||||
| 262 | Permethrin | 4 | 113.34 | 17.58 | 15.51 | 115.67 | 16.46 | 14.23 | 98.54 | 12.87 | 13.06 | ||||||
| 263 | Phenanthrene | 1.6 | 133.74 | 10.56 | 5.48 | 94.00 | 13.65 | 14.52 | 99.67 | 16.68 | 16.74 | 93.74 | 4.35 | 4.64 | |||
| 264 | Phenylbutazone | 16 | 110.32 | 17.68 | 21.84 | ||||||||||||
| 265 | Phosalone | 0.4 | 110.50 | 17.38 | 15.73 | 98.28 | 8.36 | 8.51 | 89.91 | 5.27 | 5.86 | 101.98 | 2.80 | 2.75 | 102.70 | 2.35 | 2.29 |
| 266 | Phosmet | 1.2 | 100.60 | 6.02 | 5.98 | 92.49 | 12.42 | 13.43 | 101.79 | 5.92 | 5.82 | 104.63 | 5.42 | 5.18 | |||
| 267 | Pthalamide (Folpet deg) | 8 | 103.79 | 11.71 | 11.28 | 100.45 | 6.35 | 6.32 | |||||||||
| 268 | Pirimicarb | 0.4 | 120.22 | 8.00 | 6.65 | 95.32 | 4.74 | 4.97 | 93.09 | 11.13 | 11.96 | 102.45 | 4.63 | 4.52 | 103.60 | 2.50 | 2.41 |
| 269 | Pirimicarb-desmethyl | 2 | 110.38 | 7.61 | 6.89 | 127.79 | 12.92 | 10.11 | 114.99 | 8.23 | 7.16 | 123.05 | 4.57 | 3.71 | |||
| 270 | Pirimiphos ethyl | 0.8 | 82.75 | 17.50 | 21.15 | 92.12 | 9.50 | 10.31 | 98.11 | 12.10 | 12.33 | 120.39 | 11.92 | 9.90 | 101.28 | 6.04 | 7.51 |
| 271 | Pirimiphos methyl | 0.4 | 111.79 | 15.98 | 14.29 | 95.45 | 16.02 | 16.78 | 88.67 | 10.14 | 11.44 | 102.62 | 4.30 | 4.19 | 101.90 | 6.74 | 6.61 |
| 272 | Prochloraz | 0.4 | 104.39 | 17.43 | 16.70 | 91.03 | 5.00 | 5.49 | 89.27 | 11.46 | 12.84 | 100.96 | 5.85 | 5.79 | 103.41 | 5.28 | 5.11 |
| 273 | Procymidone | 8 | 92.02 | 15.09 | 16.40 | 106.35 | 16.63 | 15.04 | |||||||||
| 274 | Profenofos | 0.8 | 115.10 | 18.63 | 17.91 | 93.40 | 17.40 | 18.63 | 88.46 | 19.43 | 21.96 | 84.42 | 5.94 | 7.04 | 108.50 | 7.24 | 6.67 |
| 275 | Propamocarb | 2 | 114.94 | 19.15 | 16.66 | 93.17 | 2.45 | 2.63 | 99.37 | 9.65 | 9.71 | 103.21 | 7.96 | 7.71 | |||
| 276 | Propargite | 0.4 | 113.51 | 7.83 | 6.90 | 97.28 | 4.47 | 4.59 | 90.04 | 6.01 | 6.67 | 99.27 | 1.99 | 2.00 | 100.59 | 5.36 | 5.33 |
| 277 | Propiconazole | 2 | 103.21 | 16.19 | 15.38 | 98.49 | 10.53 | 10.69 | 103.63 | 3.30 | 3.18 | ||||||
| 278 | Propoxur | 0.8 | 124.75 | 20.23 | 15.08 | 92.34 | 8.00 | 8.66 | 93.26 | 18.90 | 20.27 | 101.62 | 8.07 | 7.94 | 111.03 | 3.68 | 3.31 |
| 279 | Propyzamide (pronamide) | 0.8 | 104.03 | 19.42 | 19.74 | 89.58 | 16.14 | 19.18 | 93.02 | 13.39 | 14.39 | 97.32 | 5.91 | 6.07 | 102.92 | 7.64 | 7.42 |
| 280 | Proquinazid | 1.6 | 102.99 | 15.84 | 15.38 | 92.93 | 3.72 | 4.00 | 116.31 | 13.68 | 11.76 | 95.04 | 8.78 | 9.24 | |||
| 281 | Prothioconazol | 1.6 | 118.94 | 17.99 | 15.13 | 86.94 | 10.77 | 12.39 | 101.86 | 7.03 | 6.90 | 103.49 | 5.02 | 4.85 | |||
| 282 | Prothiophos | 2 | 127.35 | 17.52 | 15.03 | 91.22 | 11.43 | 12.53 | 110.73 | 16.36 | 14.77 | 90.19 | 7.42 | 8.23 | |||
| 283 | Pymetrozine | 8 | 95.53 | 3.63 | 3.80 | 99.15 | 5.84 | 5.89 | |||||||||
| 284 | Pyraclostrobin | 0.4 | 118.10 | 14.33 | 12.13 | 88.90 | 5.97 | 6.72 | 90.62 | 2.37 | 2.62 | 97.96 | 3.11 | 3.17 | 101.93 | 4.86 | 4.77 |
| 285 | Pyrazophos | 0.4 | 116.34 | 17.02 | 14.63 | 87.65 | 5.37 | 6.13 | 95.15 | 10.11 | 10.63 | 103.82 | 7.88 | 7.59 | 104.45 | 3.19 | 3.05 |
| 286 | Pyrene | 2 | 122.26 | 19.33 | 15.12 | 95.97 | 15.49 | 16.14 | 99.99 | 7.05 | 7.05 | 106.97 | 13.20 | 12.34 | |||
| 287 | Pyridaben | 0.4 | 111.80 | 8.50 | 7.60 | 96.96 | 11.34 | 11.70 | 89.29 | 7.37 | 8.25 | 97.16 | 3.54 | 3.64 | 101.94 | 5.96 | 5.85 |
| 288 | Pyridaphenthion | 0.4 | 125.33 | 10.87 | 8.67 | 88.86 | 12.18 | 13.71 | 90.69 | 9.17 | 10.11 | 103.36 | 5.75 | 5.56 | 104.97 | 2.29 | 2.18 |
| 289 | Pyrimethanil | 1.2 | 107.01 | 6.57 | 6.14 | 83.42 | 6.56 | 7.86 | 99.98 | 4.42 | 4.42 | 106.14 | 3.93 | 3.70 | |||
| 290 | Pyriproxifen | 0.4 | 108.80 | 6.84 | 6.29 | 93.68 | 5.81 | 6.20 | 84.68 | 4.33 | 5.11 | 99.26 | 4.88 | 4.92 | 97.21 | 10.36 | 10.66 |
| 291 | Quinalfos | 1.6 | 90.14 | 15.91 | 17.65 | 81.56 | 14.48 | 17.75 | 98.78 | 15.64 | 15.83 | 99.05 | 6.28 | 6.34 | |||
| 292 | Quinoxyfen | 0.8 | 106.79 | 20.65 | 20.64 | 102.29 | 14.68 | 14.27 | 92.06 | 12.98 | 14.10 | 113.55 | 3.75 | 3.30 | 88.93 | 8.36 | 9.40 |
| 293 | Rifampicin | 1.2 | 103.00 | 18.59 | 17.76 | 89.34 | 13.65 | 15.28 | 99.63 | 7.95 | 7.98 | 111.87 | 15.95 | 14.26 | |||
| 294 | Rotenone | 0.8 | 120.70 | 16.46 | 13.64 | 89.14 | 17.53 | 20.88 | 81.92 | 14.34 | 17.50 | 101.34 | 12.36 | 12.20 | 105.21 | 3.73 | 3.55 |
| 295 | Roxithromycin | 1.2 | 116.45 | 19.37 | 16.63 | 88.89 | 12.89 | 14.50 | 88.85 | 16.67 | 18.76 | 102.53 | 3.02 | 2.95 | |||
| 296 | Sarafloxacin | 20 | 102.28 | 16.90 | 16.52 | ||||||||||||
| 297 | Simazine | 0.8 | 127.26 | 22.73 | 17.86 | 89.60 | 13.25 | 14.79 | 90.53 | 13.38 | 14.78 | 101.60 | 7.91 | 7.79 | 108.74 | 8.42 | 7.74 |
| 298 | Spinosad (two isomers) | 1.6 | 111.72 | 19.81 | 17.73 | 90.14 | 14.70 | 16.31 | 89.32 | 9.26 | 10.37 | 99.29 | 5.46 | 5.50 | 100.87 | 7.97 | 7.90 |
| 299 | Spiramycin (two isomers) | 12 | 105.21 | 2.31 | 2.20 | 98.92 | 6.55 | 6.62 | |||||||||
| 300 | Spirodiclofen | 0.8 | 114.30 | 10.24 | 8.96 | 98.97 | 9.73 | 9.83 | 86.32 | 3.99 | 4.62 | 96.72 | 4.66 | 4.82 | 96.10 | 2.65 | 2.76 |
| 301 | Spiromesifen | 0.4 | 111.87 | 16.94 | 15.14 | 96.88 | 10.62 | 10.96 | 81.93 | 9.64 | 11.77 | 96.25 | 5.78 | 6.01 | 102.02 | 6.81 | 6.68 |
| 302 | Spirotetramat | 0.8 | 136.54 | 14.99 | 18.30 | 91.58 | 15.29 | 17.62 | 87.65 | 16.87 | 19.25 | 99.19 | 10.60 | 10.69 | 109.75 | 7.10 | 6.47 |
| 303 | Spiroxamine | 0.4 | 115.95 | 5.12 | 4.42 | 92.27 | 5.97 | 6.47 | 93.01 | 4.62 | 4.97 | 100.04 | 5.41 | 5.41 | 101.94 | 2.10 | 2.06 |
| 304 | Strychnine | 2 | 121.82 | 15.59 | 16.26 | 84.19 | 20.34 | 20.16 | 100.68 | 5.59 | 5.55 | 102.95 | 5.88 | 5.71 | |||
| 305 | Sulfacetamide | 16 | 94.80 | 9.96 | 10.51 | ||||||||||||
| 306 | Sulfachloropiridacine | 4 | 109.37 | 18.24 | 16.68 | 98.92 | 7.84 | 7.93 | 102.01 | 6.91 | 6.77 | ||||||
| 307 | Sulfadiacine | 8 | 91.59 | 4.52 | 4.94 | 87.73 | 9.54 | 10.87 | |||||||||
| 308 | Sulfadimetoxine | 2 | 126.54 | 15.11 | 10.31 | 109.89 | 11.08 | 10.08 | 103.69 | 8.01 | 7.72 | 103.97 | 3.74 | 3.60 | |||
| 309 | Sulfadoxine | 2 | 123.17 | 11.68 | 8.48 | 107.05 | 13.06 | 12.20 | 100.75 | 7.53 | 7.47 | 106.39 | 7.08 | 6.65 | |||
| 310 | Sulfameracine | 4 | 118.06 | 8.76 | 7.42 | 95.45 | 3.06 | 3.21 | 102.43 | 3.58 | 3.50 | ||||||
| 311 | Sulfametacine | 2 | 118.26 | 12.28 | 8.28 | 105.33 | 12.84 | 12.19 | 102.14 | 7.00 | 6.85 | 104.35 | 4.92 | 4.71 | |||
| 312 | Sulfametizole | 4 | 108.41 | 7.69 | 7.09 | 97.15 | 4.81 | 4.95 | 101.75 | 12.24 | 12.03 | ||||||
| 313 | Sulfametoxazole | 2 | 117.53 | 19.33 | 21.76 | 99.36 | 6.59 | 6.63 | 100.10 | 5.77 | 5.76 | 104.08 | 10.50 | 10.09 | |||
| 314 | Sulfametoxipiridacine | 1.6 | 122.56 | 10.02 | 7.23 | 93.76 | 15.04 | 16.04 | 95.82 | 4.12 | 4.30 | 106.04 | 6.02 | 5.68 | |||
| 315 | Sulfamonomethoxine | 4 | 115.82 | 19.10 | 16.49 | 109.03 | 9.00 | 8.25 | 102.71 | 5.89 | 5.73 | ||||||
| 316 | Sulfapyridine | 4 | 123.12 | 5.81 | 4.72 | 97.40 | 5.43 | 5.57 | 97.95 | 5.03 | 5.14 | ||||||
| 317 | Sulfaquinoxaline | 2 | 137.13 | 15.86 | 11.57 | 107.09 | 16.46 | 15.37 | 103.03 | 8.49 | 8.24 | 105.72 | 5.77 | 5.46 | |||
| 318 | Sulfatiazole | 4 | 114.09 | 12.17 | 10.67 | 89.17 | 3.00 | 3.36 | 96.75 | 9.66 | 9.98 | ||||||
| 319 | Sulfisoxazole | 4 | 123.17 | 15.55 | 12.62 | 109.15 | 6.85 | 6.28 | 102.56 | 9.83 | 9.58 | ||||||
| 320 | Tebuconazole | 2 | 124.33 | 10.86 | 8.73 | 102.25 | 9.82 | 9.60 | 103.18 | 10.27 | 9.95 | 100.50 | 6.55 | 6.52 | |||
| 321 | Tebufenocide | 0.8 | 113.40 | 19.30 | 17.02 | 94.01 | 6.42 | 6.83 | 88.38 | 6.07 | 6.87 | 99.16 | 2.48 | 2.50 | 99.92 | 5.26 | 5.26 |
| 322 | Tebufenpyrad | 0.8 | 125.31 | 18.01 | 14.37 | 106.17 | 5.54 | 5.22 | 88.47 | 11.68 | 13.20 | 96.73 | 4.95 | 5.12 | 106.06 | 2.21 | 2.08 |
| 323 | Teflubenzuron | 1.6 | 108.57 | 19.06 | 17.56 | 89.65 | 5.82 | 6.49 | 100.52 | 12.28 | 12.22 | 104.30 | 4.23 | 4.06 | |||
| 324 | Tefluthrin | 0.4 | 124.36 | 21.64 | 16.23 | 105.00 | 19.05 | 18.14 | 88.44 | 12.15 | 13.74 | 105.91 | 7.75 | 7.32 | 89.59 | 1.30 | 1.45 |
| 325 | Telodrin (isobenzan) | 2 | 121.92 | 12.85 | 21.31 | 88.03 | 14.49 | 17.82 | 108.27 | 5.89 | 5.44 | 99.09 | 17.36 | 17.52 | |||
| 326 | Terbufos | 0.8 | 104.09 | 14.21 | 13.26 | 88.68 | 15.21 | 16.32 | 81.58 | 8.12 | 9.95 | 110.15 | 9.52 | 8.64 | 95.99 | 5.50 | 5.73 |
| 327 | Terbuthylazine | 0.8 | 120.91 | 9.86 | 8.15 | 93.77 | 18.06 | 19.26 | 85.93 | 9.78 | 11.38 | 106.01 | 2.62 | 2.47 | 104.19 | 4.52 | 4.34 |
| 328 | Tetrachlorvinphos | 2 | 126.81 | 17.78 | 13.00 | 100.05 | 19.19 | 19.18 | 88.06 | 9.94 | 11.29 | 98.20 | 13.04 | 13.28 | |||
| 329 | Tetraconazole | 0.8 | 116.66 | 14.53 | 20.60 | 90.40 | 17.43 | 19.28 | 89.89 | 9.78 | 10.88 | 115.70 | 7.74 | 6.69 | 88.10 | 5.70 | 6.47 |
| 330 | Tetradifon | 1.6 | 106.92 | 12.99 | 19.50 | 91.97 | 11.87 | 12.91 | 112.56 | 5.80 | 5.15 | 94.92 | 2.68 | 2.82 | |||
| 331 | Tetramethrin | 2 | 120.30 | 14.89 | 12.38 | 113.61 | 8.45 | 7.44 | 99.35 | 15.78 | 15.88 | ||||||
| 332 | Thiabendazole | 1.2 | 96.72 | 17.21 | 16.26 | 86.00 | 6.75 | 7.85 | 109.00 | 4.57 | 4.19 | 95.59 | 7.21 | 7.54 | |||
| 333 | Thiacloprid | 0.8 | 121.34 | 7.44 | 5.66 | 90.02 | 6.38 | 7.09 | 88.41 | 14.87 | 16.82 | 108.68 | 3.58 | 3.29 | 110.31 | 5.93 | 5.38 |
| 334 | Thiamethoxam | 8 | 121.06 | 6.35 | 5.25 | 92.87 | 5.21 | 5.61 | |||||||||
| 335 | Thiophanate methyl | 2 | 124.15 | 8.09 | 6.03 | 101.95 | 12.23 | 12.00 | 106.08 | 6.23 | 5.87 | 103.96 | 7.36 | 7.08 | |||
| 336 | Tolclofos methyl | 1.6 | 123.59 | 15.07 | 12.19 | 84.62 | 5.68 | 6.71 | 109.41 | 7.30 | 6.67 | 96.18 | 5.78 | 6.01 | |||
| 337 | Tolfenamic acid | 1.6 | 105.31 | 15.54 | 20.30 | 104.76 | 16.01 | 15.28 | 96.52 | 3.58 | 3.71 | 97.33 | 5.08 | 5.22 | |||
| 338 | Triadimefon | 1.2 | 94.15 | 15.09 | 16.33 | 95.62 | 7.48 | 7.82 | 101.84 | 6.27 | 6.16 | 103.91 | 2.78 | 2.68 | |||
| 339 | Triadimenol | 0.8 | 114.21 | 11.55 | 9.26 | 106.40 | 13.80 | 14.65 | 89.34 | 12.93 | 14.47 | 103.34 | 6.45 | 6.24 | 106.44 | 5.74 | 5.39 |
| 340 | Triazophos (hostathion) | 0.4 | 112.50 | 15.63 | 12.76 | 91.77 | 6.14 | 6.69 | 91.68 | 11.49 | 12.53 | 104.86 | 3.62 | 3.45 | 102.76 | 4.43 | 4.31 |
| 341 | Trifloxystrobin | 0.4 | 112.87 | 13.12 | 11.62 | 92.56 | 5.89 | 6.36 | 93.35 | 4.78 | 5.12 | 100.81 | 2.86 | 2.84 | 102.51 | 4.76 | 4.64 |
| 342 | Triflumizole | 0.4 | 110.08 | 9.26 | 8.41 | 94.01 | 7.01 | 7.46 | 93.26 | 7.62 | 8.17 | 99.91 | 2.69 | 2.69 | 101.62 | 3.67 | 3.61 |
| 343 | Triflumuron | 1.2 | 118.66 | 19.24 | 16.21 | 86.93 | 7.78 | 8.95 | 96.65 | 3.90 | 4.04 | 106.18 | 6.37 | 6.00 | |||
| 344 | Trifluralin | 1.2 | 93.29 | 17.78 | 19.06 | 92.40 | 6.37 | 6.89 | 105.89 | 9.83 | 9.28 | 93.54 | 4.17 | 4.46 | |||
| 345 | Trimethoprim | 2 | 116.02 | 16.54 | 12.16 | 109.21 | 15.05 | 13.78 | 94.20 | 12.51 | 13.28 | 97.92 | 8.70 | 8.88 | |||
| 346 | Triticonazole | 1.2 | 99.19 | 8.60 | 8.67 | 89.71 | 12.71 | 14.17 | 100.84 | 8.67 | 8.60 | 104.04 | 4.36 | 4.19 | |||
| 347 | Tylmicosin | 4 | 107.14 | 16.70 | 15.59 | 93.58 | 6.97 | 7.45 | 101.18 | 11.19 | 11.06 | ||||||
| 348 | Tylosin | 8 | 106.35 | 4.46 | 4.19 | 104.60 | 2.79 | 2.67 | |||||||||
| 349 | Vinclozolin | 1.6 | 116.52 | 23.46 | 17.30 | 88.94 | 12.82 | 14.41 | 105.60 | 12.42 | 11.76 | 92.86 | 4.92 | 5.30 | |||
| 350 | Warfarin | 0.8 | 100.47 | 17.51 | 19.89 | 95.53 | 9.12 | 9.55 | 85.61 | 11.19 | 14.75 | 93.09 | 6.28 | 6.75 | 103.65 | 4.31 | 4.16 |
| 351 | Zoxamide | 0.8 | 121.96 | 19.52 | 18.47 | 88.50 | 11.13 | 13.88 | 90.57 | 19.33 | 20.34 | 106.93 | 5.18 | 4.84 | 105.40 | 6.44 | 6.11 |
Appendix C
| No. | Compound | Matriz effect | No. | Compound | Matriz effect | Nº | Compound | Matriz Effect |
|---|---|---|---|---|---|---|---|---|
| 1 | 2-Phenylphenol | −103.4 | 118 | Eprinomectin | −6.3 | 235 | Oxolinic acid | 0.5 |
| 2 | 4,4′-Dichlorobenzophenone (metabolite of dicofol) | 96.6 | 119 | Eritromicin | 45.4 | 236 | Oxydemeton methyl | 3.3 |
| 3 | Abamectine | −70.6 | 120 | Esfenvalerate | 23.4 | 237 | Oxyfluorfen | 42.4 |
| 4 | Acenaphthene | 14.3 | 121 | Ethion (diethion) | −13.7 | 238 | Paclobutrazol | 3.0 |
| 5 | Acenaphtylene | 17.0 | 122 | Ethirimol | 13.9 | 239 | Parathion methyl | 12.8 |
| 6 | Acephate | −90.4 | 123 | Ethofumesate | 30.5 | 240 | PCB 28 | 32.5 |
| 7 | Acetamiprid | 7.0 | 124 | Ethoprophos | −20.5 | 241 | PCB 52 | 32.7 |
| 8 | Acrinathrin | 16.2 | 125 | Etofenprox | 43.0 | 242 | PCB 77 | 52.5 |
| 9 | Albendazole | 17.7 | 126 | Etoxazole | −29.6 | 243 | PCB 81 | 43.7 |
| 10 | Aldicarb | 5.8 | 127 | Famoxadone | 27.9 | 244 | PCB 101 | 8.2 |
| 11 | Aldicarb-sulfone | −4.5 | 128 | Fenamidone | 12.7 | 245 | PCB 105 | 54.6 |
| 12 | Aldicarb-sulfoxide | −2.9 | 129 | Fenamiphos | 14.0 | 246 | PCB 114 | 46.6 |
| 13 | Aldrin | 43.7 | 130 | Fenamiphos sulfone | 10.4 | 247 | PCB 118 | 45.0 |
| 14 | Anthracene | 23.9 | 131 | Fenamiphos sulfoxide | 0.0 | 248 | PCB 123 | 45.7 |
| 15 | Atrazine | 7.8 | 132 | Fenarimol | 55.3 | 249 | PCB 126 | 52.1 |
| 16 | Azinphos-methyl | 14.3 | 133 | Fenazaquin | −108.8 | 250 | PCB 138 | 54.9 |
| 17 | Azoxystrobin | −6.9 | 134 | Fenbendazole | −7.9 | 251 | PCB 153 | 48.2 |
| 18 | BDE-28 | 47.0 | 135 | Fenbuconazole | 67.7 | 252 | PCB 156 | 59.3 |
| 19 | BDE-47 | 47.8 | 136 | Fenhexamid | 5.9 | 253 | PCB 157 | 51.6 |
| 20 | BDE-85 | 43.7 | 137 | Fenitrothion | 54.6 | 254 | PCB 167 | 60.1 |
| 21 | BDE-99 | 57.3 | 138 | Fenoxycarb | 16.4 | 255 | PCB 169 | 60.6 |
| 22 | BDE-100 | 51.3 | 139 | Fenpropathrin | −2.2 | 256 | PCB 180 | 52.5 |
| 23 | BDE-153 | 35.9 | 140 | Fenpropidin | 22.3 | 257 | PCB 189 | 54.8 |
| 24 | BDE-154 | 57.8 | 141 | Fenpropimorph | 1.7 | 258 | Penconazole | 50.4 |
| 25 | BDE-183 | −14.1 | 142 | Fenpyroximate | 19.8 | 259 | Pencycuron | −37.4 |
| 26 | Benalaxyl | −111.5 | 143 | Fenthion | −33.9 | 260 | Pendimethalin | 29.8 |
| 27 | Bendiocarb | −5.4 | 144 | Fenthion oxon | 27.1 | 261 | Penicillin V | −8.1 |
| 28 | Bendiocarb metabolite (2,2-dimethylbenzo-1,3-dioxol-4-ol) | 39.7 | 145 | Fenthion oxon sulfone | 5.3 | 262 | Permethrin | 71.5 |
| 29 | Benfuracarb | 24.0 | 146 | Fenthion oxon sulfoxide | 3.7 | 263 | Phenanthrene | 29.6 |
| 30 | Benzo[a]anthracene | 58.5 | 147 | Fenthion sulfone | 6.9 | 264 | Phenylbutazone | −4.9 |
| 31 | Benzo[a]pyrene | 45.0 | 148 | Fenthion sulfoxide | 11.9 | 265 | Phosalone | −4.0 |
| 32 | Benzo[b]fluoranthene | 59.8 | 149 | Fenvalerate | −39.6 | 266 | Phosmet | 5.8 |
| 33 | Benzo[ghi]perylene | 24.4 | 150 | Fipronil | 21.0 | 267 | Pthalamide (Folpet deg) | 59.3 |
| 34 | Benzo[k]fluoranthene | 38.1 | 151 | Fipronil sulfide | 676.5 | 268 | Pirimicarb | 1.7 |
| 35 | Bifenthrin | 80.4 | 152 | Flocoumafen | −34.5 | 269 | Pirimicarb-desmethyl | 57.7 |
| 36 | Bitertanol | −0.7 | 153 | Fluazinam | −1.5 | 270 | Pirimiphos ethyl | 60.5 |
| 37 | Boscalid (formerly nicobifen) | 73.5 | 154 | Flubendiamide | 62.7 | 271 | Pirimiphos methyl | −14.7 |
| 38 | Brodifacoum | −31.7 | 155 | Flucythrinate (two isomers) | 4.0 | 272 | Prochloraz | −37.2 |
| 39 | Bromadiolone | −2.1 | 156 | Fludioxonil | 61.2 | 273 | Procymidone | 52.9 |
| 40 | Bromopropylate | 94.0 | 157 | Flufenoxuron | 1.1 | 274 | Profenofos | −15.0 |
| 41 | Bromuconazole (two isomers) | 39.7 | 158 | Flumequine | −0.8 | 275 | Propamocarb | 16.2 |
| 42 | Bupirimate | 57.0 | 159 | Flunixin | −9.4 | 276 | Propargite | −29.3 |
| 43 | Buprofezin | −0.4 | 160 | Fluopyram | 45.4 | 277 | Propiconazole | −85.8 |
| 44 | Cadusafos (ebufos) | −54.8 | 161 | Fluoranthene | 36.0 | 278 | Propoxur | −7.1 |
| 45 | Carbaryl | 15.8 | 162 | Fluorene | 17.2 | 279 | Propyzamide (pronamide) | −2.0 |
| 46 | Carbendazim (azole) | 16.8 | 163 | Fluquinconazole | 55.3 | 280 | Proquinazid | 72.7 |
| 47 | Carbofuran | −9.0 | 164 | Flusilazole | 3.8 | 281 | Prothioconazol | 51.5 |
| 48 | Carbofuran-3-hydroxy | 5.5 | 165 | Flutolanil | −10.2 | 282 | Prothiophos | 49.5 |
| 49 | Carbosulfan | −107.2 | 166 | Flutriafol | 54.5 | 283 | Pymetrozine | 16.2 |
| 50 | Cefuroxima axetil (two isomers) | 24.0 | 167 | Fluvalinate tau | −31.7 | 284 | Pyraclostrobin | −18.0 |
| 51 | Chloramphenicol | 24.4 | 168 | Fonofos | 28.9 | 285 | Pyrazophos | 20.6 |
| 52 | Chlorantraniliprole | 4.5 | 169 | Formetanate | −38.7 | 286 | Pyrene | 45.1 |
| 53 | Chlorfenvinphos | −31.3 | 170 | Fosthiazate | 2.9 | 287 | Pyridaben | −83.6 |
| 54 | Chlorobenzilate | 95.2 | 171 | Heptachlor | −110.9 | 288 | Pyridaphenthion | 14.3 |
| 55 | Chlorophacinone | 38.1 | 172 | Hexachlorobencene | 16.9 | 289 | Pyrimethanil | 30.6 |
| 56 | Chlorpropham | 24.2 | 173 | Hexachlorocyclohexane (alpha) | −58.1 | 290 | Pyriproxifen | −24.8 |
| 57 | Chlorpyrifos | 38.0 | 174 | Hexachlorocyclohexane (beta) | −97.4 | 291 | Quinalfos | 3.7 |
| 58 | Chlorpyrifos methyl | 9.6 | 175 | Hexachlorocyclohexane (delta) | −89.1 | 292 | Quinoxyfen | −68.5 |
| 59 | Chlorthal dimethyl | 36.6 | 176 | Hexaclorocyclohexane (gamma. lindane) | −109.8 | 293 | Rifampicin | −68.9 |
| 60 | Chrysene | 38.1 | 177 | Hexaconazole (two isomers) | −4.6 | 294 | Rotenone | 22.0 |
| 61 | Clindamycin | −7.5 | 178 | Hexaflumuron | −1.3 | 295 | Roxithromycin | 0.0 |
| 62 | Clofentezine | 13.2 | 179 | Hexythiazox | −27.6 | 296 | Sarafloxacin | 114.8 |
| 63 | Clothianidin | 17.7 | 180 | Imazalil (enilconazole) | 11.5 | 297 | Simazine | −19.7 |
| 64 | Cloxacillin | 4.9 | 181 | Imidacloprid | 4.1 | 298 | Spinosad (two isomers) | −84.2 |
| 65 | Coumachlor | 46.2 | 182 | Indeno [1,2,3-cd] pyrene | −132.0 | 299 | Spiramycin (two isomers) | 16.8 |
| 66 | Coumaphos | −105.9 | 183 | Indoxacarb | 2.6 | 300 | Spirodiclofen | −12.7 |
| 67 | Coumatetralyl | −25.6 | 184 | Iprovalicarb | −4.9 | 301 | Spiromesifen | −23.6 |
| 68 | Cyazofamid | 12.7 | 185 | Isofenphos methyl | 42.0 | 302 | Spirotetramat | −34.1 |
| 69 | Cyflufenamid | −3.0 | 186 | Isoprothiolane | −13.5 | 303 | Spiroxamine | 9.4 |
| 70 | Cyfluthrin (sum of four isomers) | −6.1 | 187 | Ivermectin B1a | −82.6 | 304 | Strychnine | 11.9 |
| 71 | Cyhalothrin (lambda isomer) | −22.4 | 188 | Josamycin | −0.4 | 305 | Sulfacetamide | −27.9 |
| 72 | Cymoxanil | 13.6 | 189 | Ketoprofen | 10.0 | 306 | Sulfachloropiridacine | 61.1 |
| 73 | Cypermethrin (sum of four isomers) | 20.9 | 190 | Kresoxim methyl | 36.3 | 307 | Sulfadiacine | −15.0 |
| 74 | Cyproconazole (two isomers) | 8.2 | 191 | Levamisole | 10.0 | 308 | Sulfadimetoxine | 18.2 |
| 75 | Cyprodinil | −5.7 | 192 | Lincomycin | 135.3 | 309 | Sulfadoxine | 2.2 |
| 76 | Cyromazine | −106.0 | 193 | Linuron | 0.0 | 310 | Sulfameracine | 83.7 |
| 77 | Danofloxacin | 172.1 | 194 | Lufenuron | 7.3 | 311 | Sulfametacine | 166.8 |
| 78 | Dazomet | 33.1 | 195 | Mandipropamid | 20.6 | 312 | Sulfametizole | 170.5 |
| 79 | Deltamethrin | −33.7 | 196 | Mebendazole | 11.5 | 313 | Sulfametoxazole | 26.0 |
| 80 | Demeton-S-methyl | −27.1 | 197 | Mefenamic acid | 33.5 | 314 | Sulfametoxipiridacine | 107.7 |
| 81 | Demeton-S-methyl-sulfone (Dioxydemeton) | 7.5 | 198 | Mefenoxam (metalaxyl-M) | −5.9 | 315 | Sulfamonomethoxine | 1.7 |
| 82 | Dexamethasone | 5.4 | 199 | Meloxicam | 34.8 | 316 | Sulfapyridine | 24.6 |
| 83 | Diazinon | 22.3 | 200 | Mepanipyrim | 64.2 | 317 | Sulfaquinoxaline | 2.5 |
| 84 | Dibenzo[a.h]anthracene | 35.2 | 201 | Mepiquat | −57.2 | 318 | Sulfatiazole | 32.5 |
| 85 | Dichlorodiphenyldichloroethane (p,p′ DDD) | 14.9 | 202 | Metaflumizone | 5.4 | 319 | Sulfisoxazole | 11.6 |
| 86 | Dichlorodiphenyldichloroethylene (p,p′ DDE) | 44.5 | 203 | Metalaxyl | −47.0 | 320 | Tebuconazole | −72.1 |
| 87 | Dichlorodiphenyltrichloroethane (p,p′ DDT) | −124.9 | 204 | Metaldehyde | −25.7 | 321 | Tebufenocide | −7.8 |
| 88 | Diclofenac | 8.6 | 205 | Metconazole | −26.1 | 322 | Tebufenpyrad | 7.9 |
| 89 | Dicloran | −0.4 | 206 | Methamidophos (two isomers) | −16.6 | 323 | Teflubenzuron | −31.5 |
| 90 | Diclorvos | −42.8 | 207 | Methidathion | 7.1 | 324 | Tefluthrin | 33.9 |
| 91 | Dicloxacillin | −4.4 | 208 | Methiocarb | 6.2 | 325 | Telodrin (isobenzan) | −22.0 |
| 92 | Dieldrin | 19.7 | 209 | Methiocarb-sufone | 3.0 | 326 | Terbufos | 24.0 |
| 93 | Diethathyl ethyl | −15.6 | 210 | Methiocarb-sulfoxide | 3.0 | 327 | Terbuthylazine | 29.2 |
| 94 | Diethofencarb | 7.5 | 211 | Methomyl | 1.8 | 328 | Tetrachlorvinphos | 1.1 |
| 95 | Difenacoum | 7.8 | 212 | Methoxyfenozide | −7.0 | 329 | Tetraconazole | 48.4 |
| 96 | Difenoconazole | −21.3 | 213 | Metoxychlor | −124.6 | 330 | Tetradifon | 41.7 |
| 97 | Difethialone | 16.8 | 214 | Metrafenone | 6.1 | 331 | Tetramethrin | 154.7 |
| 98 | Difloxacin | 99.9 | 215 | Metronidazole | −13.3 | 332 | Thiabendazole | 67.7 |
| 99 | Diflubenzuron | 38.5 | 216 | Mevinphos (phosdrin) | −3.3 | 333 | Thiacloprid | 12.3 |
| 100 | Diflufenican | 8.1 | 217 | Mirex | −82.6 | 334 | Thiamethoxam | 19.7 |
| 101 | Dimethenamid-P (and its R-isomer) | 8.7 | 218 | Monocrotophos | −1.2 | 335 | Thiophanate methyl | −4.2 |
| 102 | Dimethoate | 4.8 | 219 | Moxidectin | 20.7 | 336 | Tolclofos methyl | 21.0 |
| 103 | Dimethomorph (two isomers) | 24.2 | 220 | Myclobutanil | 24.7 | 337 | Tolfenamic acid | 136.0 |
| 104 | Dimethylphenylsulfamide (DMSA, metabolite of dichlofluanid) | 4.9 | 221 | N-(2,4-dimethylphenyl)-N′-methylformamidine (DMPF, metabolite of amitraz) | 9.6 | 338 | Triadimefon | 20.6 |
| 105 | Diniconazole-M | −11.4 | 222 | N,N-Dimethyl-N′-p-tolylsulphamide (DMST, metabolite of tolyfluanid) | −8.2 | 339 | Triadimenol | 27.9 |
| 106 | Dinocap | −11.9 | 223 | Nafcillin | −8.2 | 340 | Triazophos (hostathion) | −6.3 |
| 107 | Diphacinone | 14.1 | 224 | Naphtalene | 12.9 | 341 | Trifloxystrobin | −55.8 |
| 108 | Diphenylamine | −102.4 | 225 | Naproxen | 16.5 | 342 | Triflumizole | −14.3 |
| 109 | Dodine | −92.0 | 226 | Nitenpyram | −4.5 | 343 | Triflumuron | 3.6 |
| 110 | Doramectina | −16.2 | 227 | Novobiocin | 2.9 | 344 | Trifluralin | −1.7 |
| 111 | Endosulfan alfa | −2.5 | 228 | Nuarimol | 74.1 | 345 | Trimethoprim | 25.2 |
| 112 | Endosulfan beta | −52.9 | 229 | Ofurace | −27.2 | 346 | Triticonazole | 13.7 |
| 113 | Endosulfan sulfate | −85.8 | 230 | Omethoate | −0.4 | 347 | Tylmicosin | 140.3 |
| 114 | Endrin | −59.9 | 231 | Oxadixyl | 6.5 | 348 | Tylosin | −1.7 |
| 115 | Enrofloxacin | 141.8 | 232 | Oxamyl | 2.6 | 349 | Vinclozolin | 33.9 |
| 116 | EPN | 32.6 | 233 | Oxamyl-oxime | 30.1 | 350 | Warfarin | 0.0 |
| 117 | Epoxiconazole | 10.0 | 234 | Oxfendazole | 7.0 | 351 | Zoxamide | −83.0 |
References
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef] [PubMed]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Chicoine, A.; Erdely, H.; Fattori, V.; Finnah, A.; Fletcher, S.; Lipp, M.; Sanders, P.; Scheid, S. Assessment of veterinary drug residues in food: Considerations when dealing with sub-optimal data. Regul. Toxicol. Pharmacol. 2020, 118, 104806. [Google Scholar] [CrossRef] [PubMed]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Council of the European Union EUR-Lex—31996L0023—EN. Off. J. L 125, 23/05/1996 P. 0010—0032. 1996.
- Fox, G.A. Wildlife as sentinels of human health effects in the Great Lakes-St. Lawrence basin. Environ. Health Perspect. 2001, 109, 853–861. [Google Scholar]
- Grove, R.A.; Henny, C.J.; Kaiser, J.L. Osprey: Worldwide Sentinel Species for Assessing and Monitoring Environmental Contamination in Rivers, Lakes, Reservoirs, and Estuaries. J. Toxicol. Environ. Health Part B 2009, 12, 25–44. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Silva, M.J.; Kuklenyik, Z.; Needham, L.L. Human exposure assessment to environmental chemicals using biomonitoring. Int. J. Androl. 2006, 29, 166–171. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Schwartz, A.L.W.; Shilling, F.M.; Perkins, S.E. The value of monitoring wildlife roadkill. Eur. J. Wildl. Res. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Saulovic, D.; Biocanin, R.; Rodriguez, B. Bioindicators in Human Environment; University of Belgrade: Belgrade, Serbia, 2015. [Google Scholar]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Wang, W.-X. Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Academic Press: Cambridge, MA, USA, 2016; pp. 99–119. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Dacal, A.C.A.; Zumbado, M.; Luzardo, O.P. Micro QuEChERS-based method for the simultaneous biomonitoring in whole blood of 360 toxicologically relevant pollutants for wildlife. Sci. Total Environ. 2020, 736, 139444. [Google Scholar] [CrossRef] [PubMed]
- Brancato, A.; Brocca, D.; De Lentdecker, C.; Erdos, Z.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; Leuschner, R.; Lythgo, C.; et al. Review of the existing maximum residue levels for bromadiolone according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Amutova, F.; Delannoy, M.; Baubekova, A.; Konuspayeva, G.; Jurjanz, S. Transfer of persistent organic pollutants in food of animal origin—Meta-analysis of published data. Chemosphere 2020, 262, 128351. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Kaczyński, P.; Łozowicka, B.; Perkowski, M.; Szabuńko, J. Multiclass pesticide residue analysis in fish muscle and liver on one-step extraction-cleanup strategy coupled with liquid chromatography tandem mass spectrometry. Ecotoxicol. Environ. Saf. 2017, 138, 179–189. [Google Scholar] [CrossRef]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Development and validation of an efficient automated method for the analysis of 300 pesticides in foods using two-dimensional liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1283, 98–109. [Google Scholar] [CrossRef]
- Rizzetti, T.M.; Souza, M.; Prestes, O.D.; Adaime, M.; Zanella, R. Optimization of sample preparation by central composite design for multi-class determination of veterinary drugs in bovine muscle, kidney and liver by ultra-high-performance liquid chromatographic-tandem mass spectrometry. Food Chem. 2018, 246, 404–413. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Widmer, M. Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-μm particulate high-performance liquid chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2008, 1194, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, B.; Lehotay, S.J.; Mastovska, K.; Lightfield, A.R.; Furey, A.; Danaher, M. New method for the analysis of flukicide and other anthelmintic residues in bovine milk and liver using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 196–207. [Google Scholar] [CrossRef]
- Filigenzi, M.S.; Ehrke, N.; Aston, L.S.; Poppenga, R.H. Evaluation of a rapid screening method for chemical contaminants of concern in four food-related matrices using QuEChERS extraction, UHPLC and high resolution mass spectrometry. Food Addit. Contam. Part A 2011, 28, 1324–1339. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Dacal, A.C.A.; González, F.; Pastor-Tiburón, N.; Zumbado, M.; Luzardo, O.P. Supporting dataset on the validation and verification of the analytical method for the biomonitoring of 360 toxicologically relevant pollutants in whole blood. Data Brief. 2020, 31, 105878. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, R.; Bernal-Suárez, M.D.M.; Luzardo, O.P. Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci. Total Environ. 2020, 753, 142015. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- EC Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, 180–184.
- Scientific Working Group for Forensic Toxicology Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [CrossRef] [PubMed]
- SANTE/12682 Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. Eur. Comm. Heal. Consum. Prot. Dir. 2019, 2–44.
- European Commission Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed (SANTE/12682/2019). Sante/12682/2019 2019.
- BOC Orden 1489, de 28 de Marzo de 2014, Por el Que se Aprueba la Estrategia Para la Erradicación del Uso Ilegal de Veneno en el Medio no Urbano de Canarias 2014. 9252–9324.
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, R.; Suárez, M.D.M.B.; Zumbado, M.; Henríquez-Hernández, L.A.; Luzardo, O.P. Supporting dataset on the optimization and validation of a QuEChERS-based method for the determination of 218 pesticide residues in clay loam soil. Data Brief. 2020, 33, 106393. [Google Scholar] [CrossRef]
- Maštovská, K.; Lehotay, S.J. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J. Chromatogr. A 2004, 1040, 259–272. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.; Camacho, M.; Henríquez-Hernández, L.A.; Boada, L.D.; Ruiz-Suárez, N.; Valerón, P.F.; González, M.A.; Zaccaroni, A.; Zumbado, M.; Luzardo, O.P. Assessment of human health hazards associated with the dietary exposure to organic and inorganic contaminants through the consumption of fishery products in Spain. Sci. Total Environ. 2016, 557-558, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Boada, L.D.; Mendoza, Z.; Ruiz-Suárez, N.; Valerón, P.F.; Camacho, M.; Zumbado, M.; Almeida-González, M.; Henriquez-Hernandez, L.A.; Luzardo, O.P. Consumption of organic meat does not diminish the carcinogenic potential associated with the intake of persistent organic pollutants (POPs). Environ. Sci. Pollut. Res. 2015, 24, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Camacho, M.; Henríquez-Hernández, L.A.; Boada, L.D.; Valerón, P.F.; Zaccaroni, A.; Zumbado, M.; Almeida-González, M.; Rial-Berriel, C.; Luzardo, O.P. Comparative study of the intake of toxic persistent and semi persistent pollutants through the consumption of fish and seafood from two modes of production (wild-caught and farmed). Sci. Total Environ. 2017, 575, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.; Boada, L.D.; Almeida-González, M.; Mendoza, Z.; Ruiz-Suárez, N.; Valeron, P.F.; Camacho, M.; Zumbado, M.; Henríquez-Hernández, L.A.; Luzardo, O.P. An estimation of the carcinogenic risk associated with the intake of multiple relevant carcinogens found in meat and charcuterie products. Sci. Total Environ. 2015, 514, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Montero, D.; Camacho, M.; Ginés, R.; Boada, L.D.; Bordón, B.R.; Valerón, P.F.; Almeida-González, M.; Zumbado, M.; Haroun, R.; et al. Comparative analysis of selected semi-persistent and emerging pollutants in wild-caught fish and aquaculture associated fish using Bogue (Boops boops) as sentinel species. Sci. Total Environ. 2017, 581-582, 199–208. [Google Scholar] [CrossRef]
- López-Sobaler, A.M.; Aparicio, A.; Rubio, J.; Marcos, V.; Sanchidrián, R.; Santos, S.; Pérez-Farinós, N.; Dal-Re, M.; Villar-Villalba, C.; Yusta-Boyo, M.J.; et al. Adequacy of usual macronutrient intake and macronutrient distribution in children and adolescents in Spain: A National Dietary Survey on the Child and Adolescent Population, ENALIA 2013–2014. Eur. J. Nutr. 2018, 58, 705–719. [Google Scholar] [CrossRef]
- Ruiz-Suárez, N.; Henriquez-Hernandez, L.A.; Valerón, P.F.; Boada, L.D.; Zumbado, M.; Camacho, M.; González, M.A.; Luzardo, O.P. Assessment of anticoagulant rodenticide exposure in six raptor species from the Canary Islands (Spain). Sci. Total Environ. 2014, 485-486, 371–376. [Google Scholar] [CrossRef]
- García-Álvarez, N.; Boada, L.D.; Fernández, A.; Zumbado, M.; Arbelo, M.; Sierra, E.; Xuriach, A.; Almunia, J.; Camacho, M.; Luzardo, O.P. Assessment of the levels of polycyclic aromatic hydrocarbons and organochlorine contaminants in bottlenose dolphins (Tursiops truncatus) from the Eastern Atlantic Ocean. Mar. Environ. Res. 2014, 100, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Boada, L.D.; Orós, J.; Lopez, P.; Zumbado, M.; Almeida-González, M.; Luzardo, O.P. Comparative Study of Organohalogen Contamination Between Two Populations of Eastern Atlantic Loggerhead Sea Turtles (Caretta caretta). Bull. Environ. Contam. Toxicol. 2013, 91, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Luzardo, O.P.; Arellano, J.L.P.; Carranza, C.; Sánchez, N.J.; Almeida-González, M.; Ruiz-Suárez, N.; Valerón, P.F.; Camacho, M.; Zumbado, M.; et al. Different Pattern of Contamination by Legacy POPs in Two Populations from the Same Geographical Area but with Completely Different Lifestyles: Canary Islands (Spain) vs. Morocco. Sci. Total Environ. 2016, 541, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Henríquez-Hernández, L.A.; Luzardo, O.P.; Pestano, J.; Zumbado, M.; Boada, L.D.; Valerón, P.F. Differential Gene Expression Pattern in Human Mammary Epithelial Cells Induced by Realistic Organochlorine Mixtures Described in Healthy Women and in Women Diagnosed with Breast Cancer. Toxicol. Lett. 2016, 246, 42–48. [Google Scholar] [CrossRef]
- Henríquez-Hernández, L.A.; Luzardo, O.P.; Zumbado, M.; Serra-Majem, L.; Valerón, P.F.; Camacho, M.; Álvarez-Pérez, J.; Salas-Salvadó, J.; Boada, L.D. Determinants of Increasing Serum POPs in a Population at High Risk for Cardiovascular Disease. Results from the PREDIMED-CANARIAS Study. Environ. Res. 2017, 156, 477–484. [Google Scholar] [CrossRef]
- Luzardo, O.P.; Boada, L.D.; Carranza, C.; Ruiz-Sua´rez, N.; Henri´quez-Herna´ndez, L.A.; Valero´n, P.F.; Zumbado, M.; Camacho, M.; Arellano, J.L.P. Socioeconomic Development as a Determinant of the Levels of Organochlorine Pesticides and PCBs in the Inhabitants of Western and Central African Countries. Sci. Total Environ. 2014, 497–498, 97–105. [Google Scholar] [CrossRef]
- Plaza, P.I.; Martínez-Lopez, E.; Lambertucci, S.A. The perfect threat: Pesticides and vultures. Sci. Total Environ. 2019, 687, 1207–1218. [Google Scholar] [CrossRef]
- Sánchez-Barbudo, I.S.; Camarero, P.R.; Mateo, R. Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci. Total Environ. 2012, 420, 280–288. [Google Scholar] [CrossRef]
- Seljetun, K.O.; Sandvik, M.; Vindenes, V.; Eliassen, E.; Øiestad, E.L.; Madslien, K.; Moe, L. Comparison of anticoagulant rodenticide concentrations in liver and feces from apparently healthy red foxes. J. Vet. Diagn. Investig. 2020, 32, 560–564. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Acosta-Dacal, A.; Pérez, M.C.; Suárez-Pérez, A.; Melián, A.M.; Zumbado, M.; Hernández, L.A.H.; Ruiz-Suárez, N.; Hernández, A.R.; Boada, L.D.; et al. Dataset on the concentrations of anticoagulant rodenticides in raptors from the Canary Islands with geographic information. Data Brief. 2021, 34, 106744. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Acosta-Dacal, A.; Pérez, M.C.; Suárez-Pérez, A.; Melián, A.M.; Zumbado, M.; Hernández, L.A.H.; Ruiz-Suárez, N.; Hernández, A.R.; Boada, L.D.; et al. Intensive livestock farming as a major determinant of the exposure to anticoagulant rodenticides in raptors of the Canary Islands (Spain). Sci. Total Environ. 2020, 768, 144386. [Google Scholar] [CrossRef] [PubMed]
- Alomar, H.; Chabert, A.; Coeurdassier, M.; Vey, D.; Berny, P. Accumulation of anticoagulant rodenticides (chlorophacinone, bromadiolone and brodifacoum) in a non-target invertebrate, the slug, Deroceras reticulatum. Sci. Total Environ. 2018, 610-611, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Hindmarch, S.; Albert, C.A.; Emery, J.; Mineau, P.; Maisonneuve, F. Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ. Monit. Assess. 2013, 186, 895–906. [Google Scholar] [CrossRef] [PubMed]
- EC COMMISSION DECISION of 13 June 2007 concerning the non-inclusion of carbofuran in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing that substance (notified under document number C (2007). Off. J. Eur. Union 2007, 156, 30–31.
- Ogada, D.L. The power of poison: Pesticide poisoning of Africa’s wildlife. Ann. N. Y. Acad. Sci. 2014, 1322, 1–20. [Google Scholar] [CrossRef]
- Guzmán, M.M.; Marla-Mojica, P.; Romero, D.; Martínez-Lopez, E.; García-Fernández, A.J. Intentional poisoning of animals in southeastern Spain: A review of the veterinary toxicology service from Murcia, Spain. Vet. Hum. Toxicol. 2003, 45. [Google Scholar]
- Kwon, Y.-K.; Wee, S.-H.; Kim, J.-H. Pesticide Poisoning Events in Wild Birds in Korea from 1998 to 2002. J. Wildl. Dis. 2004, 40, 737–740. [Google Scholar] [CrossRef]
- Hernandez, M.; Margalida, A. Pesticide abuse in Europe: Effects on the Cinereous vulture (Aegypius monachus) population in Spain. Ecotoxicology 2008, 17, 264–272. [Google Scholar] [CrossRef]
- Ruiz-Suárez, N.; Boada, L.D.; Henriquez-Hernandez, L.A.; González-Moreo, F.; Suárez-Pérez, A.; Camacho, M.; Zumbado, M.; González, M.A.; Travieso-Aja, M.D.M.; Luzardo, O.P. Continued implication of the banned pesticides carbofuran and aldicarb in the poisoning of domestic and wild animals of the Canary Islands (Spain). Sci. Total Environ. 2015, 505, 1093–1099. [Google Scholar] [CrossRef]
- Panderi, I.; Bernauer, U. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Revision of the opinion on o-Phenylphenol, Sodium o-phenylphenate and Potassium o-phenylphenate (OPP), in cosmetic products. Regul. Toxicol. Pharmacol. 2016, 79, 105. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, K.; Liu, J.; Fan, Y.; Tang, C.; Xiong, S. Body size-dependent bioaccumulation, tissue distribution, and trophic and maternal transfer of phenolic endocrine-disrupting contaminants in a freshwater ecosystem. Environ. Toxicol. Chem. 2018, 37, 1811–1823. [Google Scholar] [CrossRef]
- Jaspers, V.; Covaci, A.; Voorspoels, S.; Dauwe, T.; Eens, M.; Schepens, P. Brominated flame retardants and organochlorine pollutants in aquatic and terrestrial predatory birds of Belgium: Levels, patterns, tissue distribution and condition factors. Environ. Pollut. 2006, 139, 340–352. [Google Scholar] [CrossRef]
- Corcellas, C.; Andreu, A.; Máñez, M.; Sergio, F.; Hiraldo, F.; Eljarrat, E.; Barceló, D. Pyrethroid insecticides in wild bird eggs from a World Heritage Listed Park: A case study in Doñana National Park (Spain). Environ. Pollut. 2017, 228, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.A.; Eulaers, I.; Jaspers, V.L.; Chaudhry, M.J.I.; Frantz, A.; Ambus, P.; Covaci, A.; Malik, R.N. Use of feathers to assess polychlorinated biphenyl and organochlorine pesticide exposure in top predatory bird species of Pakistan. Sci. Total Environ. 2016, 569-570, 1408–1417. [Google Scholar] [CrossRef]
- Nakayama, S.M.; Morita, A.; Ikenaka, Y.; Mizukawa, H.; Ishizuka, M. A review: Poisoning by anticoagulant rodenticides in non-target animals globally. J. Vet. Med. Sci. 2019, 81, 298–313. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Wisk, J.D. Using the terrestrial residue exposure (T-REX) model to assess threatened and endangered bird exposure to and risk from pesticides. Integr. Environ. Assess. Manag. 2013, 9, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Luzardo, O.P.; Ruiz-Suárez, N.; Henriquez-Hernandez, L.A.; Valerón, P.F.; Camacho, M.; Zumbado, M.; Boada, L.D. Assessment of the exposure to organochlorine pesticides, PCBs and PAHs in six species of predatory birds of the Canary Islands, Spain. Sci. Total Environ. 2014, 472, 146–153. [Google Scholar] [CrossRef] [PubMed]
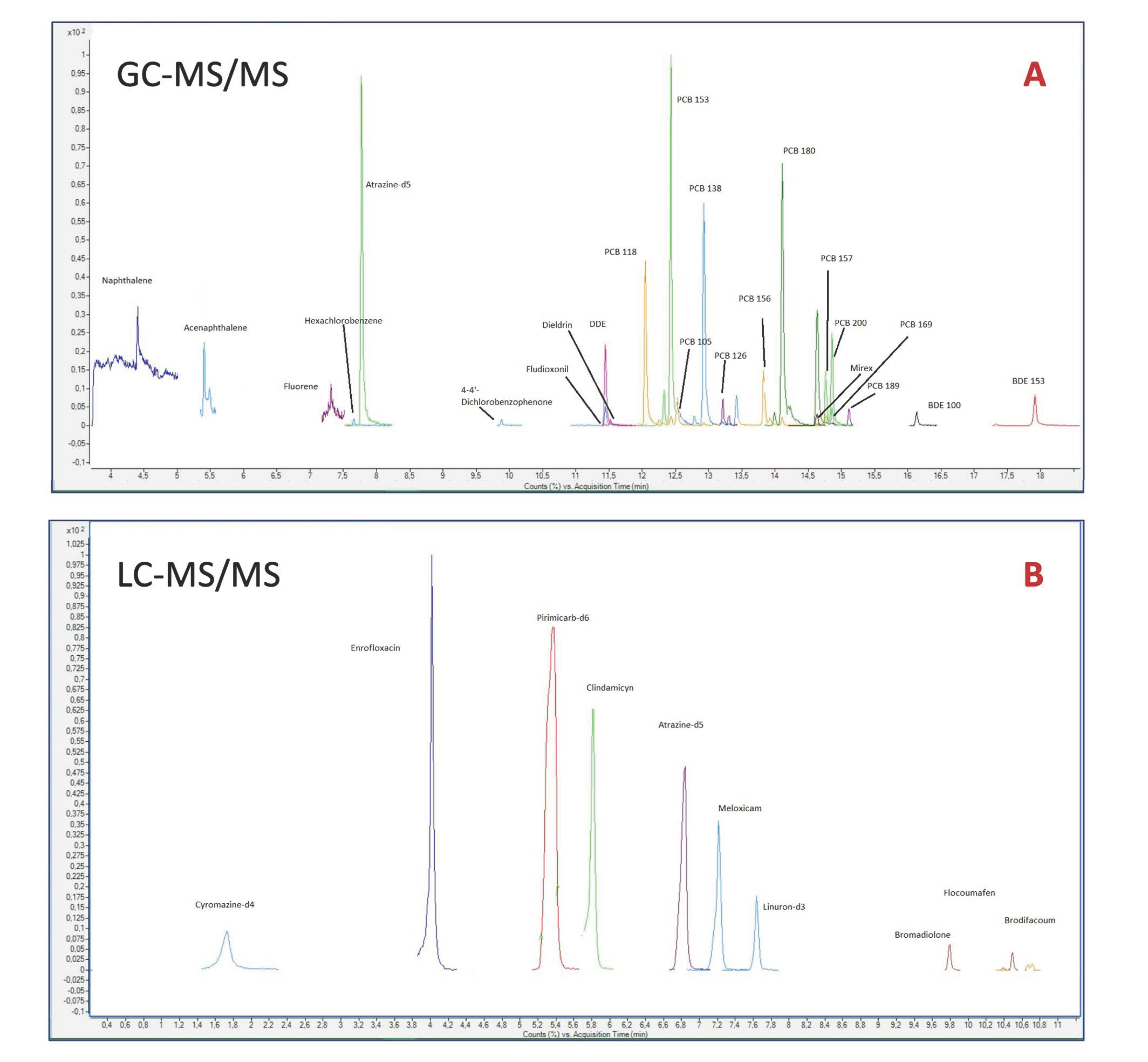
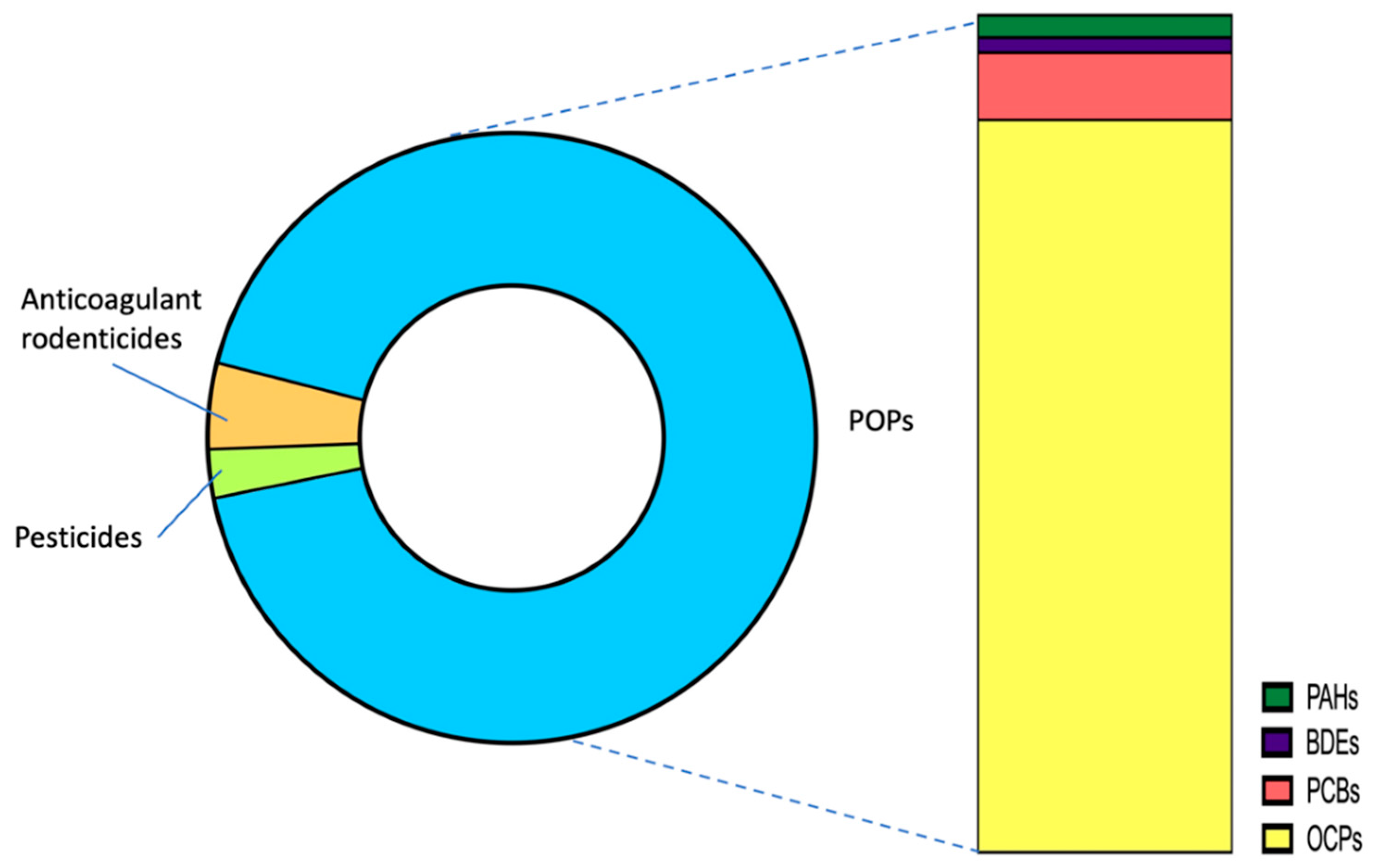
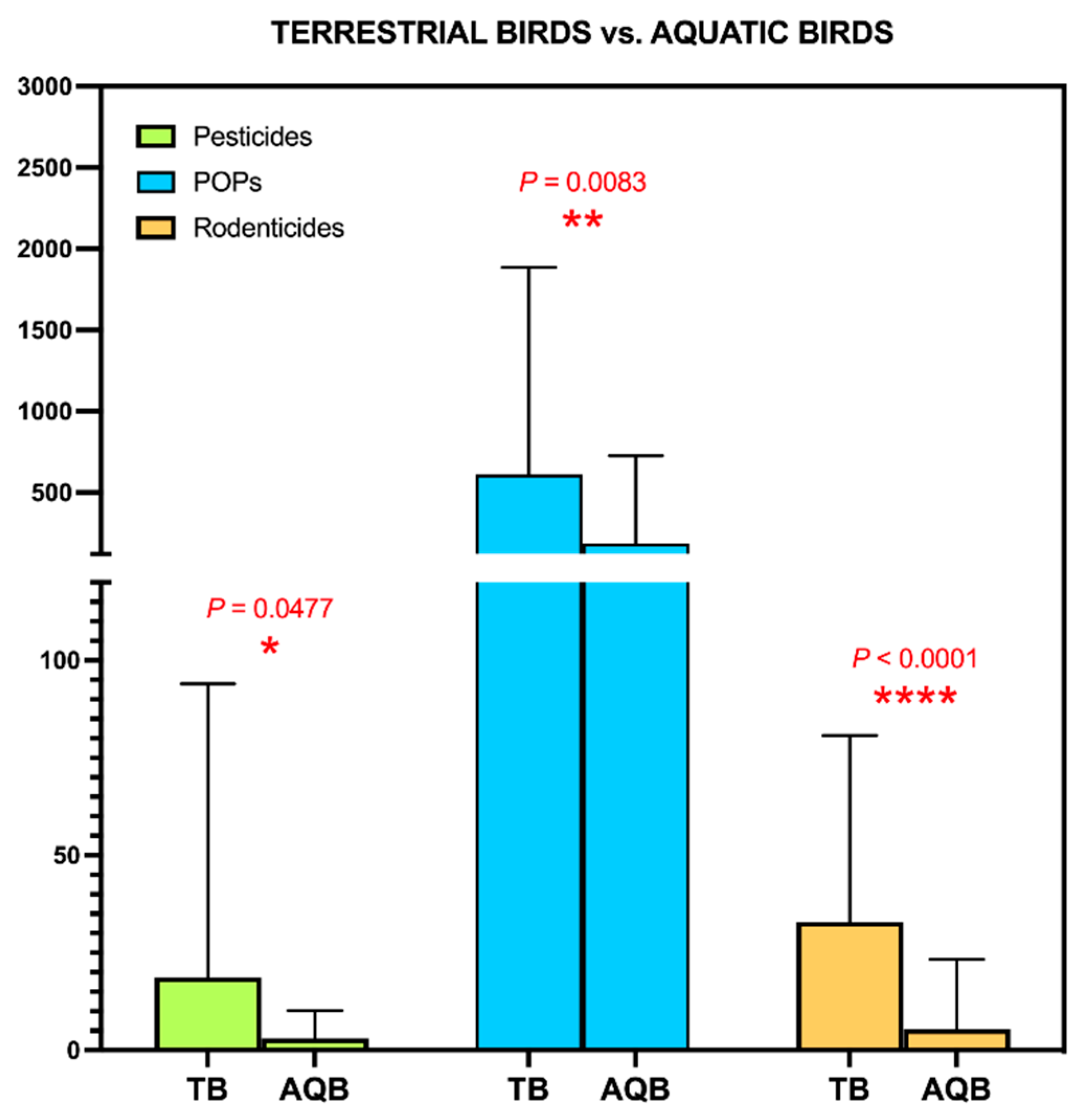
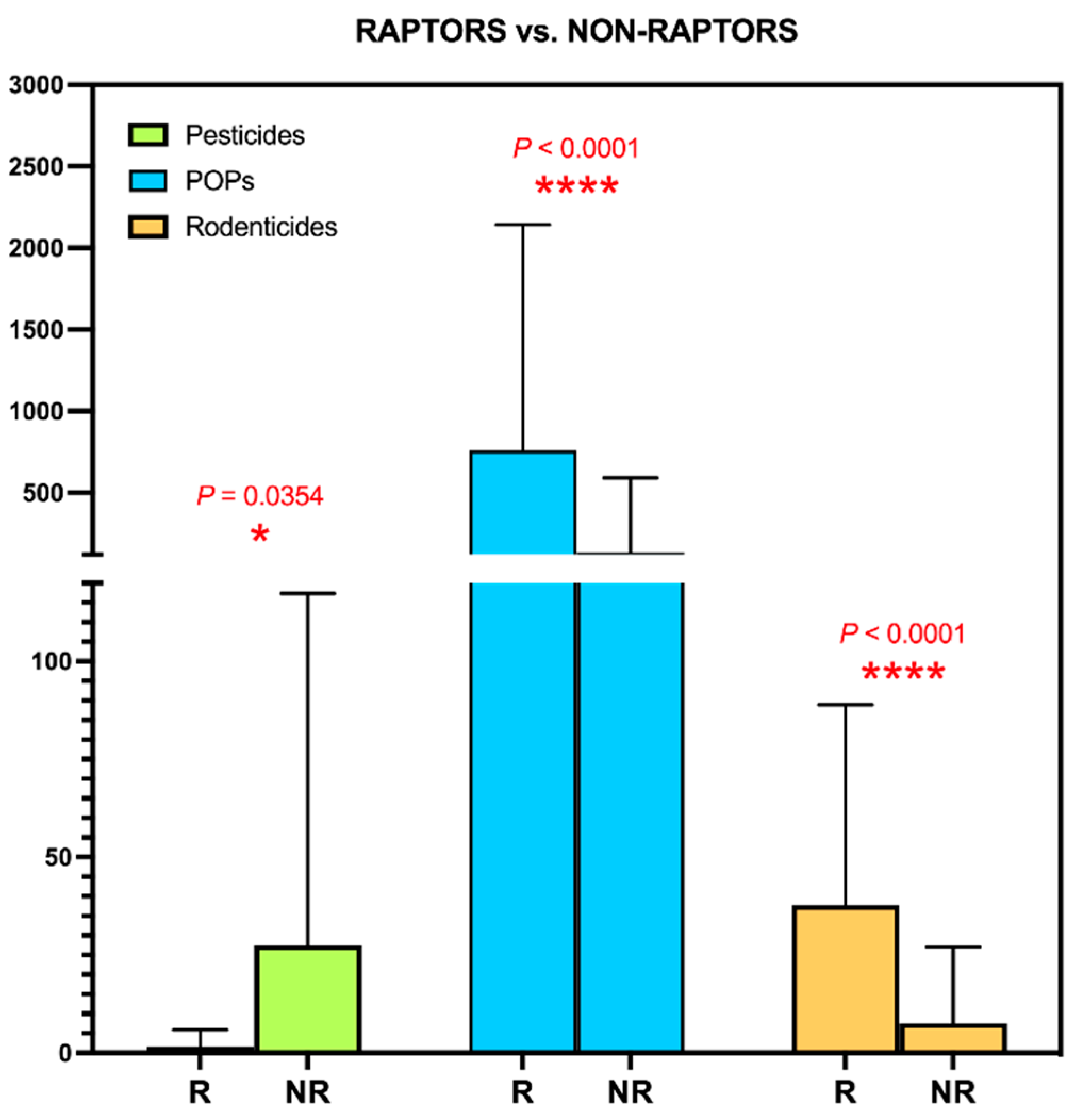
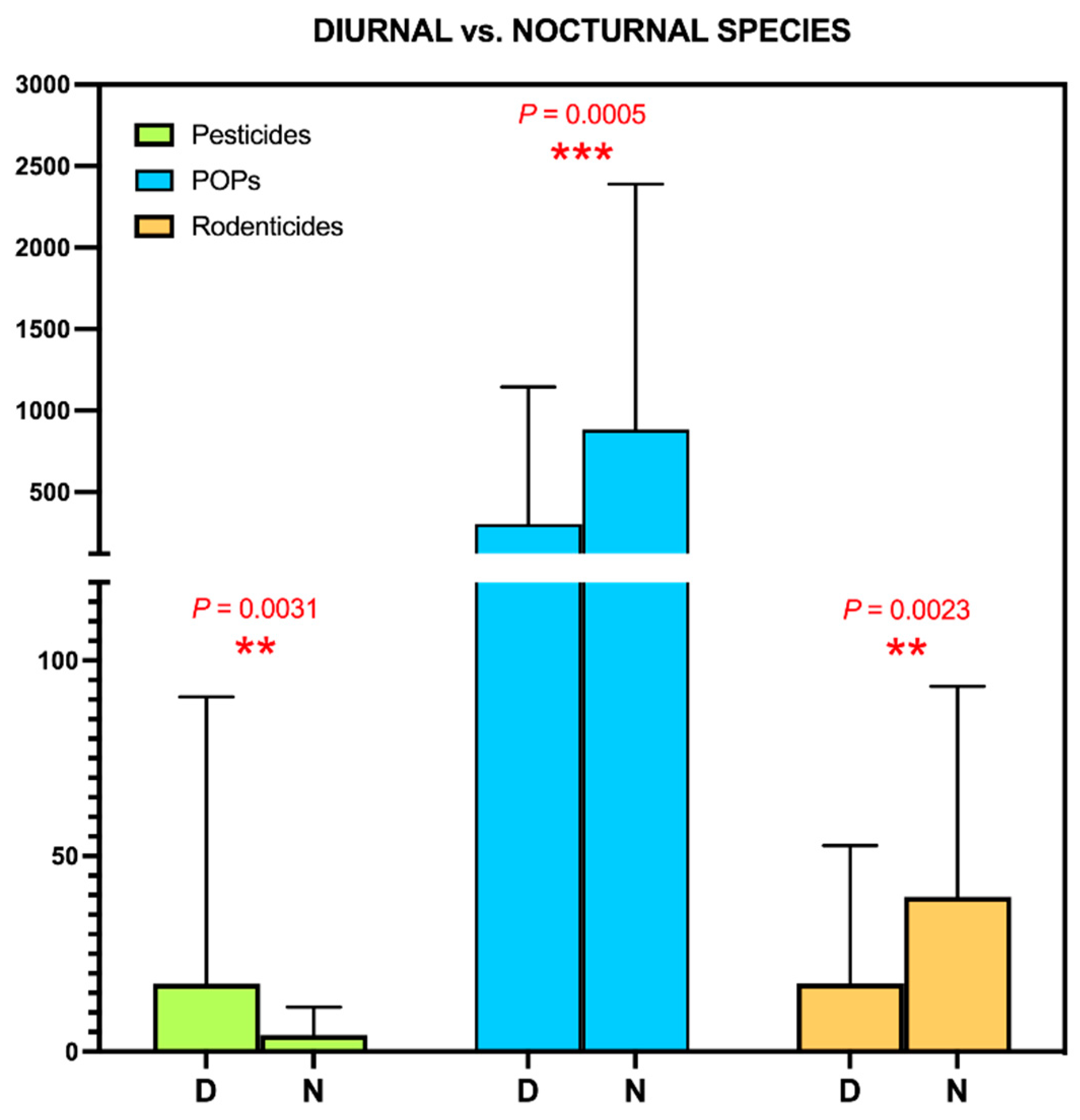
| Compound | Accipiter Nisus (n = 5) | Ardea Cinerea (n = 12) | Asio Otus (n = 34) | Burrinus Oecdinemus (n = 10) | Buteo Buteo (n = 12) | Calonectris Diomedea (n = 8) | Ciconia Ciconia (n = 2) | Corvus Corax (n = 16) | Egretta Garzetta (n = 4) | Falco Eleonorae (n = 2) | Falco Pelegrinoides (n = 6) | Falco Tinnunculus (n = 14) | Larus Michaelis (n = 14) | Turdus Merula (n = 4) | Tyto Alba (n = 8) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meloxicam | 9.9 (40) | - | 47.8 (12) | 880.3 * (20) | - | - | - | 10.3 (13) | - | - | - | 96.1 (29) | - | - | - |
| Tetraconazole | - | - | 0.6 (6) | - | - | - | - | - | - | - | - | - | - | - | - |
| Clindamycin | 1.3 (40) | - | 1423 * (18) | 1.4 (20) | 28.4 (33) | - | - | - | - | - | 2.3 (33) | - | - | - | - |
| Enrofloxacin | 5300 * (80) | - | 4739 * (29) | 4638 * (40) | 5453 * (67) | - | - | 1970 * (13) | - | 20.4 (100) | 5144 * (50) | 5531 * (57) | 10234 * (7) | - | 14508 * (50) |
| Metronidazole | - | - | - | 50.8 (10) | - | - | - | - | - | - | - | 603.3 (14) | - | - | - |
| Sulfatiazole | - | - | 15.5 (6) | - | - | - | - | - | - | - | - | - | - | - | - |
| 2-Phenylphenol | - | - | 16.3 (18) | 22.7 (20) | 2.4 (17) | - | - | - | - | - | - | - | - | - | - |
| Boscalid (formerly nicobifen) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.2 (25) |
| Fludioxonil | - | - | 0.4 (6) | - | - | - | - | - | - | - | - | - | - | - | - |
| Fluquinconazole | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Flutriafol | - | - | - | - | 4.4 (17) | - | - | - | - | - | - | - | - | - | - |
| Carbofuran | - | - | 10.3 (3) | 16.4 (10) | 7.2 (26) | - | - | 94.5 (38) | - | - | - | - | - | - | - |
| Carbofuran-3-hydroxy | - | - | - | - | - | - | - | 2.1 (38) | - | - | - | - | - | - | - |
| Fipronil | - | - | 1.4 (12) | - | - | - | - | - | - | - | - | - | - | - | - |
| Fipronil sulfide | - | - | 3.0 (6) | - | - | - | - | - | - | - | - | - | - | - | - |
| Permethrin | - | - | - | - | - | 23.4 (13) | - | - | - | - | - | - | 12.3 (7) | - | - |
| Acenaphthene | 2.4 (80) | 1.4 (17) | 0.8 (18) | - | 0.4 (17) | - | - | - | - | - | - | 2.4 (14) | - | - | - |
| Anthracene | 1.5 (40) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Chrysene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.8 (25) |
| Fluoranthene | 0.4 (40) | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.7 (25) |
| Fluorene | 5.9 (80) | - | 2.5 (12) | - | - | - | - | - | - | - | - | 5.3 (7) | - | - | - |
| Naphtalene | 1.8 (100) | - | 2.2 (6) | 5.7 (40) | 3.9 (26) | 16.6 (13) | - | 3.4 (26) | 0.7 (25) | - | - | 1.8 (14) | - | 0.9 (50) | 8.0 (25) |
| Phenanthrene | 13.3 (100) | - | 7.5 (12) | - | - | 0.4 (13) | - | 2.7 (13) | - | - | - | 7.6 (28) | - | - | 851.3 (50) |
| Pyrene | 1.8 (40) | - | 0.7 (9) | - | - | - | - | - | - | - | - | 20.0 (28) | - | - | - |
| 4,4′-Dichlorobenzophenone (metabolite of dicofol) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE-100 | - | 1.9 (33) | 0.2 (6) | - | - | - | - | - | - | - | 0.4 (33) | - | 0.2 (7) | - | - |
| BDE-153 | - | 0.3 (33) | 4.9 (41) | - | - | 1.1 (13) | - | - | - | 0.7 (50) | 19.0 (67) | 0.6 (29) | - | - | 0.8 (25) |
| BDE-154 | - | 2.1 (17) | 2.6 (6) | - | - | - | - | - | - | - | 6.9 (33) | - | - | - | - |
| BDE-183 | - | - | 0.9 (12) | - | - | - | - | - | - | - | - | - | - | - | - |
| BDE-47 | - | 1.5 (17) | 2.4 (6) | - | - | - | - | - | - | - | - | 0.6 (7) | 0.4 (14) | - | - |
| BDE-99 | - | - | 1.5 (29) | - | - | - | - | - | - | - | 3.6 (33) | 0.4 (14) | 0.6 (14) | 7.5 (100) | 0.4 (13) |
| Dichlorodiphenyldichloroethane (p,p′ DDD) | 1.2 (80) | - | - | - | - | - | - | - | - | - | - | 2.2 (21) | - | - | - |
| Dichlorodiphenyldichloroethylene (p,p′ DDE) | 211.1 (100) | 21.1 (100) | 305.6 (100) | 25.9 (60) | 5.6 (83.3) | 16.6 (100) | - | 6.7 (75) | 4.7 (100) | 68.4 (100) | 318.6 (100) | 45.3 (100) | 4.4 (100) | - | 24.1 (100) |
| Dieldrin | 7.8 (80) | 3.5 (17) | 5.6 (41.2) | 3.0 (10) | 0.9 (33) | 3.1 (13) | - | - | 2.1 (100) | 5.8 (100) | 8.5 (100) | 11.9 (100) | 2.3 (7) | - | 1.2 (75) |
| Hexachlorobencene | - | 1.4 (34) | 0.6 (24) | - | - | 7.1 (13) | - | - | 12.9 (50) | 0.6 (50) | - | 1.1 (7) | 1.1 (7) | - | 0.8 (25) |
| Hexachlorocyclohexane (beta) | - | - | 31.0 (12) | - | - | - | - | - | - | - | 3.4 (50) | - | - | - | |
| Mirex | - | - | 3.9 (12) | 3.6 (20) | 3.1 (17) | 25.0 (13) | - | - | - | - | 2.3 (33) | - | - | - | |
| PCB 105 | - | 1.2 (50) | 1.3 (35) | - | - | 2.0 (26) | - | - | 3.6 (25) | 0.6 (50) | 0.6 (67) | 1.1 (14) | 0.4 (7) | - | - |
| PCB 118 | 0.5 (40) | 5.4 (50) | 4.8 (35) | - | 0.5 (17) | 45.1 (13) | - | - | 14.9 (50) | 5.0 (100) | 2.2 (100) | 1.3 (28) | 1.4 (7) | - | 1.2 (37) |
| PCB 138 | 1.4 (80) | 7.9 (100) | 2.9 (76) | 2.2 (30) | 1.3 (34) | 8.8 (75) | - | 3.9 (38) | 24.1 (100) | 15.3 (100) | 7.7 (100) | 4.2 (71) | 2.7 (72) | - | 5.3 (75) |
| PCB 153 | 3.4 (80) | 15.5 (100) | 3.6 (94) | 0.5 (80) | 3.3 (34) | 6.8 (100) | - | 1.7 (88) | 55.3 (100) | 115.3 (100) | 16.0 (100) | 5.7 (71) | - | - | 7.8 (100) |
| PCB 156 | - | 2.0 (50) | 2.0 (33) | - | - | 8.5 (13) | - | - | 0.8 (25) | 8.8 (50) | 0.8 (100) | 0.9 (7) | - | - | 0.5 (25) |
| PCB 157 | - | 0.8 (17) | 1.1 (6) | - | - | 2.1 (13) | - | - | 9.1 (50) | 1.1 (50) | - | - | 0.4 (7) | - | - |
| PCB 167 | - | 1.7 (50) | 1.5 (35) | - | - | 6.7 (50) | - | - | 44.6 (100) | 6.7 (50) | 1.3 (67) | 0.8 (14) | 2.2 (72) | - | 0.4 (13) |
| PCB 180 | 3.9 (80) | 24.3 (67) | 3.2 (88) | - | 2.1 (50) | 3.6 (100) | - | 2.8 (88) | - | 123.6 (100) | 19.8 (100) | 5.2 (71) | - | - | 8.0 (75) |
| PCB 189 | - | - | 2.1 (6) | 1.0 (80) | - | - | - | - | - | 1.8 (50) | - | - | - | - | - |
| PCB 28 | - | - | 5.8 (3) | - | - | - | - | - | - | - | - | - | - | - | - |
| Brodifacoum | 1.7 (100) | 0.4 (100) | 32.9 (100) | 2.3 (80) | 0.9 (100) | - | - | 27.4 (75) | - | - | 20.4 (100) | 8.8 (50) | 1.4 (21) | - | 20.31 (100) |
| Bromadiolone | - | - | 1.3 (100) | 2.1 (100) | 8.5 (100) | - | - | 2.25 (38) | - | 1.1 (50) | 4.6 (100) | 2.5 (75) | - | 0.34 (25) | 2.2 (75) |
| Difenacoum | - | 0.8 (17) | 0.6 (24) | - | 1.5 (50) | - | - | 0.9 (13) | - | - | 0.9 (33) | 1.2 (57) | - | - | 3.6 (25) |
| Difethialone | - | - | 18.9 (18) | - | - | - | - | - | - | - | - | 1.9 (29) | - | - | - |
| Flocoumafen | - | - | 0.7 (24) | - | 4.1 (17) | - | - | - | - | - | - | 2.2 (7) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial-Berriel, C.; Acosta-Dacal, A.; Zumbado, M.; Henríquez-Hernández, L.A.; Rodríguez-Hernández, Á.; Macías-Montes, A.; Boada, L.D.; Travieso-Aja, M.d.M.; Martin Cruz, B.; Luzardo, O.P. A Method Scope Extension for the Simultaneous Analysis of POPs, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring. Toxics 2021, 9, 238. https://doi.org/10.3390/toxics9100238
Rial-Berriel C, Acosta-Dacal A, Zumbado M, Henríquez-Hernández LA, Rodríguez-Hernández Á, Macías-Montes A, Boada LD, Travieso-Aja MdM, Martin Cruz B, Luzardo OP. A Method Scope Extension for the Simultaneous Analysis of POPs, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring. Toxics. 2021; 9(10):238. https://doi.org/10.3390/toxics9100238
Chicago/Turabian StyleRial-Berriel, Cristian, Andrea Acosta-Dacal, Manuel Zumbado, Luis Alberto Henríquez-Hernández, Ángel Rodríguez-Hernández, Ana Macías-Montes, Luis D. Boada, María del Mar Travieso-Aja, Beatriz Martin Cruz, and Octavio P. Luzardo. 2021. "A Method Scope Extension for the Simultaneous Analysis of POPs, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring" Toxics 9, no. 10: 238. https://doi.org/10.3390/toxics9100238
APA StyleRial-Berriel, C., Acosta-Dacal, A., Zumbado, M., Henríquez-Hernández, L. A., Rodríguez-Hernández, Á., Macías-Montes, A., Boada, L. D., Travieso-Aja, M. d. M., Martin Cruz, B., & Luzardo, O. P. (2021). A Method Scope Extension for the Simultaneous Analysis of POPs, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring. Toxics, 9(10), 238. https://doi.org/10.3390/toxics9100238