Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects
Abstract
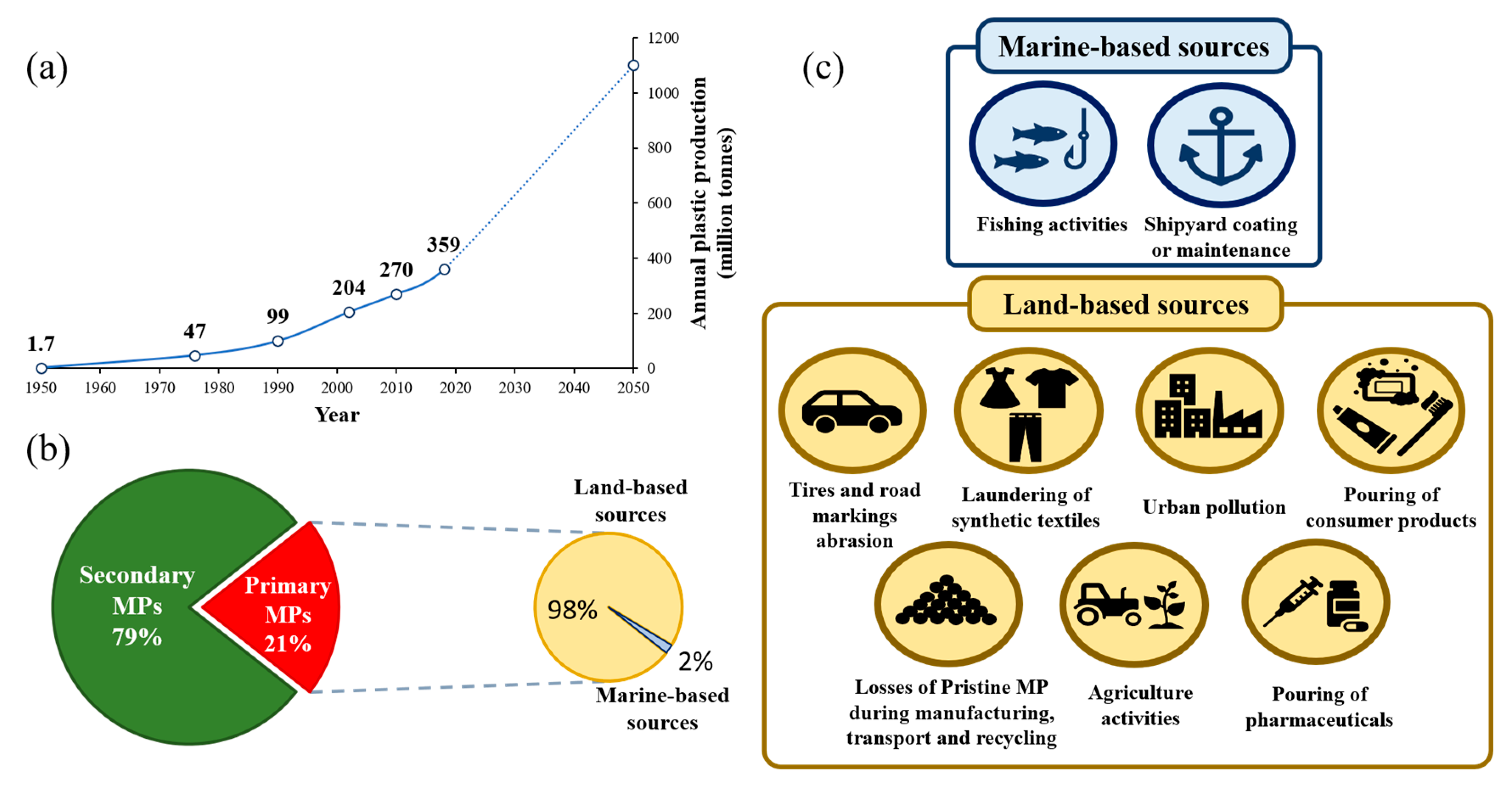
1. Introduction
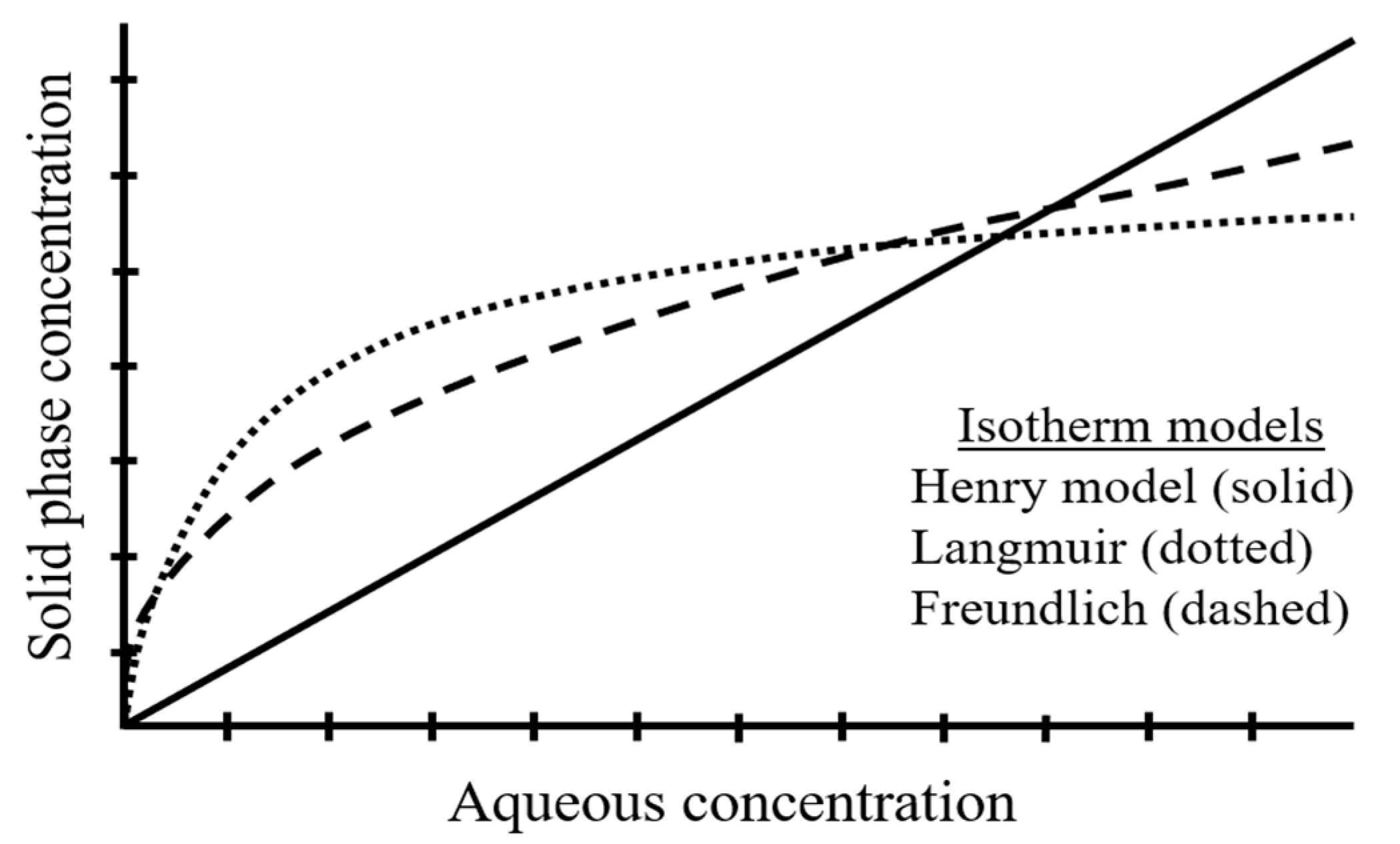
2. Factors Influencing the Sorption of Environmental Pollutants to MPs
2.1. Plastic Polymer Type
2.2. Size of the Plastic Pellets
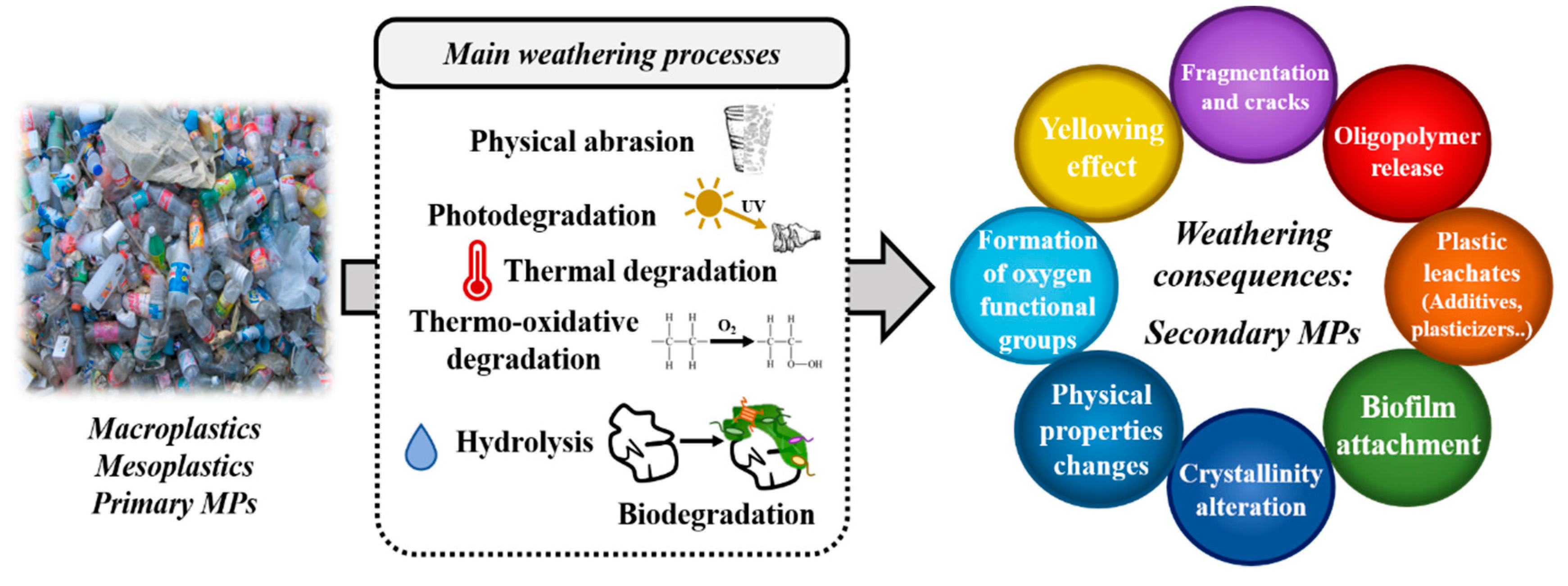
2.3. Age and Degree of Weathering of Plastic
2.4. Chemical Properties of Pollutants
2.5. Environmental Factors
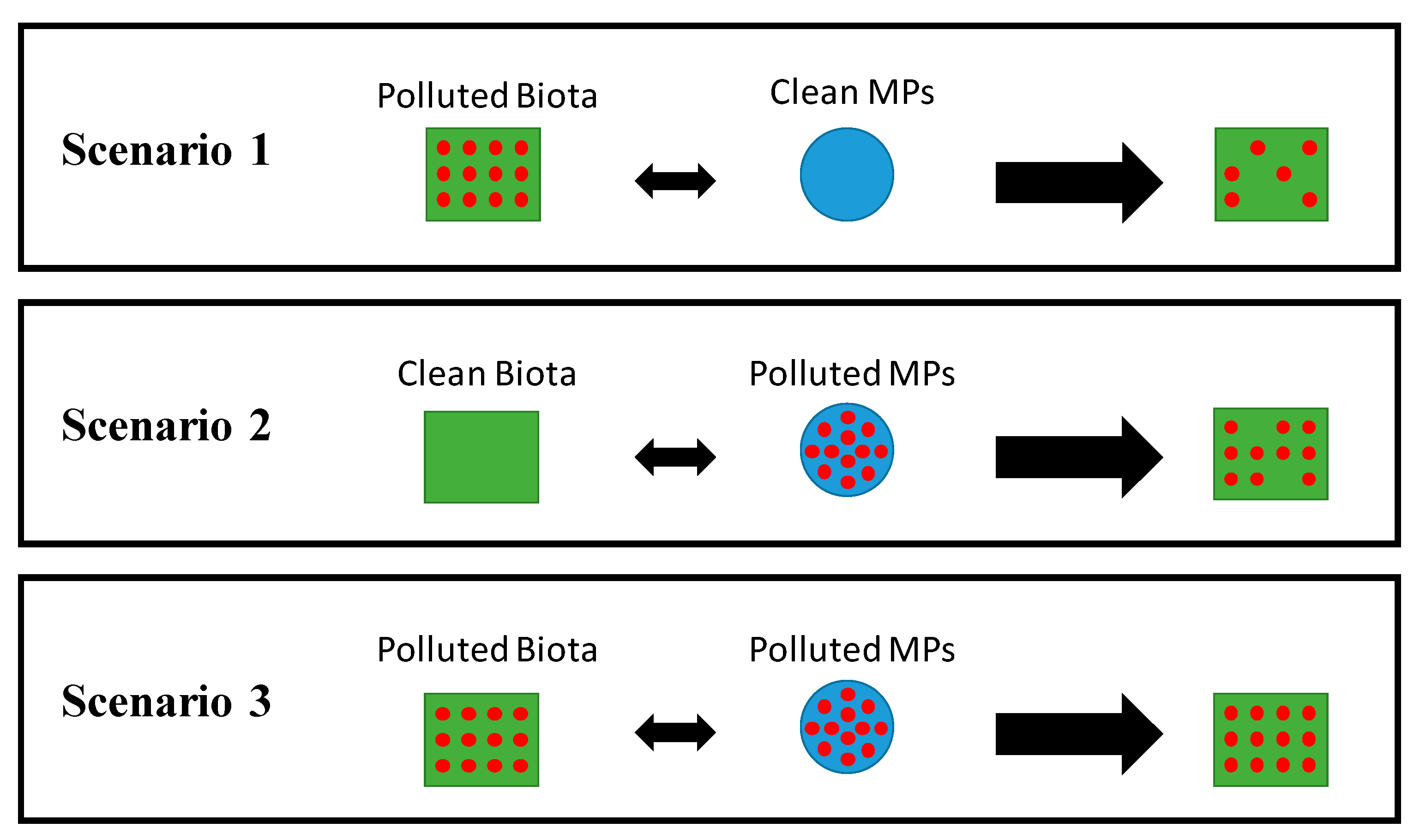
3. Effects of MPs-pollutants Interaction on Biota
3.1. Scenario 1: Contaminated Biota Eats Clean Plastics
3.2. Scenario 2: Clean Biota Consumes Contaminated Plastics
3.3. Scenario 3: Contaminated Biota Ingests Contaminated Plastics
4. Ecotoxicological Effects of MPs Combined with Sorbed Chemicals on Biota
Author Contributions
Funding
Conflicts of Interest
Abbreviations:
| 4-MBC | 4-Methylbenzylidene-camphor |
| AChE | Acetylcholinesterase |
| BPA | Bisphenol A |
| DDTs | Dichlorobiphenyl Trichloroethanes |
| DOM | Dissolved Organic Matter |
| EROD | 7-ethoxyresorufin O-deethylase |
| GSH | Glutathione |
| HCHs | Hexachlorocyclohexanes |
| HDPE | High-Density Polyethylene |
| HOCs | Hydrophobic Organic Compounds |
| Kow | Octanol-water partition coefficient |
| Kpw | Plastic-water partition coefficient |
| LDPE | Low-Density Polyethylene |
| LPO | Lipid Peroxidation |
| MDA | Malondialdehyde |
| MPs | Microplastics |
| MTLP | Metallothionein-like Proteins |
| NOAA | US National Oceanographic and Atmospheric Administration |
| NPs | Nanoplastics |
| NSAID | Nonsteroidal Anti-inflammatory Drugs |
| OPFRs | Organophosphorus Flame-Retardants |
| PA | Polyamide |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PBDEs | Polybrominated Diphenyl Ethers |
| PCBs | Polychlorinated Biphenyls |
| PE | Polyethylene |
| PET | Polyethylene Terephthalate |
| PFAS | Perfluoroalkyl substances |
| PFNA | Perfluornonanoic Acid |
| PFOS | Perfluorooctanesulfonate |
| PFOSA | Perfluorooctanesulfonamide |
| PhACs | Pharmaceuticals Active Compounds |
| POPs | Persistent Organic Pollutants |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| PVC | Polyvinyl Chloride |
| SOD | Superoxide Dismutase |
| TBBPA | Tetrabromobisphenol A |
| Tg | Glass Transition Temperature |
References
- Plastics—The Facts 2019. Available online: https://www.plasticseurope.org/es/resources/publications/1804-plastics-facts-2019 (accessed on 19 December 2019).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peng, J.; Qiu, Q.; Zhan, Z.; Wang, J.; Tan, Z. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast. Shelf Sci. 2016, 176, 102–109. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Magara, G.; Khan, F.R.; Pinti, M.; Syberg, K.; Inzirillo, A.; Elia, A.C. Effects of combined exposures of fluoranthene and polyethylene or polyhydroxybutyrate microplastics on oxidative stress biomarkers in the blue mussel (Mytilus edulis). J. Toxicol. Environ. Health—Part A Curr. Issues 2019, 82, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 17010–17020. [Google Scholar] [CrossRef] [PubMed]
- Sıkdokur, E.; Belivermiş, M.; Sezer, N.; Pekmez, M.; Bulan, Ö.K.; Kılıç, Ö. Effects of microplastics and mercury on manila clam Ruditapes philippinarum: Feeding rate, immunomodulation, histopathology and oxidative stress. Environ. Pollut. 2020, 262, 114247. [Google Scholar] [CrossRef]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of microplastics and two persistent organic pollutants alone or in combination to a bivalve species. Environ. Pollut. 2020, 258, 113845. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environment Protection; Reports Stud. GESAMP; GESAMP: London, UK, 2015; Volume 90, pp. 1–96. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environmentA review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; ISBN 9782831718279. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Orejón, L.F.; Sardá, R.; Ramis-Pujol, J. Now, you see me: High concentrations of floating plastic debris in the coastal waters of the Balearic Islands (Spain). Mar. Pollut. Bull. 2018, 133, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Meester, S.; De Landuyt, L.; Van Clerck, K.; De Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size matters more than shape: Ingestion of primary and secondary microplastics by small predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific Central Gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Fossi, M.C.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Zhu, M.; Liang, J.; Zheng, S.; Zhao, Y. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017, 115, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Pham, C.K.; Rodríguez, Y.; Dauphin, A.; Carriço, R.; Frias, J.P.G.L.; Vandeperre, F.; Otero, V.; Santos, M.R.; Martins, H.R.; Bolten, A.B.; et al. Plastic ingestion in oceanic-stage loggerhead sea turtles (Caretta caretta) off the North Atlantic subtropical gyre. Mar. Pollut. Bull. 2017, 121, 222–229. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.L.; Heubeck, M.; Jensen, J.K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Ryan, P.G.; Connell, A.D.; Gardner, B.D. Marine Pollution Bulletin Plastic Ingestion and PCBs in Seabirds: Is There a Relationship? Mar. Pollut. Bull. 1988, 19, 174–176. [Google Scholar] [CrossRef]
- Lavers, J.L.; Bond, A.L. Ingested plastic as a route for trace metals in Laysan Albatross (Phoebastria immutabilis) and Bonin Petrel (Pterodroma hypoleuca) from Midway Atoll. Mar. Pollut. Bull. 2016, 110, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC—Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Karami, A.; Groman, D.B.; Wilson, S.P.; Ismail, P.; Neela, V.K. Biomarker responses in zebrafish (Danio rerio) larvae exposed to pristine low-density polyethylene fragments. Environ. Pollut. 2017, 223, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Parnis, J.M.; Browne, M.A.; Serrato, S.; Reiner, E.J.; Robson, M.; Young, T.; Diamond, M.L.; Teh, S.J. Direct and indirect effects of different types of microplastics on freshwater prey (Corbicula fluminea) and their predator (Acipenser transmontanus). PLoS ONE 2017, 12, e0187664. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-a and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.A. A perspective on the potential health risks of PBDEs. Chemosphere 2002, 46, 745–755. [Google Scholar] [CrossRef]
- Talsness, C.E. Overview of toxicological aspects of polybrominated diphenyl ethers: A flame-retardant additive in several consumer products. Environ. Res. 2008, 108, 158–167. [Google Scholar] [CrossRef]
- Guilhermino, L.; Vieira, L.R.; Ribeiro, D.; Tavares, A.S.; Cardoso, V.; Alves, A.; Almeida, J.M. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci. Total Environ. 2018, 622–623, 1131–1142. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef]
- Rochman, C.M.; Manzano, C.; Hentschel, B.T.; Simonich, S.L.M.; Hoh, E. Polystyrene plastic: A source and sink for polycyclic aromatic hydrocarbons in the marine environment. Environ. Sci. Technol. 2013, 47, 13976–13984. [Google Scholar] [CrossRef]
- Pascall, M.A.; Zabik, M.E.; Zabik, M.J.; Hernandez, R.J. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J. Agric. Food Chem. 2005, 53, 164–169. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: Implications for plastic marine debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—but should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.K.; Leung, K.S.Y. Sorption and desorption of organic UV filters onto microplastics in single and multi-solute systems. Environ. Pollut. 2019, 254, 113066. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.S.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Koelmans, A.A. Transfer of PCBs from Microplastics under Simulated Gut Fluid Conditions Is Biphasic and Reversible. Environ. Sci. Technol. 2019, 53, 1874–1883. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]
- Herzke, D.; Anker-Nilssen, T.; Nøst, T.H.; Götsch, A.; Christensen-Dalsgaard, S.; Langset, M.; Fangel, K.; Koelmans, A.A. Negligible Impact of Ingested Microplastics on Tissue Concentrations of Persistent Organic Pollutants in Northern Fulmars off Coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e0111913. [Google Scholar] [CrossRef] [PubMed]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 2020, 708, 134841. [Google Scholar] [CrossRef]
- Barbosa, F.; Adeyemi, J.A.; Bocato, M.Z.; Comas, A.; Campiglia, A. A critical viewpoint on current issues, limitations, and future research needs on micro- and nanoplastic studies: From the detection to the toxicological assessment. Environ. Res. 2020, 182, 109089. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC—Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Kane Driscoll, S. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Endo, S.; Koelmans, A.A. Sorption of hydrophobic organic compounds in marine environments: Equilibrium. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 185–204. ISBN 9783319955667. [Google Scholar]
- Fang, S.; Yu, W.; Li, C.; Liu, Y.; Qiu, J.; Kong, F. Adsorption behavior of three triazole fungicides on polystyrene microplastics. Sci. Total Environ. 2019, 691, 1119–1126. [Google Scholar] [CrossRef]
- Xu, P.; Ge, W.; Chai, C.; Zhang, Y.; Jiang, T.; Xia, B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019, 145, 260–269. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, H.; Allgeier, A.; Zhou, Q.; Ouellet, J.D.; Crawford, S.E.; Luo, Y.; Yang, Y.; Shi, H.; Hollert, H. Marine microplastics bound dioxin-like chemicals: Model explanation and risk assessment. J. Hazard. Mater. 2019, 364, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Frias, J.G.L.; Micaelo, A.C.; Sobral, P. Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuar. Coast. Shelf Sci. 2013, 130, 62–69. [Google Scholar] [CrossRef]
- Van, A.; Rochman, C.M.; Flores, E.M.; Hill, K.L.; Vargas, E.; Vargas, S.A.; Hoh, E. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 2012, 86, 258–263. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. (China) 2020, 87, 272–280. [Google Scholar] [CrossRef]
- Johansen, M.P.; Cresswell, T.; Davis, J.; Howard, D.L.; Howell, N.R.; Prentice, E. Biofilm-enhanced adsorption of strong and weak cations onto different microplastic sample types: Use of spectroscopy, microscopy and radiotracer methods. Water Res. 2019, 158, 392–400. [Google Scholar] [CrossRef]
- Van Den Berg, M.; Birnbaum, L.; Bosveld, A.T.C.; Brunström, B.; Cook, P.; Feeley, M.; Giesy, J.P.; Hanberg, A.; Hasegawa, R.; Kennedy, S.W.; et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998, 106, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, R.; Qu, H.; Zuo, Y.; Yu, Z.; Hu, G.; Li, Z.; Chen, H.; Lin, B.; Wang, B.; et al. Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere 2020, 248, 126067. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Velázquez, A.; Subbiah, S.; Anderson, T.A.; Green, M.J.; Zhao, X.; Cañas-Carrell, J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020, 715, 136974. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Li, D.C.; Sima, X.F.; Cheng, H.Y.; Jiang, H. The effects of environmental conditions on the enrichment of antibiotics on microplastics in simulated natural water column. Environ. Res. 2018, 166, 377–383. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef]
- Gouin, T.; Roche, N.; Lohmann, R.; Hodges, G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Rainieri, S.; Rasmussen, R.R.; Kotterman, M.J.J.; Sloth, J.J.; Cederberg, T.L.; Barranco, A.; Marques, A.; Larsen, B.K. The influence of microplastics and halogenated contaminants in feed on toxicokinetics and gene expression in European seabass (Dicentrarchus labrax). Environ. Res. 2018, 164, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Adolfsson-Erici, M.; Jahnke, A.; MacLeod, M. No measurable “cleaning” of polychlorinated biphenyls from Rainbow Trout in a 9 week depuration study with dietary exposure to 40% polyethylene microspheres. Environ. Sci. Process. Impacts 2016, 18, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.I.; De Witte, B.; Vethaak, A.D.; Hostens, K.; Leslie, H.A. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Razanajatovo, R.M.; Jiang, H.; Zou, H.; Zhu, W. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 648, 1431–1439. [Google Scholar] [CrossRef]
- Qu, H.; Ma, R.; Wang, B.; Yang, J.; Duan, L.; Yu, G. Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. J. Hazard. Mater. 2019, 370, 203–211. [Google Scholar] [CrossRef]
- Beiras, R.; Muniategui-Lorenzo, S.; Rodil, R.; Tato, T.; Montes, R.; López-Ibáñez, S.; Concha-Graña, E.; Campoy-López, P.; Salgueiro-González, N.; Quintana, J.B. Polyethylene microplastics do not increase bioaccumulation or toxicity of nonylphenol and 4-MBC to marine zooplankton. Sci. Total Environ. 2019, 692, 1–9. [Google Scholar] [CrossRef]
- Sala, B.; Giménez, J.; de Stephanis, R.; Barceló, D.; Eljarrat, E. First determination of high levels of organophosphorus flame retardants and plasticizers in dolphins from Southern European waters. Environ. Res. 2019, 172, 289–295. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef]
- Wania, F.; Mackay, D. Tracking the distribution of persistent organic pollutants. Environ. Sci. Technol. 1996, 30, 390–396. [Google Scholar] [CrossRef]
- Khan, F.R.; Syberg, K.; Shashoua, Y.; Bury, N.R. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ. Pollut. 2015, 206, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Lewison, R.L.; Eriksen, M.; Allen, H.; Cook, A.M.; Teh, S.J. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014, 476, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Gassel, M.; Rochman, C.M. The complex issue of chemicals and microplastic pollution: A case study in North Pacific lanternfish. Environ. Pollut. 2019, 248, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef]
- Davarpanah, E.; Guilhermino, L. Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar. Coast. Shelf Sci. 2015, 167, 269–275. [Google Scholar] [CrossRef]
- Kim, D.; Chae, Y.; An, Y.J. Mixture Toxicity of Nickel and Microplastics with Different Functional Groups on Daphnia magna. Environ. Sci. Technol. 2017, 51, 12852–12858. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the presence of microplastics influence the acute toxicity of chromium(VI) to early juveniles of the common goby (Pomatoschistus microps)? A study with juveniles from two wild estuarine populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Oliveira, P.; Barboza, L.G.A.; Branco, V.; Figueiredo, N.; Carvalho, C.; Guilhermino, L. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): Filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicol. Environ. Saf. 2018, 164, 155–163. [Google Scholar] [CrossRef]
- Guven, O.; Bach, L.; Munk, P.; Dinh, K.V.; Mariani, P.; Nielsen, T.G. Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat. Toxicol. 2018, 198, 287–293. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Magara, G.; Elia, A.C.; Syberg, K.; Khan, F.R. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. J. Toxicol. Environ. Health Part A Curr. Issues 2018, 81, 761–773. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; D’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as vehicles of environmental PAHs to marine organisms: Combined chemical and physical hazards to the mediterranean mussels, Mytilus galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef]
- Kleinteich, J.; Seidensticker, S.; Marggrander, N.; Zarf, C. Microplastics reduce short-term effects of environmental contaminants. part II: Polyethylene particles decrease the effect of polycyclic aromatic hydrocarbons on microorganisms. Int. J. Environ. Res. Public Health 2018, 15, 287. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Gil, I. Polyethylene microplastics increase the toxicity of chlorpyrifos to the marine copepod Acartia tonsa. Environ. Pollut. 2020, 260. [Google Scholar] [CrossRef] [PubMed]
- Garrido, S.; Linares, M.; Campillo, J.A.; Albentosa, M. Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol. Environ. Saf. 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Yi, X.; Chi, T.; Li, Z.; Wang, J.; Yu, M.; Wu, M.; Zhou, H. Combined effect of polystyrene plastics and triphenyltin chloride on the green algae Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2019, 26, 15011–15018. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Effects of polyethylene microplastics on the acute toxicity of a synthetic pyrethroid to midge larvae (Chironomus tepperi) in synthetic and river water. Sci. Total Environ. 2019, 671, 971–975. [Google Scholar] [CrossRef]
- Zocchi, M.; Sommaruga, R. Microplastics modify the toxicity of glyphosate on Daphnia magna. Sci. Total Environ. 2019, 697, 1–7. [Google Scholar] [CrossRef]
- Horton, A.A.; Vijver, M.G.; Lahive, E.; Spurgeon, D.J.; Svendsen, C.; Heutink, R.; van Bodegom, P.M.; Baas, J. Acute toxicity of organic pesticides to Daphnia magna is unchanged by co-exposure to polystyrene microplastics. Ecotoxicol. Environ. Saf. 2018, 166, 26–34. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Syberg, K.; Nielsen, A.; Khan, F.R.; Banta, G.T.; Palmqvist, A.; Jepsen, P.M. Microplastic potentiates triclosan toxicity to the marine copepod Acartia tonsa (Dana). J. Toxicol. Environ. Health—Part A Curr. Issues 2017, 80, 1369–1371. [Google Scholar] [CrossRef]
- Prata, J.C.; Lavorante, B.R.; Maria da Conceição, B.S.M.; Guilhermino, L. Influence of microplastics on the toxicity of the pharmaceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquat. Toxicol. 2018, 197, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Han, Y.; Sun, S.; Tang, Y.; Zhou, W.; Du, X.; Liu, G. Immunotoxicities of microplastics and sertraline, alone and in combination, to a bivalve species: Size-dependent interaction and potential toxication mechanism. J. Hazard. Mater. 2020, 396, 122603. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Tato, T. Microplastics do not increase toxicity of a hydrophobic organic chemical to marine plankton. Mar. Pollut. Bull. 2019, 138, 58–62. [Google Scholar] [CrossRef]
- Li, Z.; Yi, X.; Zhou, H.; Chi, T.; Li, W.; Yang, K. Combined effect of polystyrene microplastics and dibutyl phthalate on the microalgae Chlorella pyrenoidosa. Environ. Pollut. 2020, 257. [Google Scholar] [CrossRef]
- Schrank, I.; Trotter, B.; Dummert, J.; Scholz-Böttcher, B.M.; Löder, M.G.J.; Laforsch, C. Effects of microplastic particles and leaching additive on the life history and morphology of Daphnia magna. Environ. Pollut. 2019, 255, 113233. [Google Scholar] [CrossRef]
- Koh, E.J.; Hwang, S.Y. Multi-omics approaches for understanding environmental exposure and human health. Mol. Cell. Toxicol. 2019, 15, 1–7. [Google Scholar] [CrossRef]

| MP Type | MP Size | Chemical Sorbate | Exposure Concentrations * | Exposure Time | Organism | Toxicological Assessment | Highlight Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| PS | Average diameter: 201.5 nm | Nickel | Ni alone [Ni] = 1, 2, 3, 4, and 5 mg/L MPs alone [MPs] = 1,5,10,20,30 mg/L Variable Ni-Fixed MPs [Ni] = 1, 2, 3, 4, and 5 mg/L [MPs]= 5 mg/L Fixed Ni-Variable MPs [Ni] = 3 mg/L [MPs] = 1,5,10,20,30 mg/L Variable Ni-Variable MPs 1 mg/L Ni-1 mg/L MP 2 mg/L Ni-5 mg/ L MP 5 mg/L Ni-30 mg/L MP | 48 h | Daphnia magna | Rate of abnormalities and changes in the morphology Rate of immobilization | Enhanced toxicity of Ni in combination with both MPs Higher immobilization effect for Ni - PS-COOH exposure Ni showed an antagonistic effect on toxicity with PS and synergistic with PS-COOH Results may indicate that the toxic effects of MPs and Ni vary depending of the properties of both pollutants | [120] |
| PS-COOH | Average diameter: 191.3 nm | |||||||
| PE | 1–5 µm | Chromium (VI) | Cr (VI) alone [Cr (VI)] = 0, 5.6, 8.4, 12.6, 18.9 and 28.4 mg/L MPs alone [MPs]= 0.184 mg/L MPs + Cr (VI) Co-exposure performed using the same concentrations of single treatments | 96 h | Early juveniles of the common goby fish (Pomatoschistus microps) | Post-predatory performance assay The activities of AChE, GST, EROD activities LPO levels | Significant decrease of the predatory performance and significant inhibition of AChE activity under simultaneous exposure Long-term exposure to different environmental conditions in developmental phases influences the response of early juveniles | [121] |
| PS | 32–40 µm | Cadmium | Cd alone [Cd]= 0 and 50 µg/L MPs alone [MPs]= 0, 50, 500 µg/L MPs + Cd 3 × 2 array configuration (MPs previously preloaded with Cd for 24 h before the exposure experiments) | 30 days | Early juveniles of discus fish (Symphysodon aequifasciatus) | Survival rate Body length The activities of SOD, CAT, GPx, LZM, ACP and ALP The level of GSH and C3 The concentrations of MDA and PC | The MP + Cd mixture induced severe oxidative damage as well as the stimulation of the immune system Co-exposure stimulate the innate immune responses of early juveniles | [122] |
| PS | 5 µm | Cadmium | Cd alone [Cd]= 10 µg/L MPs + Cd 10 µg/L Cd-20 µg/L MPs 10 µg/L Cd-200 µg/L MPs (MPs incubated during 96h before the exposure experiments) | 3 weeks | Zebrafish (Danio rerio) | Histological analysis (liver, gut and gills) GSH and MT levels SOD activity mRNA levels of 8 target genes in zebrafish tissues | Enhanced toxicity of Cd in combination with MPs Oxidative stress and early inflammatory responses observed in the mixture treatments Important changes in the gene expression observed for all co-exposure treatments | [123] |
| unknown | 1–5 µm | Mercury | Hg alone [Hg]= 0.010 and 0.016 mg/L MPs alone [MPs]= 0.26 and 0.69 mg/L MPs + Hg 4 binary mixtures using the same concentrations of single exposures | 96 h | Juvenile European seabass (Dicentrarchus labrax) | AChE, ChE, IDH and LDH activities LPO levels | A significant interaction between MPs and Hg was achieved Biomarkers’ variation was highly influenced by the concentration of MPs | [124] |
| unknown | 1–5 µm | Mercury | Hg alone [Hg] = 30 µg/L MPs alone [MPs] = 0.13 mg/L MPs + Hg Co-exposure performed using the same concentrations of single treatments | 8 days (+ 6 days in clean medium) | Freshwater bivalve (Corbicula fluminea) | The post-exposure filtration rate ChE, IDH, GST, GSR, GPx, ODH and CAT activities LPO levels | Antagonistic behaviour between MPs and Hg in several biomarkers Six days of post-exposure recovery in the clean medium was not enough to reverse the toxic effects induced by both pollutants | [125] |
| PE | 10–45 µm | Mercury | Hg alone [Hg] = 10 µg/L MPs alone [MPs] = 25 µg/L MPs + Hg Co-exposure and incubation treatments performed using the same concentrations of single treatments (incubation for 96h) | 7 days | Manila clam (Ruditapes philippinarum) | Histological analysis (gill and digestive gland) Filtration rates Immunomodulation Oxidative stress | The filtration rates decreased as a result of the co-exposure A higher decrease in haemocyte viability was detected in co-exposure treatments Antioxidant parameters remain unchanged in the mixture in comparison to single treatments | [7] |
| PE | 1–5 µm | Copper | Cu alone [Cu]= 0.02, 0.04, 0.08, 0.16, 0.32 and 0.64 mg/L MPs alone [MPs] = 0.046, 0.092, 0.184, 0.368, 0.736 and 1.472 mg/L MPs + Cu 6 binary mixtures using the same concentrations of Cu combined with 0.184 mg/L of MPs | 96 h | Marine microalgae (Tetraselmis chuii) | The average specific growth rate and the percentage of growth inhibition | No significant differences were observed between treatments with and without MPs MPs did not influence the Cu toxicity | [119] |
| Virgin PVC | D50: 139 µm | Copper | Cu alone [Cu]= 0, 0.2, 0.5 and 1 mg/L MPs alone (virgin and aged) [MPs] = 10, 100 and 1000 mg/L MPs + Cu 0.5 mg/L Cu-10 mg/L aged MPs | 10 days | Microalgae (Chlorella vulgaris) | The growth inhibition ratio (IR) and biomass productivity The enzymatic activities of SOD and MDA | Mixture exposure enhances the cell growth in comparison to single treatments The ageing of MPs poses stronger inhibitory effects in microalgae than virgin pellets | [92] |
| Aged PVC | D50:132 µm | |||||||
| PS | 0.1 µm 20 µm | Copper | Cu alone [Cu] = 50 µg/L MPs alone [MPs] = 200 µg/L MPs + Cu Combination of concentrations used in single treatments | 14 days | Zebrafish (Danio rerio) | SOD, MDA and MT levels Transcriptomic analysis | Synergetic effects in co-exposure treatments of small MPs were observed The presence of MPs and DOM aggravates the Cu-toxicity | [102] |
| MP Type | MP Size | Chemical Sorbate | Exposure Concentrations | Exposure Time | Organism | Toxicological Assessment | Highlight Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| PS-divinilbenzene | 97 μm | Pyrene | Pyrene alone [Pyrene] = 0.1 µM MPs alone [MPs] = 100 particles/L MPs + Pyrene 100 nM pyrene + 100 particles/L MPs | 24 h | Tropical fish juveniles (Lates calcarifer) | Mortality rate Juveniles behaviour Predatory performance Size differences | Individuals exposed to both pollutants were the most affected group, but the negative impact was relatively small | [126] |
| PE | < 100 µm | Pyrene | MPs alone [MPs]= 20 g/L MPs + pyrene Before the experiment, a solution of PE or PS were incubated with pyrene (50 µg/L) for 6 days | 7 days | Marine mussel (Mytilus galloprovincialis) | Histological analysis (gills and digestive glands) Gene transcription analyses Genotoxic effects Immunological alterations Neurotoxic responses Oxidative stress Antioxidant defences | Clear separation between control and MPs exposed mussels Biological variations were influenced by the typology of polymer (PE vs PS) Only genotoxic responses separated virgin from pyrene- contaminated polymers | [107] |
| PS | ||||||||
| PE | 1–5 µm | Pyrene | Pyrene alone [Pyrene]= 20 and 200 µg/L MPs alone [MPs]= 0, 18.4 and 184 µg/L MPs + Pyrene 0 µg/L pyrene-18.4 µg/L MPs 200 µg/L pyrene-184 µg/L MPs 200 µg/L pyrene-184 µg/L MPs | 96 h | Juveniles of the common Goby (Pomatoschitus microps) | Protein content AChE, IDH, GST activities - LPO levels Bile samples were analysed for pyrene metabolites | The presence of MPs was found to delay the pyrene-induced mortality Enhanced concentration of pyrene-metabolites was detected in co-exposure treatments Results suggest toxicologically relevant interactions between both pollutants | [130] |
| PE | 10–90 µm | Fluoranthene (Flu.) | Flu. alone [Flu]= 100 µg/L MPs alone [MPs]= 1000 particles/mL MPs + Flu. Flu – PE/PHB co-exposure or incubation at the same concentrations tested in single exposures (incubation during overnight) | 96 h | Blue Mussel (Mytilus edulis) | Protein content in the cytosol The cytosolic concentration of GSH SOD, CAT, GPx and SeGPx activities | In co-exposure and incubation treatments, biochemical responses were generally comparable with those exerted MPs only Apparent absence of combined effects of MPs with the pollutant. | [5] |
| PHB | 10–90 µm | |||||||
| PE | 10–90 µm | Fluoranthene (Flu.) | Flu. alone [Flu]= 50 and 100 µg/L MPs alone [MPs]= 100 and 1000 particles/mL MPs+ Flu. 50 µg/L Flu.-100 particle/mL 100 µg/L Flu.-1000 particle/mL (For both mixtures, co-exposure and incubation experiments (incubation during overnight)) | 96 h | Blue mussel (Mytilus edulis) | Total GSH + 2GSSG levels SOD, CAT, GPx and SeGPx activities | No synergistic or antagonistic effect was seen in the co-exposure or the incubation experiments | [131] |
| PS | Mix of 2 and 6 µm | Fluoranthene (Flu.) | Flu. alone [Flu]= 30 µg/L day MPs alone [MPs]= 32 mg/L day MPs + Flu. 30 µg/L day Flu.-32 mg/L day PS | 7 days (+ 7 days of depuration) | Marine mussel (Mytilus spp.) | Morphological and functional analyses of hemocytes Hemocyte mortality Circulating hemocytes concentration Phagocytosis activity Histopathological assessment (digestive tract and intestine) ROS production Levels of LPO SOD, CAT, GR and GST activities Gene expression analysis | Increase in the total histopathological lesions/ abnormalities was demonstrated in co-exposure treatments After depuration, a higher fluoranthene concentration was detected in mussels exposed to the mixture of MPs and Flu Results suggested that MPs led to modulated fluoranthene kinetics and toxicity in marine mussels. | [132] |
| PS | 500 nm 30 µm | Benzo[a]pyrene (B[a]P) 17β-estradiol (E2) | B[a]P alone [B[a] P] = 5 and 50 mg/L E2 alone [E2] = 0.1 and 1 mg/L MPs alone [MPs]= 1 mg/L MPs + Pollutant Combination of individual concentrations of MPs of both sizes and the organic contaminants | 4 days | Bivalve specie (Tegillarca granosa) | Analysis of total counts, cell-type composition, and phagocytic activity of haemocytes ROS and Ca2+ concentration from haemocytes LZM content and activity Gene expression of three major types of genes | POPs toxicity was aggravated by smaller MPs and mitigated by larger MPs The deleterious impacts of B[a]P or E2 were mitigated by the presence of larger sized MPs and aggravated smaller ones | [8] |
| LD-PE | 20–25 µm | Benzo(a)pyrene (B[a]P) | B[a]P alone [B[a] P] = 150 µg/L MPs alone [MPs]= 10 mg/L MPs+ B[a]P 15 µg/g B[a]P-10 mg/L MPs (To reach this B[a]P sorbed concentration, 2 days of incubation was performed) | 7, 14 and 28 days | Marine mussel (Mytilus galloprovincialis) | Immunological alterations of hemocytes Neurotoxic responses in hemocytes and gills Oxidative stress Antioxidant defences, Genotoxic effects Transcriptional responses | The overall evaluation provided a clear separation between times and typologies of exposure Significant alterations measured on the immune system Results suggested that the toxicological risk of MPs for marine organisms is probably low, but not negligible | [133] |
| PE | 212–250 µm | Phenanthrene (Phe.) Anthracene | Phe. alone [Phe.] = 0.12 µM Anthracene alone [Anthracene] = 0.14 µM MPs alone [MPs]= 0.02 and 0.2 g/g sediment MPs + Phe. / Anthracene Lower dose of PE combined with pollutants preloaded for 96h | 2 weeks | Bacterial community of sediments | Gene expression assessment | The presence of MP reduced the effect of the two PAHs on microbial community composition and the degradation of these organic compounds | [134] |
| LD-PE non-uniformly shaped | < 60 µm | Phenanthrene (Phe.) | Phe. alone [Phe.] = 10 and 100 µg/L MPs alone [MPs]= 50 and 500 µg/L MPs + Phe. Combination of individual concentrations of MPs the organic contaminant | 96 h | African catfish (Clarias gariepinus) | Histopathological analysis (liver and gill) Glycogen stores of the liver Biomarkers responses of AST, ALT, LDH, ALP, γGT Contents of total protein, total albumin, lipase, glucose, lactate, direct bilirubin, HDL, LDL, TG and cholesterol Gene expression analysis | Changes in biomarker responses of co-exposure treatment might be due to the facilitated transportation of Phe into the fish body Findings suggested toxicologically relevant interactions between MPs and Phe | [135] |
| PE | 50 nm 500nm 5 µm 10 µm 15 µm | Phenanthrene (Phe.) | Phe. alone [Phe.] = 0, 0.05, 0.1, 0.2, 0.4, 0.8 and 1.2 mg/L MPs alone [MPs]= 0, 2.5, 5, 10 and 50 mg/L NPs alone [NPs]= 0, 2.5, 5, 8.5, 11 and 14.5 mg/L MPs/NPs + Phe. Combination of the individual concentrations tested for both pollutants | 48 h | Daphnia magna | Immobilization rate of the daphnids | Enhanced immobilization of daphnia was observed in co-exposure treatments (especially for NPs) The presence of NPs inhibited the dissipation of phenanthrene of the environment | [61] |
| LD-PE | 125–250 µm | α-HBCD 2,4,6-tribromophenol PBDEs mix (PBDE 47, 99, 153, 154) PCB congeners (28, 52, 101, 118, 138, 153, 180) methyl mercury PFOS PFOA PFOSA PFNA | Feed A: Basic feed (control) Feed B: Basic feed + contaminants sorbed to MPs before the incorporation of 2% into pellets (incubation for overnight) Feed C: Basic feed + contaminants without MPs Feed D: Basic feed + contaminants and clean MPs | 80 days (+ 51 days of depuration) | European seabass (Dicentrarchus labrax) | Growth factors Feeding rates Gene expression analysis | Results indicated that MPs inhibit or induce detoxification in the liver and influence the lipid distribution Gene expression results also indicated that MPs might indeed potentiate the adverse effect of some chemical contaminants | [105] |
| LD-PE | 125–250 µm | Methylmercury Perfluoroctanesulfonate Perfluorooctanoat PFOSA PFNA α-HBCD 2,4,6-Tribromphenol PBDE 47 PBDE 99 PBDE 153 PBDE 154 PCB 28 PCB 52 PCB 101 PCB 118 PCB 138 PCB 153 PCB 180 | Feed A: Basic feed Feed B: Basic feed + 4% of clean MPs Feed C: Basic feed + 2% of MPs with sorbed POPs Feed D: Basic feed + POPs | 3 weeks | Zebrafish (Danio rerio) | Visual observation (microscopic level and Hispathological analysis) Evaluation of differential gene expression of some selected biomarkers | Feed C produced the most evident effects, especially on the liver Combined effects of MPs and chemicals significantly altered the homeostasis in greater manner respect both pollutants alone | [136] |
| LD-PE | marine exposition: 3 mm feed exposition: 0.5 mm | PAHs, PCBs and PBDEs congeners | Feed A: Basic feed Feed B: Basic feed + virgin LD-PE Feed C: Basic feed + marine-plastic treatment (LDPE deployed in San Diego Bay for 3 months) | 2 months | Japanese medaka (Oryzias latipes) | Histopathological analysis (gonads) Gene expression analysis on selected liver’s genes and biomarkers for endocrine disruption | Results show early- warning signs of endocrine disruption in fish exposed to a mixture of plastic and sorbed contaminants | [127] |
| LD-PE | marine exposition: 3 mm feed exposition: 0.5 mm | PAHs, PCBs and PBDEs congeners | Feed A: Basic feed Feed B: Basic feed + virgin LD-PE Feed C: Basic feed + marine-plastic treatment (LDPE deployed in San Diego Bay for 3 months) | 2 months | Japanese medaka (Oryzias latipes) | Histopathological analysis (gonads) Gene expression analysis on selected liver’s genes and biomarkers for endocrine disruption | Hepatic stress in medaka exposed to the combination of plastic and sorbed contaminants was demonstrated No significant differences in the expression of CYP1A were found between treatments | [31] |
| MP Type | MP Size | Chemical Sorbate | Exposure Concentrations | Exposure Time | Organism | Toxicological Assessment | Highlight Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pesticides | ||||||||
| HD-PE with irregular shape | mean size: 7.73 µm | Chlorpyrifos (CPF) | CPF alone [CPF] = 0, 0.1, 1, 10, 100 µg/L MPs alone [MPs]= 0, 0.1, 1, 10, 100 µg/L MPs + CPF 100 µg/L CPF-100 µg/L MPs co-exposure and incubation treatments (incubated for 2 h) | 48 h | Marine copepod (Acartia tonsa) | The survival rates Fecundity, feeding and egg viability | CPF showed higher toxicity when combined with MP than alone for all tested biological responses Higher toxicity was observed with the co-exposure treatment | [137] |
| PE | mean size: ranging from 2–6 µm maximum particle size: 22 µm | Chlorpyrifos | CPF alone [CPF] = 0 to 4 mg/L MPs alone [MPs]= 0.5, 1, 10 and 25 mg/L MPs + CPF (co-exposure) 0–3 mg/L CPF-1 mg/L MPs co-exposure and incubation treatments (incubated for 2 h) | 72 h | Microalgae (Isochrysis galbana clone T-ISO) | Microalgae daily growth rate Inhibition of microalgae growth | MPs reduced the toxicity of CPF MPs were not small enough to penetrate the microalgal cell and cause any damage | [138] |
| PS | 0.1 mm, 0.55 mm 5 mm | Triphenyltin chloride (TPTCl) | TPTCl alone [TPTCl] = 30 μg/L MPs alone [MPs]= 0.05, 0.5, 5 mg/L MPs + TPTCl Combination of the individual concentrations tested for both pollutants | 96 h | Microalgae (Chlorella pyrenoidosa) | Morphology and structural damage Grown inhibition | PS particles toxicity to the green algae was size-dependent Toxicity of the mixture was size-dependent: MPs with smaller particle size increased the toxicity of TPTCl | [139] |
| Pristine PE | 10–27 µm | Bifenthrin | Bifenthrin alone [Bifenthrin] = 0.1 to 3.2 µg/L MPs alone [MPs]= 5 mg/L MPs+ Bifentrin (co-exposure) 0.1–3.2 µg/L CPF-5 mg/L MPs | 48 h | Freshwater larvae organism (Chironomus tepperi) | Immobilization rates | The addition of MPs to synthetic water reduced the toxicity of bifenthrin The addition of MPs to river water did not mitigate bifenthrin toxicity due to the greater interaction of bifenthrin with DOM | [140] |
| PET/PA fibers | length: 10 µm width: 2 µm | Three different glyphosate chemical formulations | Glyphosate alone [Glyphosate] = 2.5 mg/L PE alone [MPs]= 0.01 mg/mL Fibers alone [MPs]= 0.045–0.136 µg/L MPs + glyphosate Single treatments were combined | 1 week | Daphnia magna | Mortality rate | The toxicity of the mixture was more influenced by the type and size of the MPs than their abundance Toxicity of glyphosate was enhanced by the presence of MPs | [141] |
| PE | 1–10 µm | |||||||
| PS | 1 µm | Dimetholate Deltamethrin | Pesticides alone [Dimetholate] = 0.156,0.313, 0.625, 1.25 and 5 mg/L [Deltamethrin]: 0.016, 0.08, 0.4, 2,5,10 µg/L MPs alone [MPs]= 300,000 particles/mL MPs + glyphosate Single treatments were combined | 72 h | Daphnia magna | Mortality rate Impaired mobility | The concentrations at which detrimental effects occurred were not influenced by the presence of MPs | [142] |
| Pharmaceuticals | ||||||||
| PE | 1–5 µm | Cefalexin | Cefalexin alone [Cefalexin]= 1.3, 2.5, 5 and 10 mg/L MPs alone [MPs]= 0.184 mg/L MPs + Cefalexin Combination of individual exposure concentrations (Exposure experiments performed at 20 and 25 °C) | 96 h | Common goby juveniles (Pomatoschistus microps) | Mortality rate Post-predatory performance AChE activity LPO levels | The temperature rise increased the toxicity for both pollutants alone and in MPs mix No significant differences between cefalexin treatment alone and in MPs mix | [143] |
| PE | 10–90 µm | Triclosan | Triclosan alone [Triclosan] = 0–300 µg/L MPs alone [MPs]= 0–25,000 MPs/mL MPs+ Cefalexin Combination of individual exposure concentrations of Triclosan and 500 MPs/mL | 48 h | Marine copepod (Acartia tonsa) | Mortality of marine copepods | The LC50-values of individual pollutants and mixture were significantly different (synergistic effect) | [144] |
| PS | 1 µm 10 µm | Roxithromycin (ROX) | ROX alone [ROX] = 0.1, 1, 5, 10, 50, 100, and 150 mg/L MPs alone [MPs] = 0.005, 0.05, 0.1, 0.2, 2, 15, 20, 25, 30, 35, and 40 mg/L MPs + ROX Mix 1: 0.1 mg/L 1-μm PS + 0.01 mg/L ROX Mix 2: 0.1 mg/L 10-μm PS + 0.01 mg/L ROX. | 48 h | Daphnia magna | Mortality rate MDA levels Activities of: SOD, CAT, GST and GPx | Small-size PS was more toxic to D. magna than the large-size PS Co-exposure to 1-μm PS and ROX led to the strongest biological responses in D. magna | [6] |
| PS | 0.1 µm | Roxithromycin (ROX) | Roxithromycin alone [ROX] = 50 µg/L MPs + ROX Mix 1: 1 µg/L MPs+50 µg/L ROX Mix 2: 10 µg/L MPs + 50 µg/L ROX. Mix 3: 100 µg/L MPs + 50 µg/L ROX. | 14 days | Water fish red tilapia (Oreochromis niloticus) | Histopathological analysis (liver, gills, guts and brain) AChE, EROD, BFCOD, SOD and MDA activities | The neurotoxicity caused by ROX was alleviated due to the presence of MPs The presence of MPs may affect the metabolism of ROX in tilapia Oxidative damage in situations of co-exposure to MPs and ROX was mitigated in fish livers This study suggests that the effects of MPs combined with other pollutants cannot be ignored | [109] |
| unknown | 1–5 µm | Florfenicol | Florfenicol alone [Florfenicol] = 1.8 and 7.1 mg/L MPs alone [MPs] = 0.2 and 0.7 mg/L MPs + Florfenicol Combination of individual exposure concentrations of both pollutants | 96 h | Marine bivalve (Corbicula fluminea) | Feeding inhibition Histopathological alterations (digestive system and gills) Enzymatic activities of ChE, IDH, ODH, GST, GR, GPx and CATLPO levels | Enhanced toxicity of florfenicol in combination with MPs Differences in the toxicological effects induced by mixtures containing the lowest or the highest concentrations of both substances | [48] |
| PVC | < 10 µm | Venlafaxine O-desmethylvenlafaxine | Venlafaxine and derivate alone [Venlafaxine] = 0–500 µg/L O-desmethylvenlafaxine alone [O-desmethylvenlafaxine] = 0–500 µg/L MPs + chemicals Combination of individual exposure concentrations of both pollutants and 50 mg/L of MPs | 4 days | Loach (Misgurnus anguillicaudatus) | SOD and MDA activities | In liver subcellular structure, MPs may help to transport pollutants into subtle areas and postpone the contaminants metabolism Mixtures enhance the oxidative stress in loach Enantioselective effects were observed in high dose exposure groups MPs combined with chemicals might cause more adverse effects to organisms compared with only chemicals themselves. | [110] |
| unknown | 1–5 µm | Procainamide Doxycycline | Procainamide alone [Procainamide] = 4, 8, 16, 32, 64, 128 and 256 mg/l Doxycycline alone [Doxicycline] = 4, 8, 16, 32, 64 and 128 mg/l MPs alone [MPs] = 0.75, 1.5, 3, 6, 12, 24 and 48 mg/l MPs + chemicals Combination of individual exposure concentrations of both chemicals and 1.5 mg/L of MPs | 96 h | Marine microalga (Tetraselmis chuii) | Inhibition of average specific grow per day Chlorophyll concentration decrease | Significant toxicity enhancement of each pharmaceutical in mixture with MPs was found for procainamide (chlorophyll), and doxycycline (both parameters) | [145] |
| PS | 30 µm 500 nm | Sertraline (Ser) | Ser. alone [Ser]=100ng/L MPs alone [MPs]= 0.29 mg/L MPs+ Ser. Combination of individual exposure concentrations of both pollutants | 14 days | Bivalve mollusk (Tegillarca granosa) | ROS generation Apoptosis status MDA, ACh and GABA levels Plama cortisol content ATP content and PK activity Transcriptomic analysis | Evident synergistic immuno-toxic effect was observed between Ser. and NPs NPs could exert more toxic effects than larger MPs | [146] |
| Others (UV Filters, Surfactants, Plasticizers, …) | ||||||||
| PE irregular shape | 3.4 µm 9.9 µm | 4-Nonylphenol (4-NP) 4- MBC | 4-NP alone [4-NP] = 4, 25 and 70 µg/l 4-Nonyphenol alone [4-MBC] = 70, 150 and 350 µg/l MPs + chemicals Combination of individual exposure concentrations of both chemicals with 1 and 10 mg/L of MPs | 48 h | Marine zooplanktons | Effective concentration reducing the larval size Mortality rate | The presence of MPs did not increase the toxicity of both chemicals tested | [111] |
| PE irregular shape | 3.4 µm 9.9 µm | 4-Nonylphenol (4-NP) | 4-NP alone [4-NP] = 20 and 60 µg/l MPs+ 4-NP Combination of individual exposure concentrations of 4-NP with 1 and 10 mg/L of MPs | 48 h | Planktonic sea-urchin larvae | Filtering rate Effective concentration reducing larval growth | The ingestion of MPs did not increase the toxicity of 4-NP | [147] |
| PE | 50 nm | BPA | BPA alone [BPA] = 0.78 and 1 µg/l NPs alone [NPs]= 1 mg/l NPs+ BPA 1 µg/L BPA + 1 mg/L NPs | 3 days | Zebrafish (Danio rerio) | Gene expression analysis AChE activity Dopamine level Protein content | The co-exposure of NPs and BPA led to increased neurotoxic effects in both CNS and dopaminergic system The reduction of the AChE activity in co-exposure treatment was alleviated in comparison to single experiments | [113] |
| PS | 0.1 mm 0.55 mm 5 mm | Dibutyl phthalate (DBP) | DBP alone [DBP] = 0.25, 0.5, 1, 2, 4, 8 and 16 mg/l MPs alone [MPs]= 0.5, 1, 2, 4, 8, 16, 32 and 64 mg/l MPs + BPA Combination of individual exposure concentrations of both pollutants (MPs size: 0.1 mm) | 96 h | Microalgae (Chlorella pyrenoidosa) | Grow inhibition rate Changes in morphology and structural damage Chlorophyll levels | The interaction between MPs and DBP was antagonistic at low concentrations of DBP Synergistic effect was found at relatively high concentrations of DBP when [MPs]< 10mg/L Antagonistic effect was found across all concentrations of MPs above 10 mg/L | [148] |
| Rigid PVC | 4–141 µm | Diisononylphthalate (DiNP) | Rigid PVC (PVC) 4320 MP particles/100mL Flexible PVC with DiNP 4320 particles/100 mL ([DiNP] in PVC was 30% of plastic weight) | 25–31 days | Daphnia magna | Mortality rate Morphology changes and body length Reproductive output | MPs containing DiNP significantly affect the number of offspring as well as the growth of D. magna The relevance of long-term chronic exposure experiments, as effects did emerge relatively late in the experiment | [149] |
| Flexible PVC | 12–276 µm | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. https://doi.org/10.3390/toxics8020040
Menéndez-Pedriza A, Jaumot J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics. 2020; 8(2):40. https://doi.org/10.3390/toxics8020040
Chicago/Turabian StyleMenéndez-Pedriza, Albert, and Joaquim Jaumot. 2020. "Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects" Toxics 8, no. 2: 40. https://doi.org/10.3390/toxics8020040
APA StyleMenéndez-Pedriza, A., & Jaumot, J. (2020). Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics, 8(2), 40. https://doi.org/10.3390/toxics8020040






