Reactive Oxygen Species Detection Using Fluorescence in Enchytraeus crypticus—Method Implementation through Ag NM300K Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Species
2.2. Test Soil
2.3. Test Materials, Characterization, and Spiking Procedures
2.4. ROS Formation Assessment (Fluorescence Detection)
2.5. Data Analysis
3. Results
3.1. Implementation: H2O2 as Positive Control
3.2. Ag NM300K Case Study
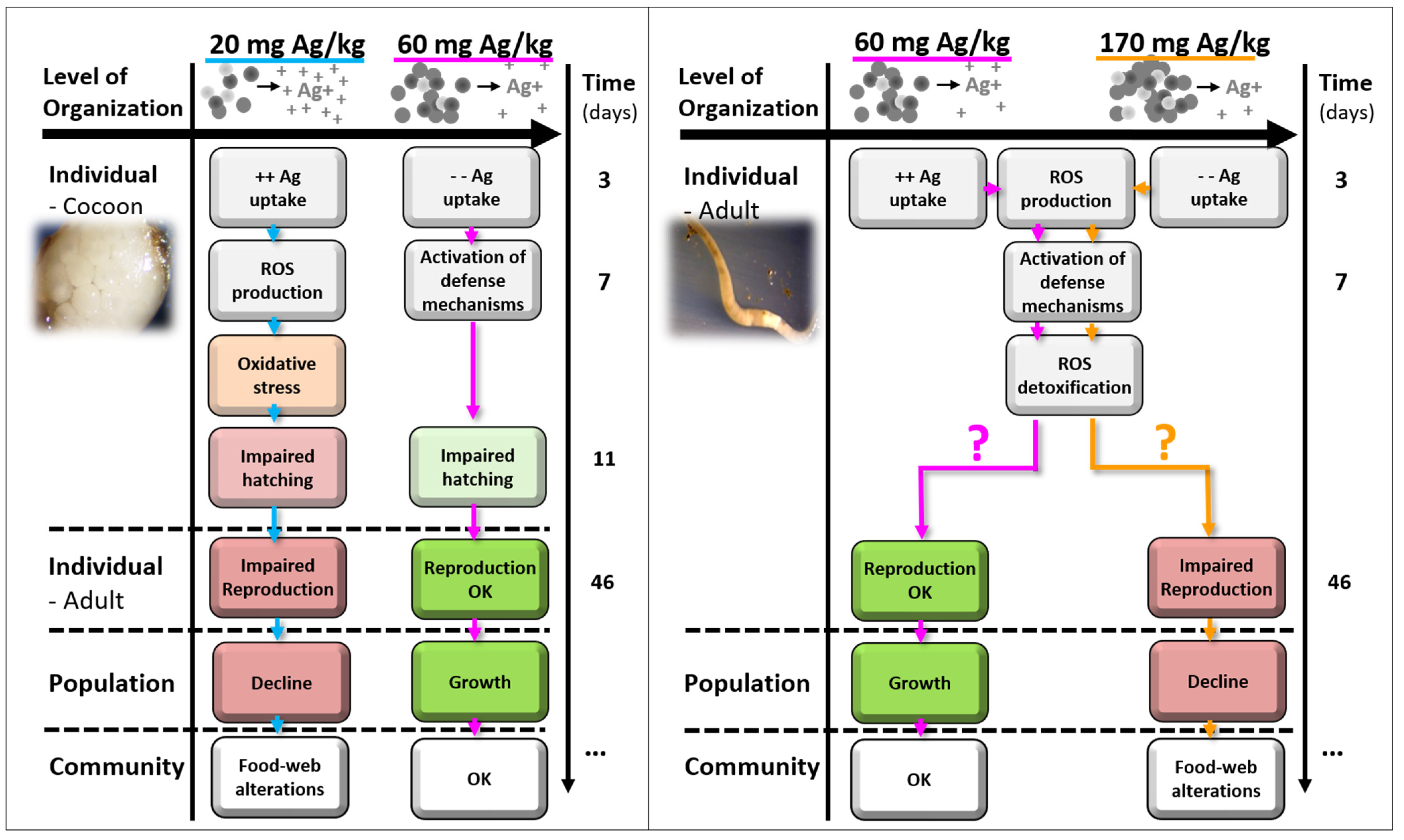
4. Discussion
4.1. Implementation
4.2. Ag NM300K Case Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Saint-Denis, M.; Narbonne, J.F.; Arnaud, C.; Ribera, D. Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: Effects of lead acetate. Soil Biol. Biochem. 2001, 33, 395–404. [Google Scholar] [CrossRef]
- Laszczyca, P.; Augustyniak, M.; Babczynska, A.; Bednarska, K.; Kafel, A.; Migula, P.; Wilczek, G.; Witas, I. Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland). Environ. Int. 2004, 30, 901–910. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Hansen, D.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of silver nanoparticles to soil invertebrates: Oxidative stress biomarkers in Eisenia fetida. Environ. Pollut. 2015, 199, 49–55. [Google Scholar] [CrossRef]
- Saleeb, N.; Robinson, B.; Cavanagh, J.; Ross, J.; Munir, K.; Gooneratne, R. Antioxidant Enzyme Activity and Lipid Peroxidation in Aporrectodea caliginosa Earthworms Exposed to Silver Nanoparticles and Silver Nitrate in Spiked Soil. Environ. Toxicol. Chem. 2020, 39, 1257–1266. [Google Scholar] [CrossRef]
- Novais, S.C.; Gomes, S.I.L.; Gravato, C.; Guilhermino, L.; De Coen, W.; Soares, A.M.V.M.; Amorim, M.J.B. Reproduction and biochemical responses in Enchytraeus albidus (Oligochaeta) to zinc or cadmium exposures. Environ. Pollut. 2011, 159, 1836–1843. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Novais, S.C.; Gravato, C.; Guilhermino, L.; Scott-Fordsmand, J.J.; Soares, A.M.V.M.; Amorim, M.J.B. Effect of Cu-nanoparticles versus one Cu-salt: Analysis of stress biomarkers response in Enchytraeus albidus (Oligochaeta). Nanotoxicology 2012, 6, 134–143. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Maria, V.; Scott-Fordsmand, J.; Amorim, M.J.B. Oxidative Stress Mechanisms Caused by Ag Nanoparticles (NM300K) are Different from Those of AgNO3: Effects in the Soil Invertebrate Enchytraeus crypticus. Int. J. Environ. Res. Public Health 2015, 12, 9589–9602. [Google Scholar] [CrossRef] [Green Version]
- Novais, S.C.; Gomes, N.C.; Soares, A.M.V.M.; Amorim, M.J.B. Antioxidant and neurotoxicity markers in the model organism Enchytraeus albidus (Oligochaeta): Mechanisms of response to atrazine, dimethoate and carbendazim. Ecotoxicology 2014, 23, 1220–1233. [Google Scholar] [CrossRef]
- Maria, V.L.; Ribeiro, M.J.; Amorim, M.J.B. Oxidative stress biomarkers and metallothionein in Folsomia candida—responses to Cu and Cd. Environ. Res. 2014, 133, 164–169. [Google Scholar] [CrossRef]
- Mendes, L.; Maria, V.; Scott-Fordsmand, J.; Amorim, M. Ag Nanoparticles (Ag NM300K) in the Terrestrial Environment: Effects at Population and Cellular Level in Folsomia candida (Collembola). Int. J. Environ. Res. Public Health 2015, 12, 12530–12542. [Google Scholar] [CrossRef] [Green Version]
- Sillapawattana, P.; Gruhlke, M.C.H.; Schäffer, A. Effect of silver nanoparticles on the standard soil arthropod Folsomia candida (Collembola) and the eukaryote model organism Saccharomyces cerevisiae. Environ. Sci. Eur. 2016, 28, 27. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-W.; Li, S.-W.; Hsiu-Chuan Liao, V. Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans. Environ. Pollut. 2017, 220, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Du, Z.; Li, B.; Zhu, L.; Wang, J.; Zhang, S. Toxic effect of [Omim]BF 4 and [Omim]Br on antioxidant stress and oxidative damage in earthworms (Eisenia fetida). Environ. Toxicol. Pharmacol. 2018, 60, 37–44. [Google Scholar] [CrossRef]
- Wilson, M.R.; Foucaud, L.; Barlow, P.G.; Hutchison, G.R.; Sales, J.; Simpson, R.J.; Stone, V. Nanoparticle interactions with zinc and iron: Implications for toxicology and inflammation. Toxicol. Appl. Pharmacol. 2007, 225, 80–89. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development. OECD 220 Guidelines for the testing of chemicals No. 220. In Enchytraeid Reproduction Test; Organization for Economic Cooperation and Development: Paris, France, 2016. [Google Scholar]
- Gomes, S.I.L.; Roca, C.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. High-throughput transcriptomics reveals uniquely affected pathways: AgNPs, PVP-coated AgNPs and Ag NM300K case studies. Environ. Sci. Nano 2017, 4, 929–937. [Google Scholar] [CrossRef]
- Maria, V.L.; Ribeiro, M.J.; Guilherme, S.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Silver (nano)materials cause genotoxicity in Enchytraeus crypticus, as determined by the comet assay. Environ. Toxicol. Chem. 2018, 37, 184–191. [Google Scholar] [CrossRef]
- Bicho, R.C.; Ribeiro, T.; Rodrigues, N.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus. J. Hazard. Mater. 2016, 318, 608–614. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Westheide, W.; Graefe, U. Two new terrestrial Enchytraeus species (Oligochaeta, Annelida). J. Nat. Hist. 1992, 26, 479–488. [Google Scholar] [CrossRef]
- Bicho, R.C.; Santos, F.C.F.; Gonçalves, M.F.M.; Soares, A.M.V.M.; Amorim, M.J.B. Enchytraeid Reproduction TestPLUS: Hatching, growth and full life cycle test—an optional multi-endpoint test with Enchytraeus crypticus. Ecotoxicology 2015, 24, 1053–1063. [Google Scholar] [CrossRef]
- Klein, C.L.; Comero, S.; Stahlmecke, B.; Romazanov, J.; Kuhlbusch, T.A.J.; Doren, E.V.; De Temmerman, P.-J.; Mast, T.J.; Wick, P.; Krug, H.; et al. NM-Series of Representative Manufactured Nanomaterials NM-300 Silver Characterisation, Stability, Homogeneity. JRC Publ. Repos. 2011. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef]
- Paatero, I.; Casals, E.; Niemi, R.; Özliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- El Battawy, K.; Linhart, O. Cryopreservation of Common Carp (Cyprinus carpio) Embryos using Vitrification. Middle East J. Appl. Sci. 2014, 4, 1155–1160. [Google Scholar]
- Kim, K.-T.; Tanguay, R.L. The role of chorion on toxicity of silver nanoparticles in the embryonic zebrafish assay. Environ. Health Toxicol. 2014, 29, e2014021. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Bicho, R.C.; Rêma, A.; Soares, A.M.V.M.; Faustino, A.M.R.; Amorim, M.J.B. Development of an embryotoxicity test for Enchytraeus crypticus—the effect of Cd. Chemosphere 2015, 139, 386–392. [Google Scholar] [CrossRef]
- George, S.G.; Hodgson, P.A.; Tytler, P.; Todd, K. Inducibility of Metallothionein mRNA Expression and Cadmium Tolerance in Larvae of a Marine Teleost, the Turbot (Scophthalmus maximus). Toxicol. Sci. 1996, 33, 91–99. [Google Scholar] [CrossRef]
- Verriopoulos, G.; Moraïtou-Apostolopoulou, M. Differentiation of the sensitivity to copper and cadmium in different life stages of a copepod. Mar. Pollut. Bull. 1982, 13, 123–125. [Google Scholar] [CrossRef]
- Santos, F.C.F.; Gomes, S.I.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Hazard assessment of nickel nanoparticles in soil-The use of a full life cycle test with Enchytraeus crypticus. Environ. Toxicol. Chem. 2017, 36, 2934–2941. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Q. Dose- and time-related changes in aerobic metabolism, chorionic disruption, and oxidative stress in embryonic medaka (Oryzias latipes): Underlying mechanisms for silver nanoparticle developmental toxicity. Aquat. Toxicol. 2012, 124–125, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.I.L.; Roca, C.P.; Scott-Fordsmand, J.J.; Amorim, M.J.B. High-throughput transcriptomics: Insights into the pathways involved in (nano) nickel toxicity in a key invertebrate test species. Environ. Pollut. 2019, 245, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Novo, M.; Lahive, E.; Díez-Ortiz, M.; Matzke, M.; Morgan, A.J.; Spurgeon, D.J.; Svendsen, C.; Kille, P. Different routes, same pathways: Molecular mechanisms under silver ion and nanoparticle exposures in the soil sentinel Eisenia fetida. Environ. Pollut. 2015, 205, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Ortiz, M.; Lahive, E.; George, S.; Ter Schure, A.; Van Gestel, C.A.M.; Jurkschat, K.; Svendsen, C.; Spurgeon, D.J. Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils. Environ. Pollut. 2015, 203, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bicho, R.C.; Faustino, A.M.R.; Carvalho, F.; Soares, A.M.V.M.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Embryotoxicity of silver nanomaterials (Ag NM300k) in the soil invertebrate Enchytraeus crypticus—Functional assay detects Ca channels shutdown. NanoImpact 2021, 21, 100300. [Google Scholar] [CrossRef]

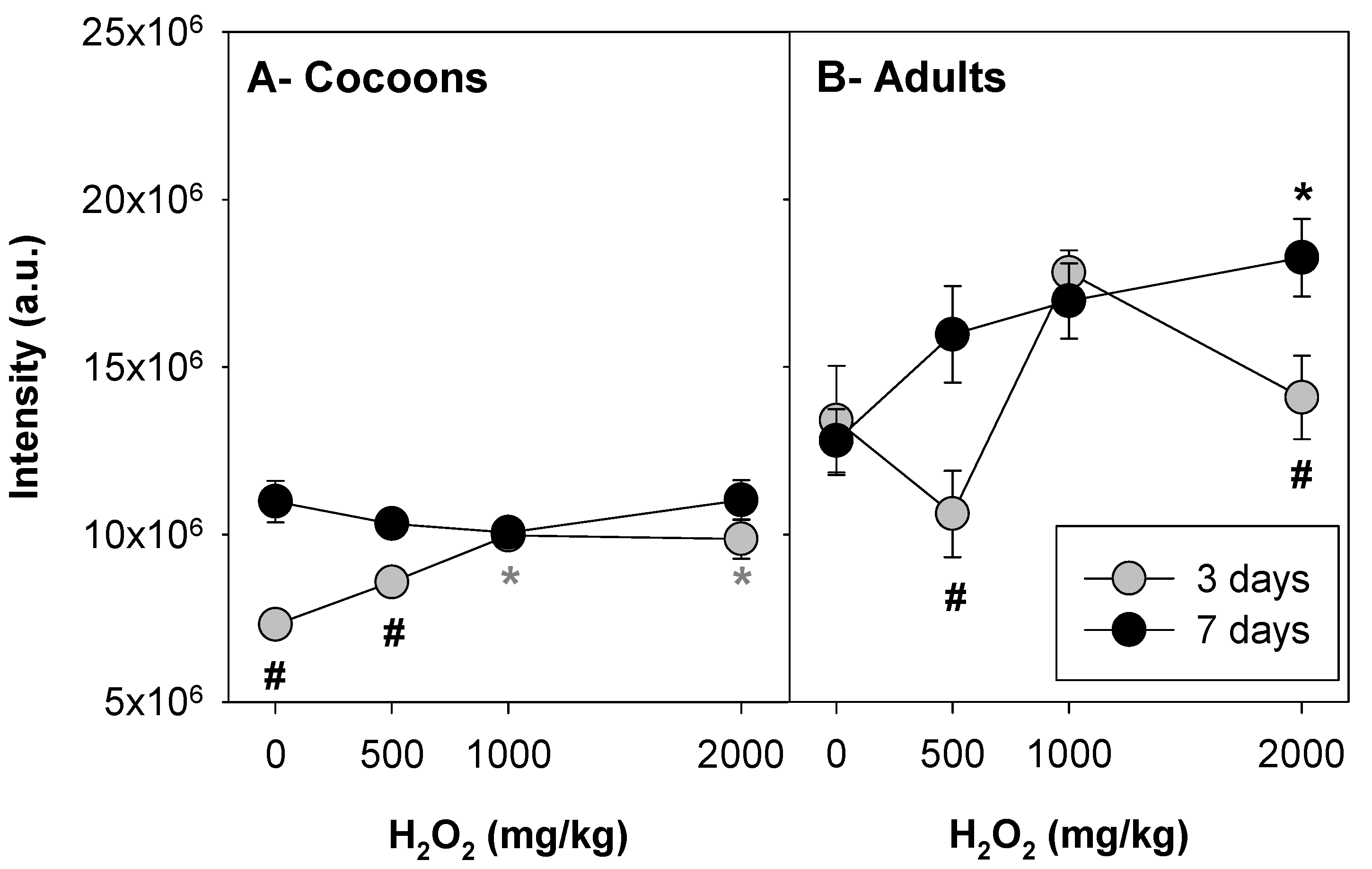
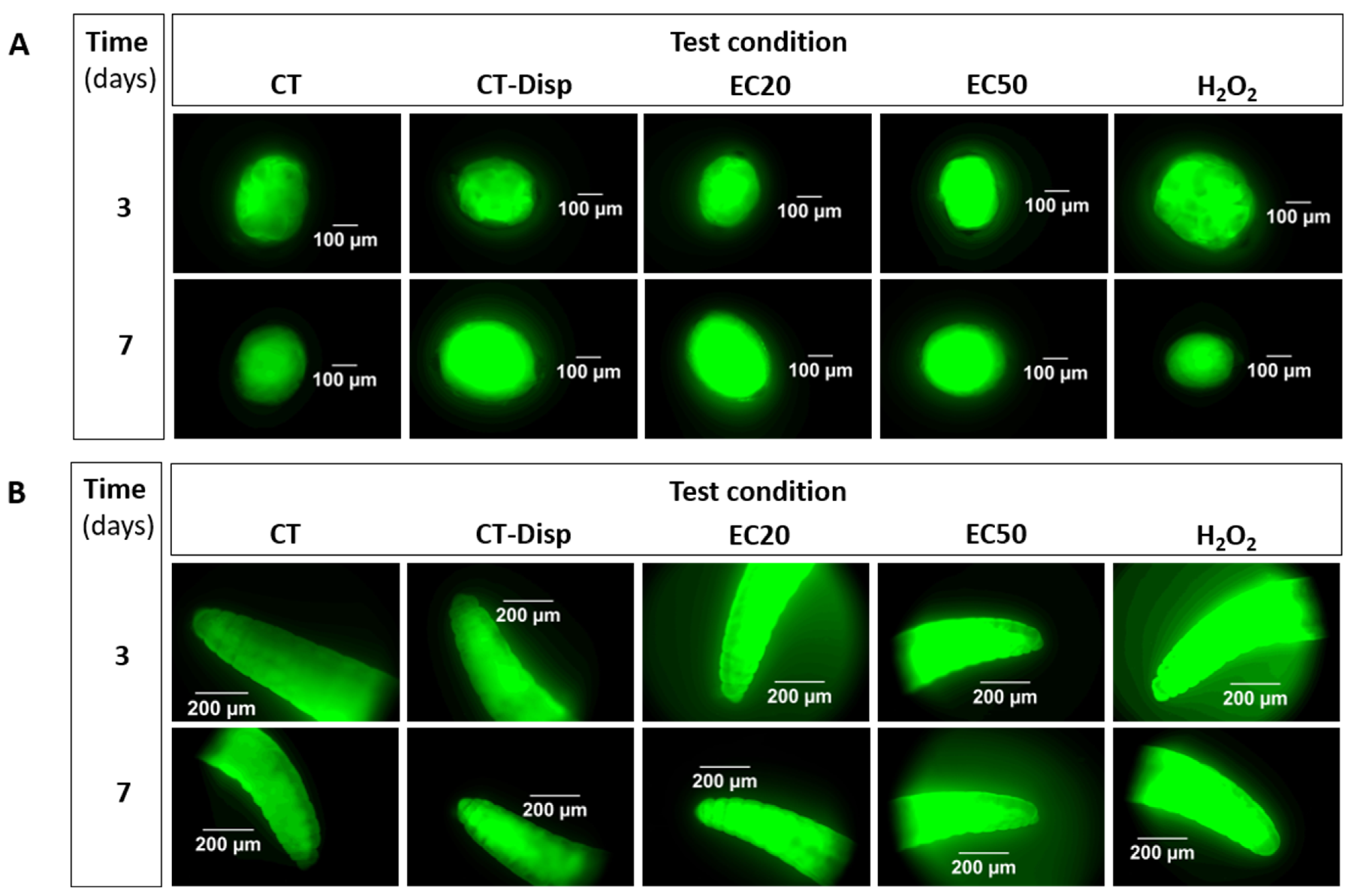
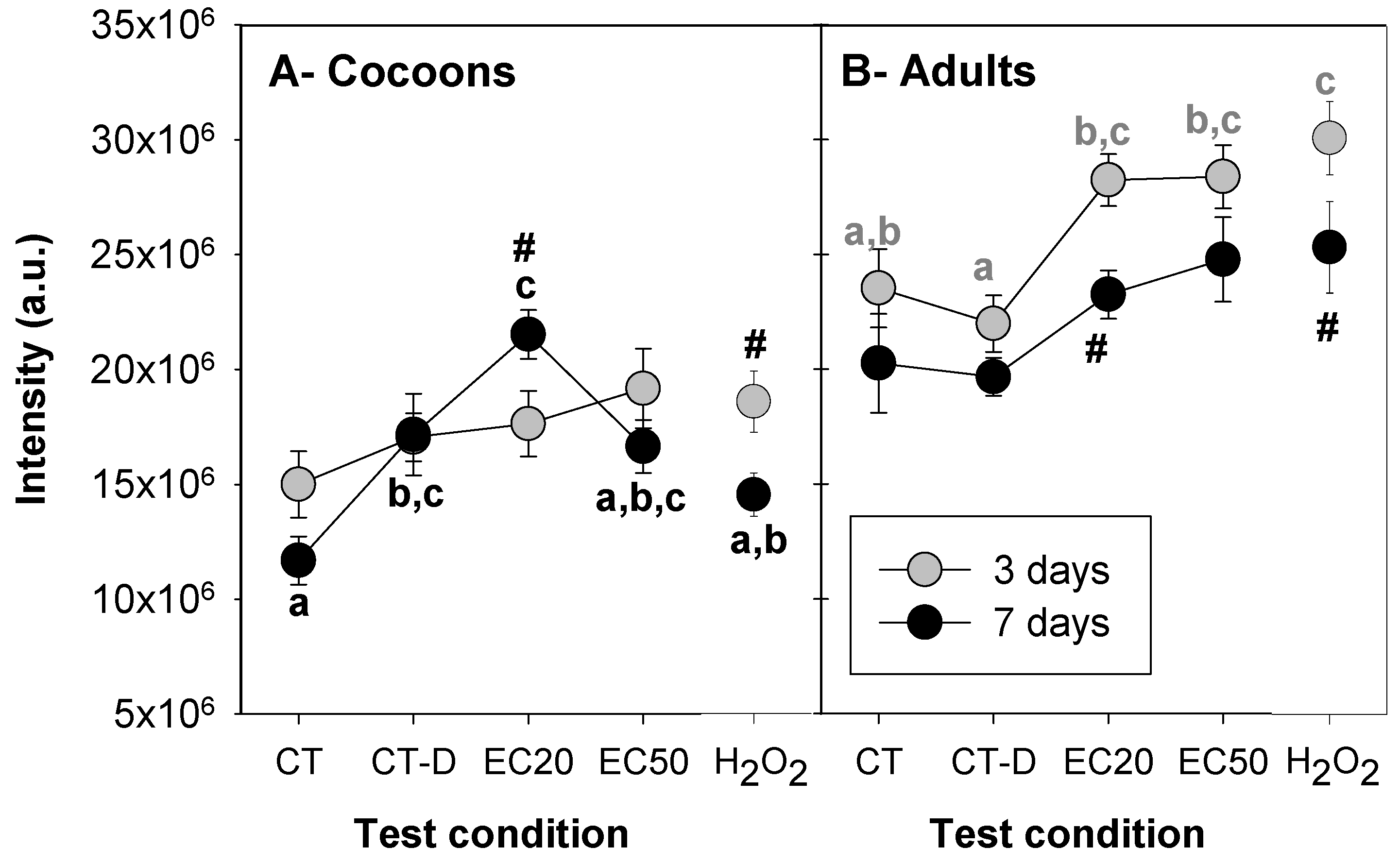
| # | Test Level | Test Substance/Material | E. crypticus Development Stage | Concentrations (mg/kg) | Exposure Time (Days) |
|---|---|---|---|---|---|
| 1 | implementation | H2O2 | adults | 0, 500, 1000, 2000 | 3, 7 |
| 2 | cocoons | ||||
| 3 | case study | Ag NM300K | adults | disp.#, 60 *, 170 * | 3, 7 |
| 4 | cocoons | disp.#, 20 §, 60 § | |||
| 5 | H2O2 (positive control) | adults | 0, 2000 | 3, 7 | |
| 6 | cocoons |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.I.L.; Neves, A.B.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Reactive Oxygen Species Detection Using Fluorescence in Enchytraeus crypticus—Method Implementation through Ag NM300K Case Study. Toxics 2021, 9, 232. https://doi.org/10.3390/toxics9100232
Gomes SIL, Neves AB, Scott-Fordsmand JJ, Amorim MJB. Reactive Oxygen Species Detection Using Fluorescence in Enchytraeus crypticus—Method Implementation through Ag NM300K Case Study. Toxics. 2021; 9(10):232. https://doi.org/10.3390/toxics9100232
Chicago/Turabian StyleGomes, Susana I. L., Ana B. Neves, Janeck J. Scott-Fordsmand, and Mónica J. B. Amorim. 2021. "Reactive Oxygen Species Detection Using Fluorescence in Enchytraeus crypticus—Method Implementation through Ag NM300K Case Study" Toxics 9, no. 10: 232. https://doi.org/10.3390/toxics9100232
APA StyleGomes, S. I. L., Neves, A. B., Scott-Fordsmand, J. J., & Amorim, M. J. B. (2021). Reactive Oxygen Species Detection Using Fluorescence in Enchytraeus crypticus—Method Implementation through Ag NM300K Case Study. Toxics, 9(10), 232. https://doi.org/10.3390/toxics9100232








