Effect of Exposure of Plastic Infant Feeding Bottle Leached Water on Biochemical, Morphological and Oxidative Stress Parameters in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
- In Group 1 (control group), the rats were provided with food and RO-filtered tap water ad libitum.
- In Group 2 (BPA treated), BPA dissolved in olive oil (20 µg/kg bodyweight/day) was fed to the rats, orally, by gavage feeding.
- In Group 3 (plastic leached water treated), water leached from plastic infant bottles as mentioned under section “Drugs and Solutions” was provided to the rats. The rats consumed plastic leached water instead of RO-filtered tap water throughout the period of treatment. Each rat drank 30–35 mL of this leached water per day.
2.2. Drugs and Solutions
- The infant feeding bottles used in the present study were made of plastic. These bottles were purchased from a local shop. The information regarding the type of plastic used in these bottles was not mentioned.
- Initially, the plastic infant bottles were cleansed with detergent and washed under tap water.
- These bottles were soaked in hot water for sterilization.
- Thereafter, the bottles were allowed to dry.
- The dried bottles were filled with water, which was previously boiled to 100 °C in a separate glass container. The water was boiled to 100 °C, as in real practice, water/milk is initially boiled by people for sterilization and, thereafter, it was allowed to cool so that it can be administered to infants, where it attains a lukewarm temperature.
- These filled infant bottles were kept in a water bath with the temperature maintained at 40 °C for an hour, so that lukewarm temperature was maintained.
- This water was given to the rats of Group 3.
- The leached water thus prepared was subjected to HPLC analysis to determine the concentration of BPA.
2.3. HPLC Analysis
2.4. Histological Examination and Biochemical Parameter
2.5. Oxidative Stress Markers
2.6. Statistical Analysis
3. Results
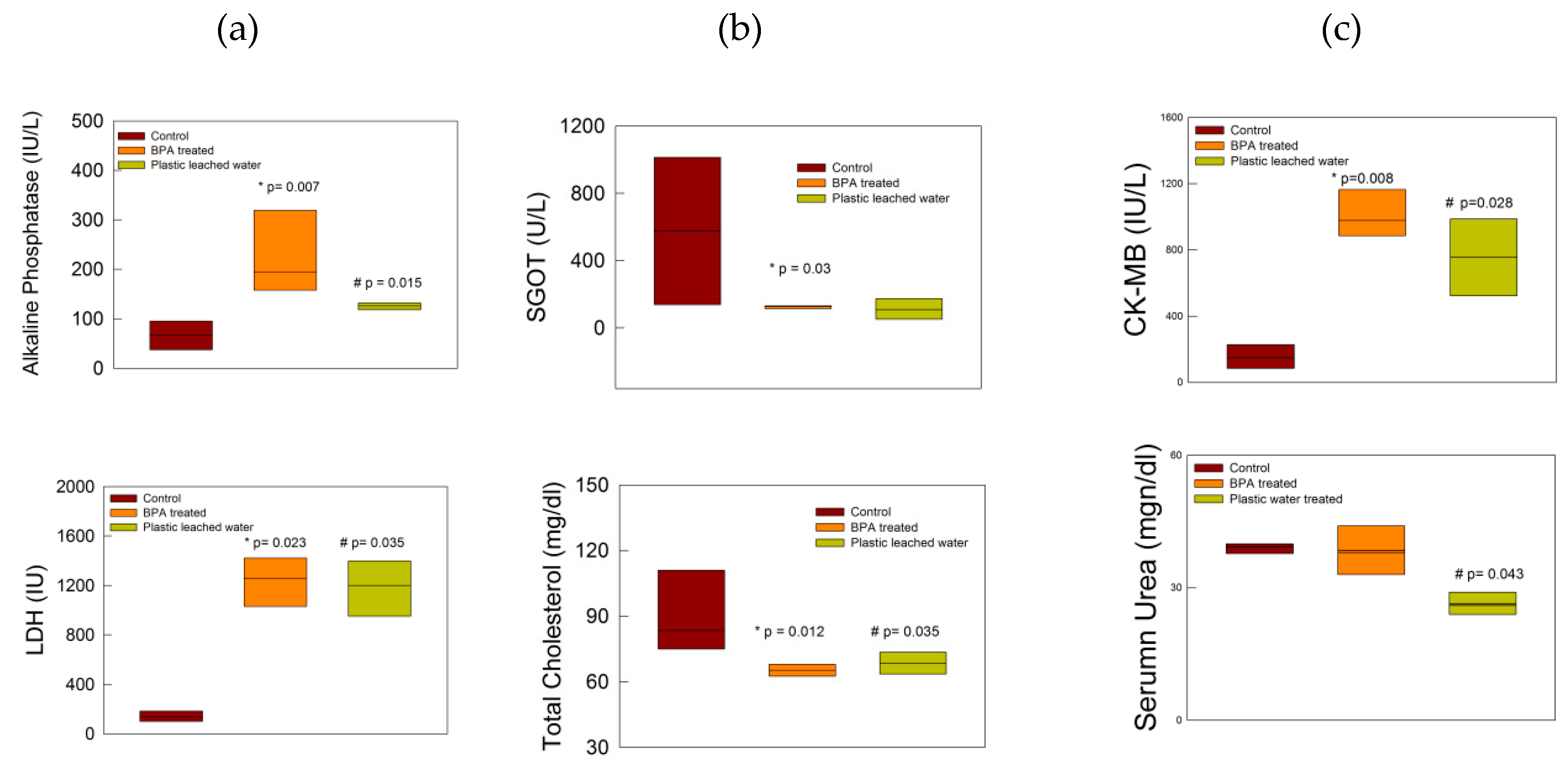
3.1. Biochemical Changes
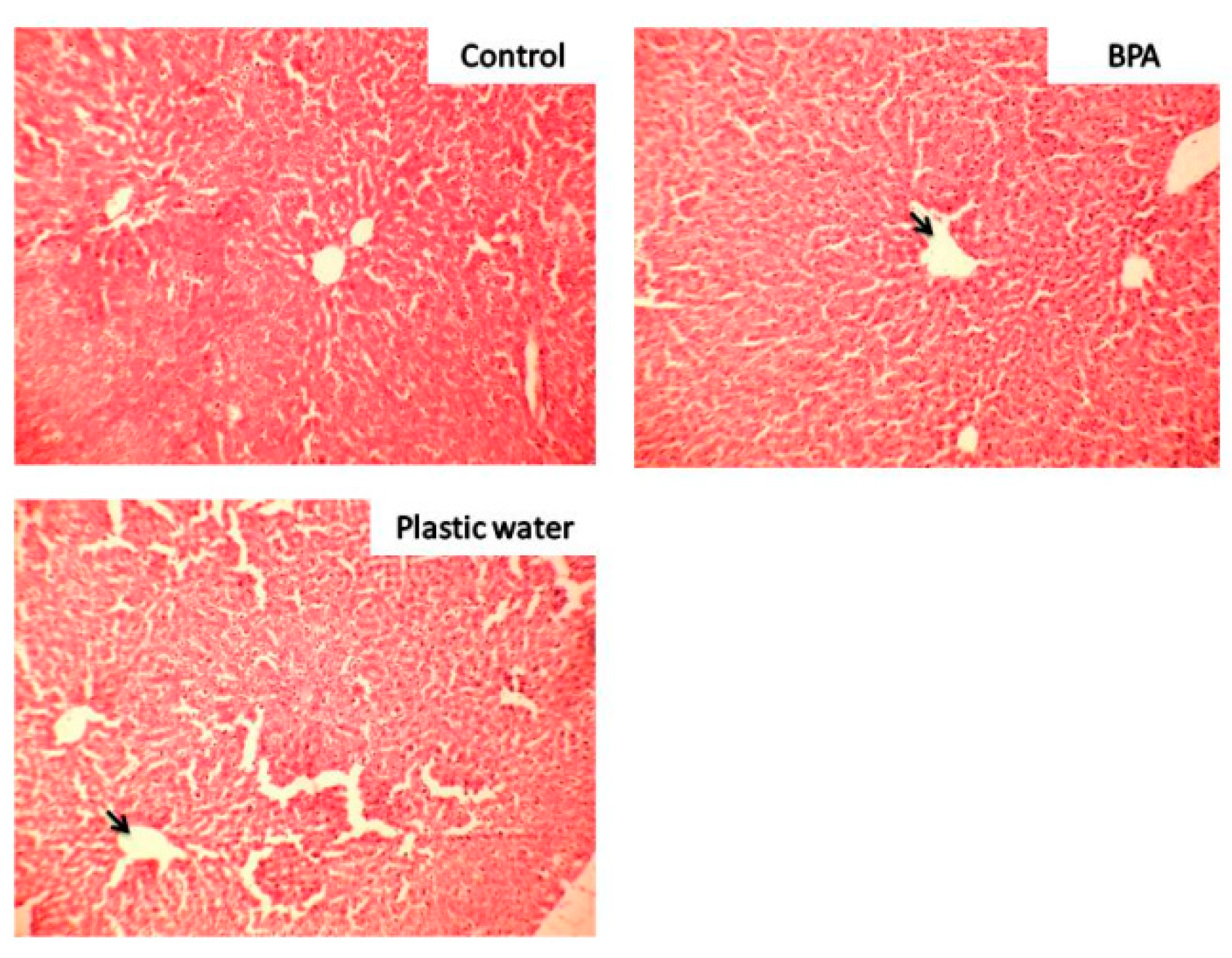
3.2. Cytoarchitectural Changes
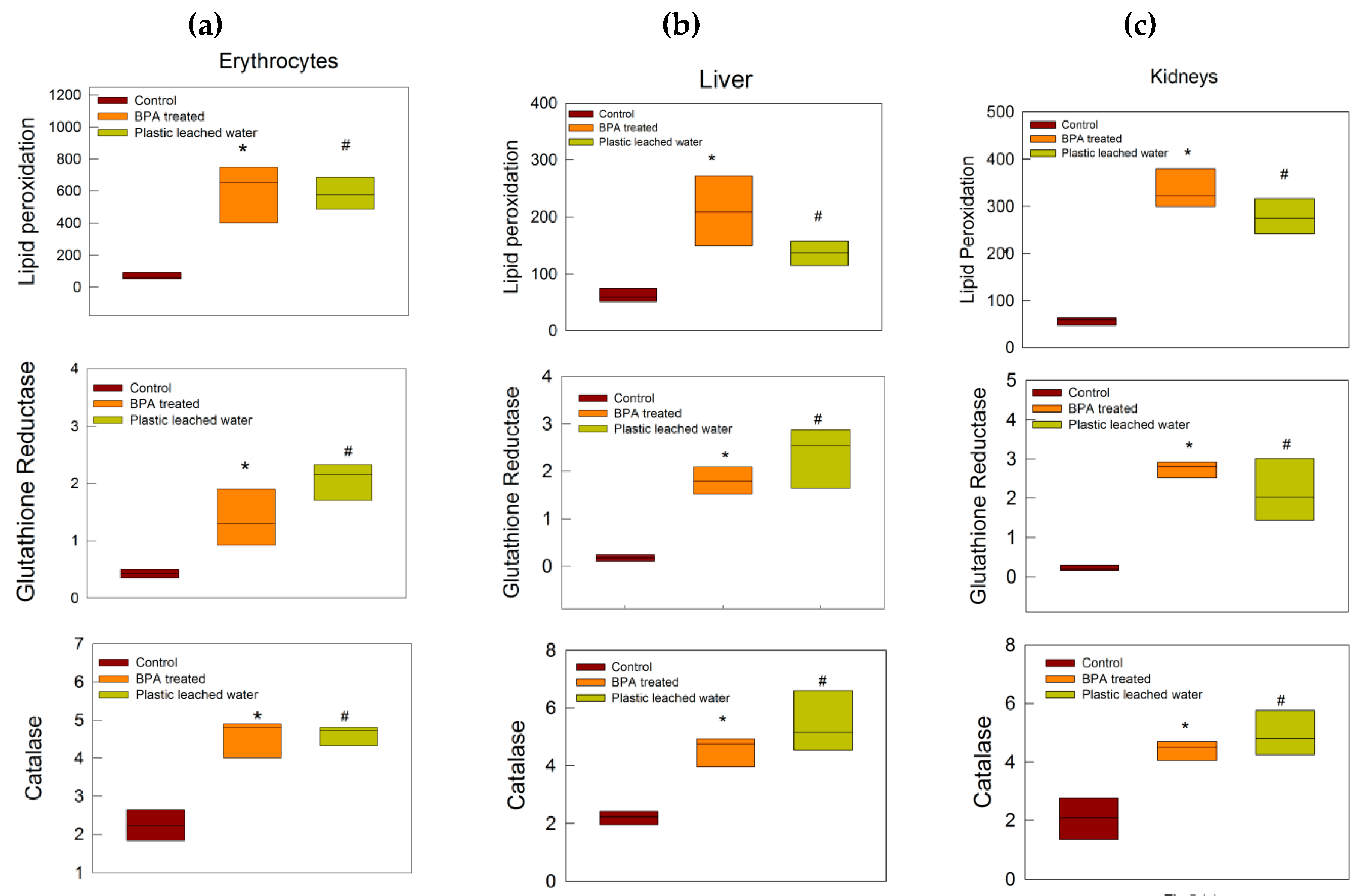
3.3. Oxidative Stress Tests
3.4. BPA Estimation in Plastic Leached Water by HPLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, C.; Kannan, K. High levels of Bisphenol A in paper currencies from several countries, and implications for dermal exposure. Environ. Sci. Technol. 2011, 45, 6761–6768. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and their Implications for Human Exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 319–329. [Google Scholar] [CrossRef]
- Brede, C.; Fjeldal, P.; Skjevrak, I.; Herikstad, H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003, 20, 684–689. [Google Scholar] [CrossRef]
- Kataria, A.; Levine, D.; Wertenteil, S.; Vento, S.; Xue, J.; Rajendiran, K.; Kannan, K.; Thurman, J.M.; Morrison, D.; Brody, R.; et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatric Res. 2017, 81, 857–864. [Google Scholar] [CrossRef]
- Mendonca, K.; Hauser, R.; Calafat, A.M.; Arbuckle, T.E.; Dutya, S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health 2014, 87, 13–20. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelika, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence. Human Exposure, and Toxicity- A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Lyons, G. Bisphenol A: A known endocrine Disruptor; WWF European Toxics Programme Report; WWF: Gland, Switzerland, 2000. [Google Scholar]
- Tinwell, H.; Haseman, J.; Lefevre, P.A.; Wallis, N.; Ashby, J. Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol. Sci. 2002, 68, 339–348. [Google Scholar] [CrossRef]
- Smarr, M.M.; Grantz, K.L.; Sundaram, R.; Maisog, J.M.; Kannan, K.; Buck Louis, G.M. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ. Health 2015, 14, 73. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.; Makino, T.; Suzumori, K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005, 20, 2325–2329. [Google Scholar] [CrossRef]
- Yamasaki, K.; Sawaki, M.; Noda, S.; Imatanaka, N.; Takatsuki, M. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch. Toxicol. 2002, 76, 65–74. [Google Scholar] [CrossRef]
- Brehm, E.; Flaws, J.A. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- NTP. Bisphenol A: Reproduction and Fertility Assessment in CD-1 Mice when Administered in the Feed; NTP-85-192. National Toxicology Program; National Institute of Environmental Health Sciences: Research Triangle, NC, USA, 1985.
- Chapin, R.; Adams, J.; Boekelheide, K.; Gray, L.; Hayward, S.; Lees, P. NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A. 2007. Available online: http://cerhr.niehs.nih.gov/chemicals/bisphenol/bisphenol.html (accessed on 12 January 2020).
- Patisaul, H.B.; Fortino, A.E.; Polston, E.K. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol A. Neurotoxicology 2007, 28, 1–12. [Google Scholar] [CrossRef]
- Kiguchi, M.; Fujita, S.; Lee, J.; Shimizu, N.; Koshikawa, N. Behavioral responses to methylphenidate and apomorphine in rats exposed neonatally to bisphenol A. J. Oral Sci. 2007, 49, 311–318. [Google Scholar] [CrossRef][Green Version]
- Toufexis, D. Region and sex specific modulation of anxiety behaviours in the rat. J. Neuroendocr. 2007, 19, 461–473. [Google Scholar] [CrossRef]
- Grohs, M.N.; Reynolds, J.E.; Liu, J.; Martin, J.W.; Pollock, T.; Lebel, C.; Dewey, D. Prenatal maternal and childhood bisphenol A exposure and brain structure and behavior of young children. Environ. Health 2019, 18, 85. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Balmes, J.; Kogut, K.; Holland, N.; Calafat, A.M.; Harley, K.G. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr. Allergy Immunol. 2019, 30, 36–46. [Google Scholar] [CrossRef]
- Ho, S.M.; Tang, W.Y.; Belmonte, D.F.J.; Prins, G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006, 66, 5624–5632. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Petra, C.E.; Monk, K.R.; Puga, A.; Knudsen, K.E. The xenoestrogenbisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostate adenocarcinoma cells. Mol. Cancer 2002, 1, 515–524. [Google Scholar]
- Reeves, K.W.; Schneider, S.; Xue, J.; Kannan, K.; Mason, H.; Johnson, M.; Makari-Judson, G.; Santana, M.D. Bisphenol-A in breast adipose tissue of breast cancer cases and controls. Environ. Res. 2018, 167, 735–738. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of urinary bisphenol A with heart disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef]
- Braun, J.M.; Li, N.; Arbuckle, T.E.; Dodds, L.; Massarelli, I.; Fraser, W.D.; Lanphear, B.P.; Muckle, G. Association between gestational urinary bisphenol A concentrations and adiposity in young children: The MIREC study. Environ. Res. 2019, 172, 454–461. [Google Scholar] [CrossRef]
- Pant, J.; Ranjan, P.; Deshpande, S.B. BisphenolA decreases atrial contractility involving NO-dependent G-cyclasesignaling pathway. J. Appl. Toxicol. 2011, 31, 698–702. [Google Scholar] [CrossRef]
- Pant, J.; Pant, M.K.; Deshpande, S.B. Bisphenol A attenuates phenylbiguanide-induced cardio-respiratory reflexes in anaesthetized rats. Neurosci. Lett. 2012, 530, 69–74. [Google Scholar] [CrossRef]
- Pant, J.; Pant, M.K.; Chouhan, S.; Singh, S.P.; Deshpande, S.B. Toxic Chemical from Plastics Attenuates Phenylbiguanide-induced Cardio-respiratory Reflexes in Anaesthetized Rats. Ind J. Physiol. Pharm. 2015, 59, 204–210. [Google Scholar]
- Nahar, M.S.; Liao, C.; Kannan, K.; Dolinoy, D.C. Fetal Liver Bisphenol A Concentrations and Biotransformation Gene Expression Reveal Variable Exposure and Reduced Capacity for Metabolism in Humans. J. Biochem. Mol. Toxicol. 2013, 27, 116–123. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Fernanada, B.A.P.; Cibele, M.C.P.G.; Patricia, P.A.; Ione, S. Protective action of hexane crude extract of Pterodonemarginatus fruits against oxidative nitrosative stress induced by acute exercise in rats. BMC Complement. Altern. Med. 2005, 5, 17–21. [Google Scholar]
- Rehman, S.U. Lead-induced regional lipid peroxidation in brain. Toxicol. Lett. 1984, 21, 333–337. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Spooner, R.J. Assay of Glutathione Reductase. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyen, H.V., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Carvalho, G.; Rassi, S. The Prognostic Value of CK-MB in Acute Myocardial Infarction in Developing Countries: A Descriptive Study. Angiol 2016, 4, 3. [Google Scholar] [CrossRef]
- Higgins, C. Urea and the Clinical Value Measuring Blood Urea Concentration. 2016. Available online: www.acutecaretesting.org (accessed on 4 February 2019).
- Geetharathan, T.; Josthna, P. Effect of BPA on Protein, Lipid Profile and Immuno-Histo Chemical Changes in Placenta and Uterine Tissues of Albino Rat. IJPCR 2016, 8, 260–268. [Google Scholar]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenolA on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Ozaydın, T.; Oznurlu, Y.; Sur, E.; Celik, I.; Uluısık, D.; Dayan, M.O. Effects of Bisphenol A on Antioxidant System and Lipid Profile in Rats. Biotech. Histochem. 2018, 93, 231–238. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell Longev. 2014. [Google Scholar] [CrossRef]
- Shinde, A.; Ganu, J.; Naik, P.; Sawant, A. Oxidative stress and antioxidative status in patients with alcoholic liver disease. Biomed. Res. 2012, 23, 105–108. [Google Scholar]
- Kumata, H.; Wakui, K.; Suzuki, H.; Sugawara, T.; Lim, I. Glutathione reductase activity in serum and liver tissue of human and rat with hepatic damage. Tohoku J. Exp. Med. 1975, 116, 127–132. [Google Scholar] [CrossRef][Green Version]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Goth, L.; Meszaros, I.; Nemeth, H. Serum catalase enzyme activity in liver diseases. Acta Biol. Hung. 1987, 38, 287–290. [Google Scholar]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell Longev. 2019. [Google Scholar] [CrossRef]
- Bottles Can be Toxic—Part, II. Available online: http://www.toxicslink.org/?q=content/bottles-can-be-toxic-part-ii (accessed on 25 April 2020).
- Andaluri, G.; Manickavachagam, M.; Suri, R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities. Environ. Monit. Assess. 2018, 190, 65. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Elangovan, M.; Kannan, K. Migration of Parabens, Bisphenols, benzophenone-Type UV Filters, Triclosan, and Triclocarban from Teethers and Its Implications forInfant Exposure. Environ. Sci. Technol. 2016, 50, 13539–13547. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2016, 4, 1600248. [Google Scholar] [CrossRef]
- Prins, G.S.; Patisaul, H.B.; Belcher, S.M.; Vandenberg, L.N. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin. Pharm. Toxicol. 2019, 125 (Suppl. 3), 14–31. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pant, M.K.; Ahmad, A.H.; Naithani, M.; Pandey, H.S.; Pandey, M.; Pant, J. Effect of Exposure of Plastic Infant Feeding Bottle Leached Water on Biochemical, Morphological and Oxidative Stress Parameters in Rats. Toxics 2020, 8, 34. https://doi.org/10.3390/toxics8020034
Pant MK, Ahmad AH, Naithani M, Pandey HS, Pandey M, Pant J. Effect of Exposure of Plastic Infant Feeding Bottle Leached Water on Biochemical, Morphological and Oxidative Stress Parameters in Rats. Toxics. 2020; 8(2):34. https://doi.org/10.3390/toxics8020034
Chicago/Turabian StylePant, Mahendra K., Abul H. Ahmad, Manisha Naithani, Hari S. Pandey, Monika Pandey, and Jayanti Pant. 2020. "Effect of Exposure of Plastic Infant Feeding Bottle Leached Water on Biochemical, Morphological and Oxidative Stress Parameters in Rats" Toxics 8, no. 2: 34. https://doi.org/10.3390/toxics8020034
APA StylePant, M. K., Ahmad, A. H., Naithani, M., Pandey, H. S., Pandey, M., & Pant, J. (2020). Effect of Exposure of Plastic Infant Feeding Bottle Leached Water on Biochemical, Morphological and Oxidative Stress Parameters in Rats. Toxics, 8(2), 34. https://doi.org/10.3390/toxics8020034




