Clinical and Epidemiological Characteristics of Severe Acute Adult Poisoning Cases in Martinique: Implicated Toxic Exposures and Their Outcomes
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mégarbane, B.; Donetti, L.; Blanc, T.; Chéron, G.; Jacobs, F. Intoxications graves par médicaments et substances illicites en réanimation. Reanimation 2006, 5, 332–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, N.; Li, T. Acute poisoning in Shenyang, China: A retrospective and descriptive study from 2012 to2016. BMJ Open 2018, 8, e021881. [Google Scholar] [CrossRef] [PubMed]
- Sorge, M.; Weidhase, L.; Bernhard, M.; Gries, A.; Petros, S. Self-poisoning in the acute care medicine 2005–2012. Anaesthesist 2015, 64, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, A.M.; Purdy, C.H.; Blondell, R.D. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J. Addict. Dis. 2008, 27, 1–11. [Google Scholar] [CrossRef]
- Burillo-Putze, G.; Munne, P.; Dueñas, A.; Pinillos, M.A.; Naveiro, J.M.; Cobo, J.; Alonso, J.; The Clinical Toxicology Working Group; Spanish Society of Emergency Medicine (SEMESTOX). National multicentre study of acute intoxication in emergency departments of Spain. Eur. J. Emerg. Med. 2003, 10, 101–104. [Google Scholar] [CrossRef]
- Kristinsson, J.; Palsson, R.; Gudjonsdottir, G.A.; Blondal, M.; Gudmundsson, S.; Snook, C.P. Acute poisonings in Iceland: A prospective nationwide study. Clin. Toxicol. (Phila) 2008, 46, 126–132. [Google Scholar] [CrossRef]
- Heyerdahl, F.; Bjornas, M.A.; Hovda, K.E.; Skog, K.; Opdahl, A.; Wium, C.; Ekeberg, O.; Jacobsen, D. Acute poisonings treated in hospitals in Oslo: A one-year prospective study (II): Clinical outcome. Clin. Toxicol. (Phila) 2008, 46, 42–49. [Google Scholar] [CrossRef]
- Warner, M.; Chen, L.H.; Makuc, D.M.; Anderson, R.N.; Miniño, A.M. Drug poisoning deaths in the United States, 1980–2008. NCHS Data Brief 2011, 81, 1–8. [Google Scholar]
- Chen, L.H.; Hedegaard, H.; Warner, M. Drug-poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. NCHS Data Brief 2014, 166, 1–8. [Google Scholar]
- Heyman, E.N.; LoCastro, D.E.; Gouse, L.H.; Morris, D.L.; Lombardo, B.A.; Montenegro, H.D.; Takacs, M. Intentional drug overdose: Predictors of clinical course in the intensive care unit. Heart Lung. 1996, 25, 246–252. [Google Scholar] [CrossRef]
- Bosch, T.M.; van der Werf, T.S.; Uges, D.R.A.; Ligtenberg, J.J.M.; Fijen, J.W.; Tulleken, J.E.; Zijlstra, J.G. Antidepressants self-poisoning and ICU admissions in a university hospital in The Netherlands. Pharm. World Sci. 2000, 22, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Strøm, J.; Thisted, B.; Krantz, T.; Bredgaard Sørensen, M. Self-poisoning treated in an ICU: Drug pattern, acute mortality and short-term survival. Acta Anaesthesiol. Scand. 1986, 30, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Cochet, A.; Guyodo, G. Poison episodes reported to French poison control centers in 2006. Rev. Prat. 2008, 58, 825–831. [Google Scholar] [PubMed]
- Lai, M.W.; Klein-Schwartz, W.; Rodgers, G.C.; Abrams, J.Y.; Haber, D.A.; Bronstein, A.C.; Wruk, K.M. 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exposure database. Clin. Toxicol. (Phila) 2006, 44, 803–932. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.E.; Sjöberg, G.K.; Haines, J.A.; Pronczuk de Garbino, J. Poisoning severity score. Grading of acute poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 14, 29–36. [Google Scholar] [CrossRef]
- Thomas, S.; Lewis, S.; Bevan, L.; Bhattacharyya, S.; Bramble, M.G.; Chew, K.; Connolly, J.; Dorani, B.; Han, K.H.; Horner, J.E.; et al. Factors affecting hospital admission and length of stay of poisoned patients in the north east of England. Hum. Exp. Toxicol. 1996, 15, 915–919. [Google Scholar] [CrossRef]
- Kapur, N.; House, A.; Creed, F.; Feldman, E.; Friedman, T.; Guthrie, E. Management of deliberate self poisoning in adults in four teaching hospitals: Descriptive study. BMJ 1998, 316, 831–832. [Google Scholar] [CrossRef]
- Gunnell, D.; Ho, D.; Murray, V. Medical management of deliberate drug overdose: A neglected area for suicide prevention? Emerg. Med. J. 2004, 21, 35–38. [Google Scholar] [CrossRef]
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in Drug and Opioid-Involved Overdose Deaths-United States, 2010–2015. MMWR Morb. Mortal. Wkly Rep. 2016, 6, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Wright, M.; Pond, S.M. Experience with 732 acute overdose patients admitted to an intensive care unit over six years. Med. J. Aust. 1993, 158, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Maignan, M.; Pommier, P.; Clot, S.; Saviuc, P.; Debaty, G.; Briot, R.; Carpentier, F.; Danel, V. Deliberate drug poisoning with slight symptoms on admission: Are there predictive factors for intensive care unit referral? A three-year retrospective study. Basic Clin. Pharmacol. Toxicol. 2014, 114, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.E.; Wagner, D.P.; Draper, E.A.; Wright, L.; Alzola, C.; Knaus, W.A. Evaluation of acute physiology and chronic health evaluation III predictions of hospital mortality in an independent database. Crit. Care Med. 1998, 26, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, R.; Brinkman, S.; de Keizer, N.F.; Meulenbelt, J.; de Lange, D.W. In-Hospital Mortality and Long-Term Survival of Patients with Acute Intoxication Admitted to the ICU*. Crit. Care Med. 2014, 42, 1471–1479. [Google Scholar] [CrossRef]
- Schwake, L.; Wollenschläger, I.; Stremmel, W.; Encke, J. Adverse drug reactions and deliberate self-poisoning as cause of admission to the intensive care unit: A 1-year prospective observational cohort study. Intensive Care Med. 2009, 35, 266–274. [Google Scholar] [CrossRef]
- Mühlberg, W.; Becher, K.; Heppner, H.-J.; Wicklein, S.; Sieber, C. Acute poisoning in old and very old patients: A longitudinal retrospective study of 5883 patients in a toxicological intensive care unit. Z Gerontol. Geriatr. 2005, 38, 182–189. [Google Scholar] [CrossRef]
- Hamad, A.E.; Al-Ghadban, A.; Carvounis, C.P.; Soliman, E.; Coritsidis, G.N. Predicting the Need for Medical Intensive Care Monitoring in Drug-Overdosed Patients. Intensive Care Med. 2000, 15, 321–328. [Google Scholar] [CrossRef]
- Liisanantti, J.H.; Ohtonen, P.; Kiviniemi, O.; Laurila, J.J.; Ala-Kokko, T.I. Risk factors for prolonged intensive care unit stay and hospital mortality in acute drug-poisoned patients: An evaluation of the physiologic and laboratory parameters on admission. J. Crit. Care 2011, 26, 160–165. [Google Scholar] [CrossRef]
- Harchelroad, F.; Clark, R.F.; Dean, B.; Krenzelok, E.P. Treated vs reported toxic exposures: Discrepancies between a poison control center and a member hospital. Vet. Hum. Toxicol. 1990, 32, 156–159. [Google Scholar]
- Brinkman, S.; Bakhshi-Raiez, F.; Abu-Hanna, A.; de Jonge, E.; Bosman, R.J.; Peelen, L.; de Keizer, N.F. External validation of Acute Physiology and Chronic Health Evaluation IV in Dutch intensive care units and comparison with Acute Physiology and Chronic Health Evaluation II and Simplified Acute Physiology Score II. J. Crit. Care 2011, 26, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Juneja, D.; Singh, O.; Nasa, P.; Dang, R. Comparison of newer scoring systems with the conventional scoring systems in general intensive care population. Minerva Anestesiol. 2012, 78, 194–200. [Google Scholar] [PubMed]
- Liisanantti, J.; Kaukoranta, P.; Martikainen, M.; Ala-Kokko, T. Aspiration pneumonia following severe self-poisoning. Resuscitation. 2003, 56, 49–53. [Google Scholar] [CrossRef]
- Reisinger, A.; Rabensteiner, J.; Hackl, G. Diagnosis of acute intoxications in critically ill patients: Focus on biomarkers-part 1: Epidemiology, methodology and general overview. Biomarkers 2020, 25, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, R.; Brinkman, S.; de Keizer, N.F.; Kesecioglu, J.; Meulenbelt, J.; de Lange, D.W. The need for ICU admission in intoxicated patients: A prediction model. Clin. Toxicol. (Phila) 2017, 55, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Baird-Gunning, J.; Lea-Henry, T.; Hoegberg, L.C.G.; Gosselin, S.; Roberts, D.M. Lithium Poisoning. J. Intensive Care Med. 2017, 32, 249–263. [Google Scholar] [CrossRef]
- Majori, S.; Ricci, G.; Capretta, F.; Loss, R.; Baldovin, T.; Cigolini, D.; Tardivo, S.; Zannoni, M. The impact of acute intoxications in a toxicological unit care in north east Italy. J. Prev. Med. Hyg. 2012, 58, 8–13. [Google Scholar]
- Koskela, L.; Raatiniemi, L.; Bakke, H.K.; Ala-Kokko, T.; Liisanantti, J. Fatal poisonings in Northern Finland: Causes, incidence, and rural-urban differences. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 90. [Google Scholar] [CrossRef]
- Lindqvist, E.; Edman, G.; Hollenberg, J.; Nordberg, P.; Ösby, U.; Forsberg, S. Intensive care admissions due to poisoning. Acta Anaesthesiol. Scand. 2017, 61, 1296–1304. [Google Scholar] [CrossRef]
- Prashar, A.; Ramesh, M. Assessment of pattern and outcomes of pesticides poisoning in a tertiary care hospital. Trop. Med. Int. Health 2018, 23, 1401–1407. [Google Scholar] [CrossRef]
- Herbland, A.; El Zein, I.; Valentino, R.; Cassinotto, C.; Meunier, C.; Rieux, D.; Mehdaoui, H. Star fruit poisoning is potentially life-threatening in patients with moderate chronic renal failure. Intensive Care Med. 2009, 35, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Signaté, A.; Olindo, S.; Chausson, N.; Cassinoto, C.; EdimoNana, M.; Saint Vil, M.; Cabre, P.; Smadja, D. Star fruit (Averrhoa carambola) toxic encephalopathy. Rev. Neurol. (Paris) 2009, 165, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Damien, D.A.; Thomas, N.; Hélène, P.; Sara, K.; Yves, L. First evaluation of illicit and licit drug consumption based on wastewater analysis in Fort de France urban area (Martinique, Caribbean), a transit area for drug smuggling. Sci. Total. Environ. 2014, 490, 970–978. [Google Scholar] [CrossRef]
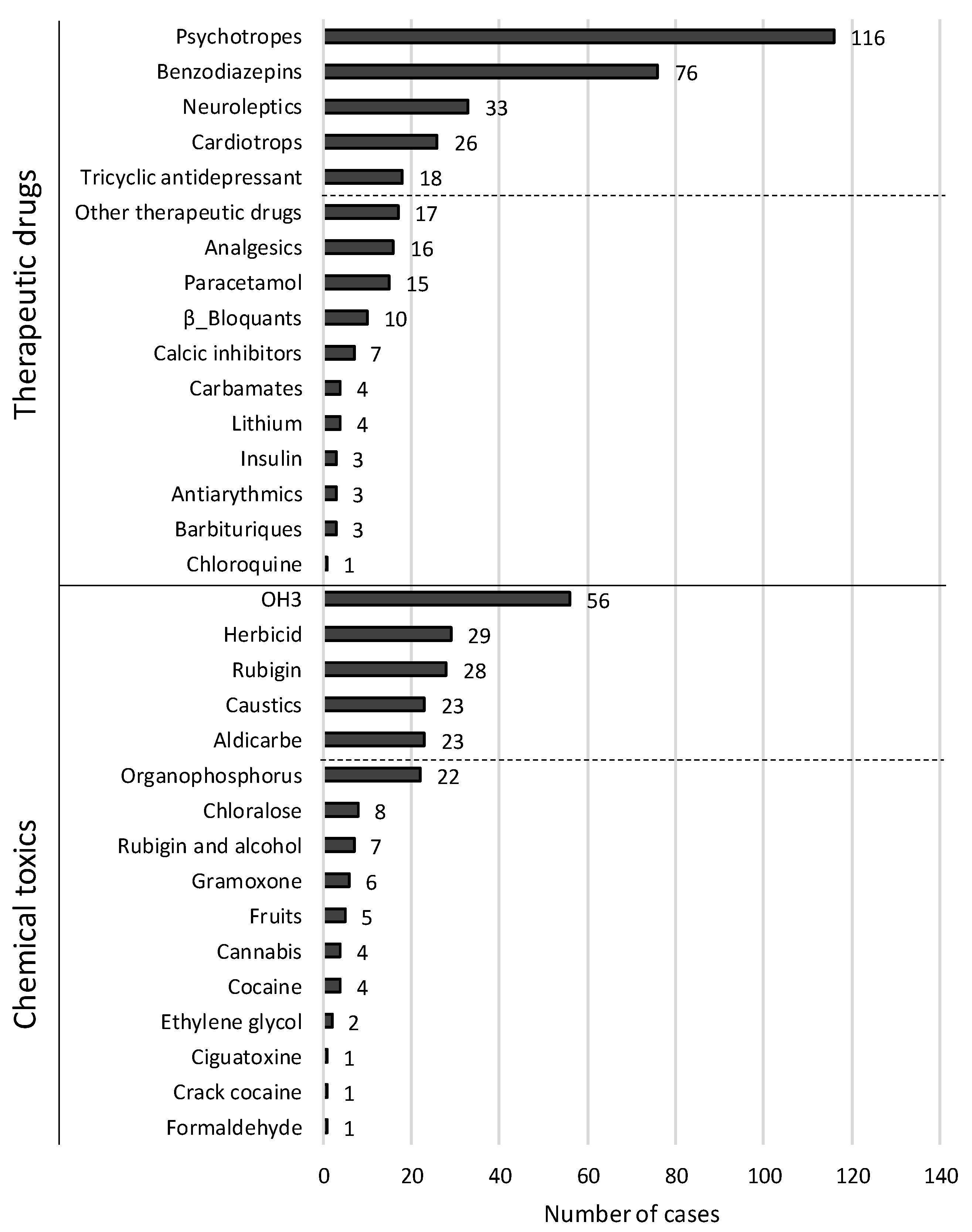
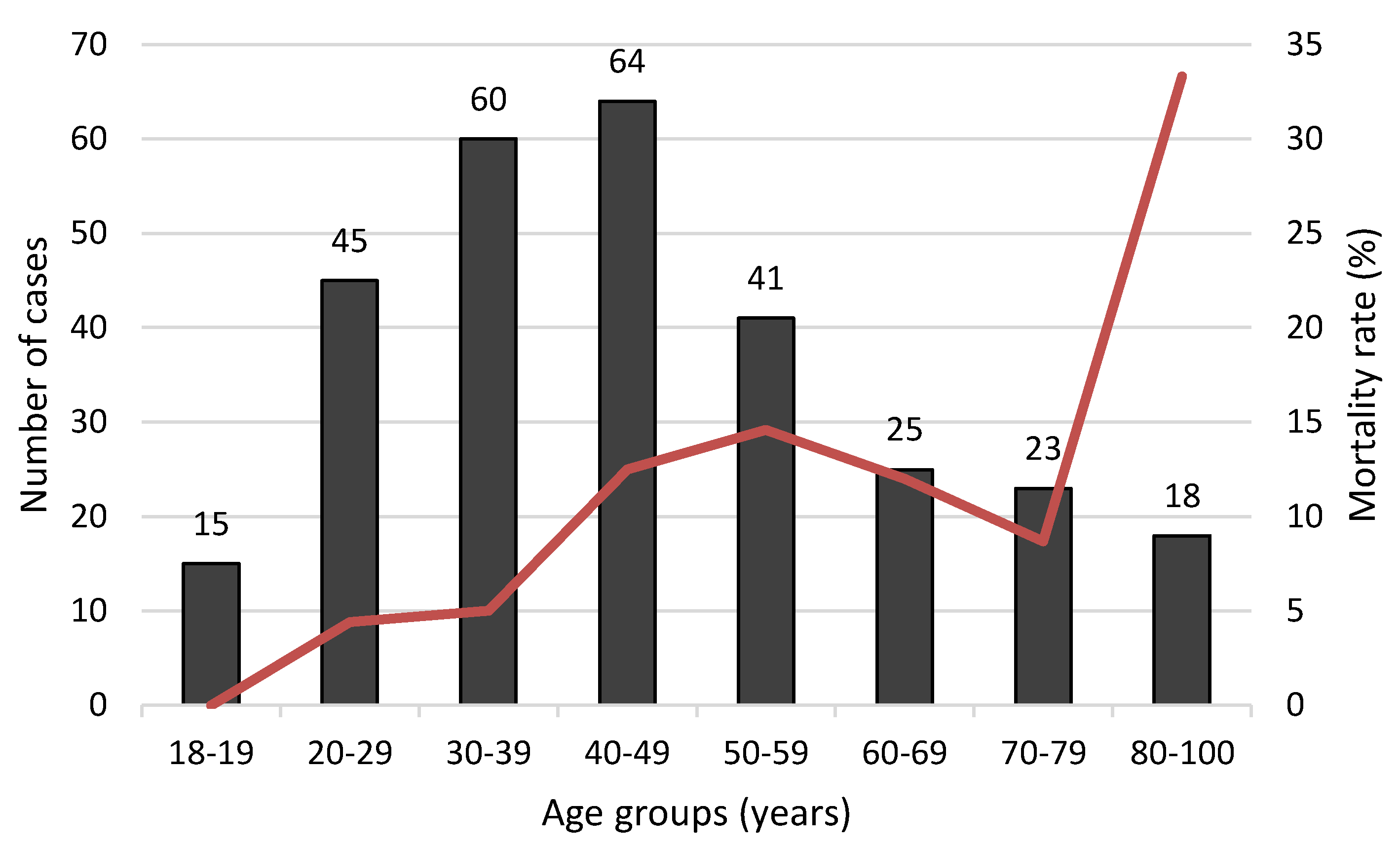
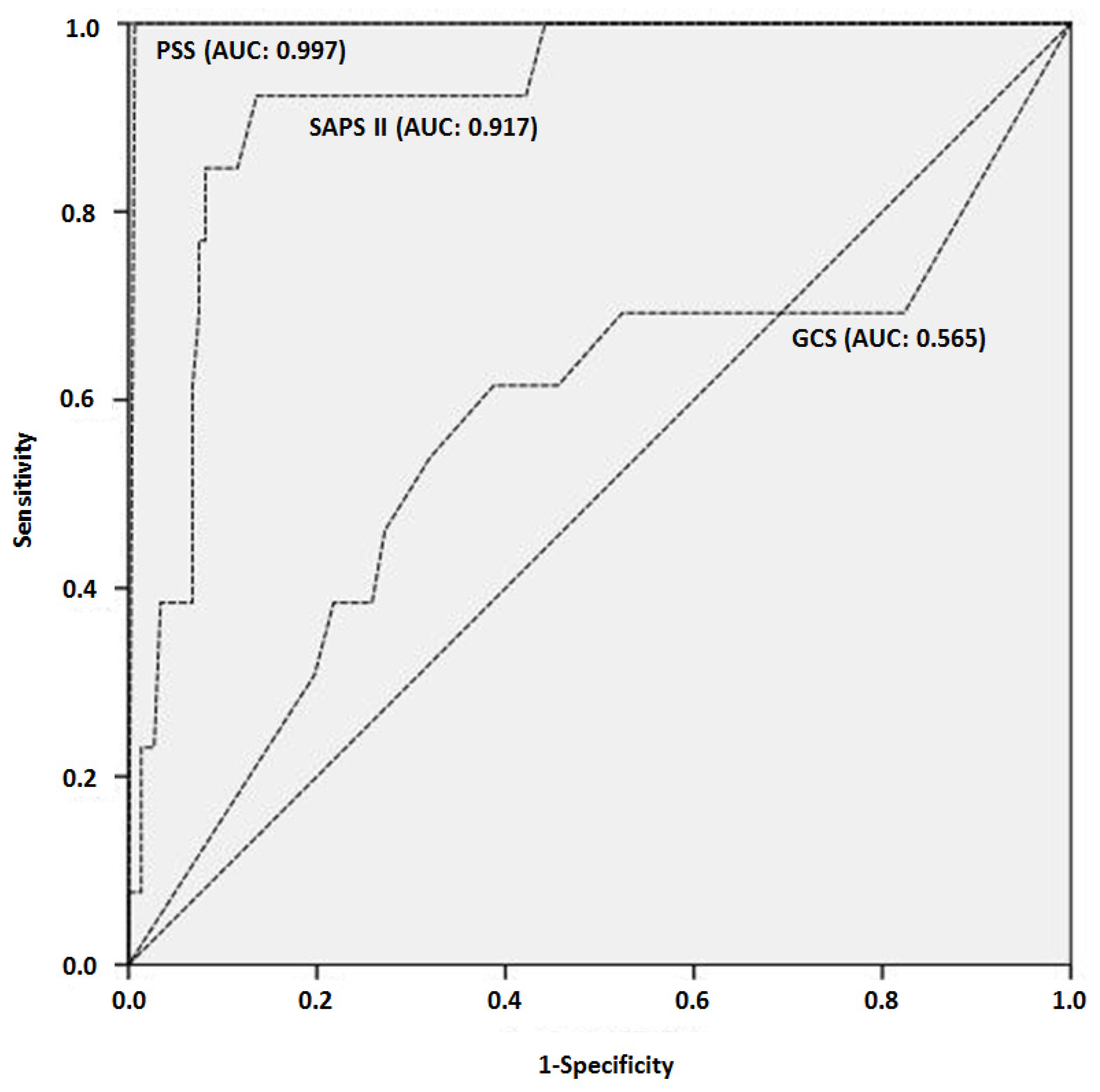
| Variable | Total Population | Non Survivors | Survivors | p | |||
|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | ||
| Age (years) | 291 | 46 ± 19 | 30 | 56 ± 18 | 261 | 45 ± 18 | 0.001 |
| Male gender | 291 | 166 (57%) | 30 | 17 (56.7%) | 261 | 149 (57.1%) | 0.965 |
| Medical history | |||||||
| Psychiatric disorder | 287 | 143 (49.8%) | 30 | 14 (46.7%) | 257 | 129 (50.2%) | 0.865 |
| Drug intoxication | 284 | 65 (22.9%) | 29 | 1 (3.4%) | 255 | 64 (25.1%) | 0.031 |
| Alcohol abuse | 284 | 59 (20.8%) | 29 | 6 (20.7%) | 255 | 53 (20.8%) | 0.940 |
| Hospitalization | |||||||
| Emergency Department | 287 | 185 (64.5%) | 30 | 19 (63.3%) | 257 | 166 (64.6%) | 0.785 |
| Medical ward | 288 | 26 (9%) | 29 | 4 (13.8%) | 259 | 22 (8.5%) | 0.271 |
| Level 3 ICU | 288 | 227 (78.8%) | 30 | 23 (76.7%) | 258 | 204 (79.1%) | 0.802 |
| Level 2 ICU | 290 | 71 (24.5%) | 30 | 6 (20%) | 260 | 65 (25%) | 0.787 |
| Severity | |||||||
| PSS | 289 | 2.7 ± 0.8 | 30 | 4 ± 0 | 259 | 2.5 ± 0.7 | 0.000 |
| SAPS II | 191 | 39 ± 23 | 19 | 71 ± 19 | 172 | 36 ± 20 | 0.000 |
| Issue | |||||||
| Hospital Length of stay | 290 | 6 ± 7 | 30 | 5 ± 8 | 260 | 6 ± 7 | 0.750 |
| 1 day | 290 | 18 (6.2%) | 30 | 8 (26.7%) | 260 | 10 (3.8%) | - |
| 2 to 7 days | 290 | 225 (77.3%) | 30 | 17 (56.7%) | 260 | 208 (80%) | - |
| 8 to 14 days | 290 | 21 (7.2%) | 30 | 3 (10%) | 260 | 18 (6.9%) | - |
| >15 days | 290 | 26 (8.9%) | 30 | 2 (6.7%) | 260 | 24 (9.2%) | - |
| Cardiac arrest | 291 | 34 (11.7%) | 30 | 30 (100%) | 261 | 4 (1.5%) | 0.000 |
| Death | 291 | 30 (10.3%) | 30 | 30 (100%) | 261 | 0 (0%) | - |
| Variable | Total Population | Non-Survivors | Survivors | p | |||
|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | ||
| Therapeutic drug intoxication | 291 | 169 (58.1%) | 30 | 11 (36.7%) | 261 | 158 (60.5%) | 0.012 |
| Nb of therapeutic drug involved | 291 | 1.2 ± 1.3 | 30 | 0.6 ± 0.9 | 261 | 1.3 ± 1.3 | 0.090 |
| Antipsychotics | 291 | 116 (39.9%) | 30 | 4 (13.3%) | 261 | 112 (42.9%) | 0.002 |
| Benzodiazepines | 291 | 76 (26.1%) | 30 | 2 (6.7%) | 261 | 74 (28.4%) | 0.010 |
| Barbiturates | 291 | 3 (1%) | 30 | 0 (0%) | 261 | 3 (1.1%) | 0.555 |
| Neuroleptics | 291 | 33 (11.3%) | 30 | 1 (3.3%) | 261 | 32 (12.3%) | 0.144 |
| Lithium | 291 | 4 (1.4%) | 30 | 0 (0%) | 261 | 4 (1.5%) | 0.495 |
| Tricyclic antidepressants | 291 | 18 (6.2%) | 30 | 1 (3.3%) | 261 | 17 (6.5%) | 0.493 |
| Carbamate | 291 | 4 (1.4%) | 30 | 0 (0%) | 261 | 4 (1.5%) | 0.495 |
| Cardiotrops | 291 | 26 (8.9%) | 30 | 3 (10%) | 261 | 23 (8.8%) | 0.829 |
| Antiarrhythmics | 291 | 3 (1%) | 30 | 0 (0%) | 261 | 3 (1.1%) | 0.555 |
| Β-Blockers | 291 | 10 (3.4%) | 30 | 2 (6.7%) | 261 | 8 (3.1%) | 0.305 |
| Calcium inhibitors | 291 | 7 (2.4%) | 30 | 1 (3.3%) | 261 | 6 (2.3%) | 0.726 |
| Analgesics | 291 | 16 (5.5%) | 30 | 1 (3.3%) | 261 | 15 (5.7%) | 0.583 |
| Paracetamol | 291 | 15 (5.2%) | 30 | 1 (3.3%) | 261 | 14 (5.4%) | 0.634 |
| Chloroquine | 291 | 1 (0.3%) | 30 | 0 (0%) | 261 | 1 (0.4%) | 0.734 |
| Insulin | 291 | 3 (1%) | 30 | 0 (0%) | 261 | 3 (1.1%) | 0.555 |
| Other therapeutic drugs | 291 | 17 (5.8%) | 30 | 3 (10%) | 261 | 14 (5.4%) | 0.305 |
| Chemical intoxication | 291 | 152 (52.2%) | 30 | 19 (63.3%) | 261 | 133 (51%) | 0.199 |
| Nb of chemical products involved | 291 | 0.8 ± 0.9 | 30 | 0.9 ± 0.9 | 261 | 0.7 ± 0.9 | 0.250 |
| Herbicides | 291 | 29 (10%) | 30 | 6 (20%) | 261 | 23 (8.8%) | 0.053 |
| Aldicarb | 291 | 23 (7.9%) | 30 | 0 (0%) | 261 | 23 (8.8%) | 0.090 |
| Organophosphorus | 291 | 22 (7.6%) | 30 | 2 (6.7%) | 261 | 20 (7.7%) | 0.845 |
| Gramoxone | 291 | 6 (2.1%) | 30 | 5 (16.7%) | 261 | 1 (0.4%) | 0.000 |
| Chloralose | 291 | 8 (2.7%) | 30 | 0 (0%) | 261 | 8 (3.1%) | 0.331 |
| Rubigine® | 291 | 28 (9.6%) | 30 | 4 (13.3%) | 261 | 24 (9.2%) | 0.467 |
| Caustics | 291 | 23 (7.9%) | 30 | 1 (3.3%) | 261 | 22 (8.4%) | 0.327 |
| Ethylene glycol | 291 | 2 (0.7%) | 30 | 0 (0%) | 261 | 2 (0.8%) | 0.630 |
| Formaldehyde | 291 | 1 (0.3%) | 30 | 1 (3.3%) | 261 | 0 (0%) | 0.003 |
| Crack cocaine | 291 | 1 (0.3%) | 30 | 0 (0%) | 261 | 1 (0.4%) | 0.734 |
| Cocaine | 291 | 4 (1.4%) | 30 | 1 (3.3%) | 261 | 3 (1.1%) | 0.331 |
| Cannabis | 291 | 4 (1.4%) | 30 | 0 (0%) | 261 | 4 (1.5%) | 0.495 |
| OH3 | 291 | 56 (19.2%) | 30 | 5 (16.7%) | 261 | 51 (19.5%) | 0.705 |
| Fruits | 291 | 5 (1.7%) | 30 | 2 (6.7%) | 261 | 3 (1.1%) | 0.028 |
| Ciguatoxine | 291 | 1 (0.3%) | 30 | 0 (0%) | 261 | 1 (0.4%) | 0.734 |
| Mixed therapeutic and chemical toxic | 291 | 30 (10.3%) | 30 | 0 (0%) | 261 | 30 (11.5%) | 0.050 |
| Nb of toxicant involved | 291 | 1.1 ± 0.3 | 30 | 1 ± 0 | 261 | 1.1 ± 0.3 | 0.005 |
| Multiple therapeutic drugs intoxication | 169 | 61 (36.1%) | 11 | 2 (18.2%) | 158 | 59 (37.3%) | 0.025 |
| Multiple chemical intoxication | 152 | 48 (31.6%) | 19 | 7 (36.8%) | 133 | 41 (30.8%) | 0.371 |
| Drug overdose | 291 | 37 (12.7%) | 30 | 7 (23.3%) | 261 | 30 (11.5%) | 0.065 |
| Intentional self-poisoning | 289 | 250 (86.5%) | 30 | 24 (80%) | 259 | 226 (87.3%) | 0.484 |
| Parameter | Normal Range | Total Population | Non Survivors | Survivors | p | |||
|---|---|---|---|---|---|---|---|---|
| Number | Result | Number | Result | Number | Result | |||
| Temperature (°C) | 36.1–37.5 | 74 | 36.6 ± 1.6 | 11 | 36.2 ± 2.3 | 63 | 36.7 ± 1.4 | 0.361 |
| Breath rhythm (breath/min) | 16–20 | 50 | 25 ± 19 | 9 | 44 ± 37 | 41 | 21 ± 7 | 0.000 |
| SaO2 (%) | 92–100 | 94 | 94 ± 9 | 10 | 90 ± 10 | 84 | 94 ± 9 | 0.099 |
| Cardiac rhythm (beat/min) | 60–90 | 179 | 89 ± 28 | 23 | 85 ± 30 | 156 | 90 ± 28 | 0.472 |
| Shock | - | 291 | 78 (26.8%) | 30 | 28 (93.3%) | 261 | 50 (19.2%) | 0.000 |
| Ventricular fibrillation or tachycardia | - | 291 | 8 (2.7%) | 30 | 7 (23.3%) | 261 | 1 (0.4%) | 0.000 |
| GCS | 15 | 227 | 9 ± 4 | 21 | 9 ± 5 | 206 | 8 ± 4 | 0.860 |
| Seizures | - | 291 | 31 (10.7%) | 30 | 5 (16.7%) | 261 | 26 (10%) | 0.260 |
| Myoclonus | - | 291 | 28 (9.6%) | 30 | 1 (3.3%) | 261 | 27 (10.3%) | 0.217 |
| Gastro-intestinal disorders | - | 291 | 78 (26.8%) | 30 | 14 (46.7%) | 261 | 64 (24.5%) | 0.010 |
| Pneumonia | - | 291 | 49 (16.8%) | 30 | 7 (23.3%) | 261 | 42 (16.1%) | 0.315 |
| Conjunctivitis | - | 291 | 3 (1%) | 30 | 0 (0%) | 261 | 3 (1.1%) | 0.555 |
| Other infections | - | 291 | 77 (26.5%) | 30 | 10 (33.3%) | 261 | 67 (25.7%) | 0.173 |
| Biology | ||||||||
| pH | 7.40 ± 0.02 | 81 | 7.30 ± 0.20 | 17 | 7.20 ± 0.20 | 64 | 7.30 ± 0.20 | 0.092 |
| Alkaline reserve (mmol/L) | 22–29 | 66 | 19.3 ± 7.1 | 13 | 15.2 ± 6.3 | 53 | 20.3 ± 7 | 0.019 |
| PaCO2 (mmHg) | 35–45 | 68 | 39.8 ± 15 | 10 | 40.5 ± 18.8 | 58 | 39.6 ± 14.4 | 0.867 |
| Lactate (mmol/L) | 0.5–2.2 | 53 | 6.4 ± 5.9 | 9 | 11.1 ± 7.7 | 44 | 5.4 ± 5 | 0.006 |
| Hyper lactacidemia | - | 53 | 37 (69.8%) | 9 | 8 (88.9%) | 44 | 29 (65.9%) | 0.171 |
| Sodium (mmol/L) | 136–145 | 59 | 139 ± 8 | 8 | 136 ± 9 | 51 | 139 ± 7 | 0.225 |
| Potassium (mmol/L) | 3.5–5.1 | 82 | 4 ± 1.3 | 12 | 5.4 ± 1.5 | 70 | 3.8 ± 1.1 | 0.000 |
| Hypokalemia | - | 82 | 26 (31.7%) | 12 | 1 (8.3%) | 70 | 25 (35.7%) | 0.029 |
| Hyperkalemia | - | 82 | 13 (15.9%) | 12 | 7 (58.3%) | 70 | 6 (8.6%) | 0.000 |
| Calcium (mmol/L) | 2.15–2.55 | 33 | 2.1 ± 0.4 | 2 | 1.4 ± 0.2 | 31 | 2.1 ± 0.4 | 0.029 |
| Magnesium (mmol/L) | 0.75–0.90 | 10 | 0.9 ± 0.2 | 2 | 0.8 ± 0.1 | 8 | 0.9 ± 0.3 | 0.497 |
| Phosphorus (mmol/L) | 0.80–1.35 | 22 | 1.1 ± 0.6 | 1 | 1.27 | 21 | 1.1 ± 0.6 | 0.805 |
| Creatinine (µmol/L) | 62–106 | 90 | 220 ± 213 | 15 | 270 ± 179 | 75 | 210 ± 219 | 0.317 |
| Urea nitrogen (mmol/L) | 1.7–8.3 | 73 | 15 ± 34 | 10 | 43 ± 87.8 | 63 | 10.6 ± 8.9 | 0.004 |
| Creatine Kinase (IU/L) | 38–174 | 34 | 15,974 ± 45,164 | 5 | 38,193 ± 44,310 | 29 | 12,143 ± 44,947 | 0.000 |
| Rhabdomyolysis | - | 34 | 20 (58.8%) | 5 | 5 (100%) | 29 | 15 (51.7%) | 0.041 |
| Troponins (µg/L) | 0–0.014 | 15 | 0.8 ± 1.9 | 4 | 0.5 ± 0.6 | 11 | 0.9 ± 2.2 | 0.700 |
| Elevated troponins | - | 3 | 3 (100%) | 1 | 1 (100%) | 2 | 2 (100%) | 0.187 |
| Glycaemia (mmol/L) | 3.9–7.1 | 12 | 8.3 ± 5.1 | 2 | 4.6 ± 6 | 10 | 9.1 ± 4.9 | 0.270 |
| Aspartate aminotransferases (AST) (IU/L) | <37 | 43 | 1968 ± 3894 | 6 | 5452 ± 4317 | 37 | 1403 ± 3570 | 0.042 |
| Alanine aminotransferases (ALT) (IU/L) | <40 | 43 | 961 ± 2211 | 6 | 2431 ± 2574 | 37 | 723 ± 2089 | 0.239 |
| Platelet count (Giga/L) | 150–400 | 29 | 152 ± 95 | 7 | 75 ± 74 | 22 | 177 ± 88 | 0.010 |
| Prothrombine time (%) | 60–100 | 38 | 42 ± 31 | 8 | 31 ± 18 | 30 | 45 ± 34 | 0.262 |
| SvO2 (%) | 75–85 | 2 | 82 ± 4.2 | 1 | 85 | 1 | 79 | - |
| Toxicologic analysis | - | 284 | 111 (39.1%) | 30 | 8 (26.7%) | 254 | 103 (40.6%) | 0.218 |
| Parameter | Total Population | Non Survivors | Survivors | p | |||
|---|---|---|---|---|---|---|---|
| Nb | Result | Nb | Result | Nb | Result | ||
| Activated charcoal | 291 | 44 (15.1%) | 30 | 2 (6.7%) | 261 | 42 (16.1%) | 0.172 |
| Gastric lavage | 291 | 57 (19.6%) | 30 | 8 (26.7%) | 261 | 49 (18.8%) | 0.302 |
| Antidotes | 291 | 60 (20.6%) | 30 | 2 (6.7%) | 261 | 58 (22.2%) | 0.046 |
| Mechanical ventilation | 291 | 171 (58.8%) | 30 | 29 (96.7%) | 261 | 142 (54.4%) | 0.000 |
| ARDS | 172 | 12 (7%) | 30 | 6 (20%) | 142 | 6 (4.2%) | 0.000 |
| MV duration (days) | 171 | 6 ± 8 | 29 | 5 ± 8 | 142 | 6 ± 8 | 0.433 |
| Catecholamines | 291 | 78 (26.8%) | 30 | 28 (93.3%) | 261 | 50 (19.2%) | 0.000 |
| Renal replacement therapy | 291 | 32 (11%) | 30 | 13 (43.3%) | 261 | 19 (7.3%) | 0.000 |
| ECMO | 291 | 3 (1%) | 30 | 2 (6.7%) | 261 | 1 (0.4%) | 0.001 |
| Treatment | 291 | 133 (45.7%) | 30 | 20 (66.7%) | 261 | 113 (43.3%) | 0.015 |
| Main Toxicants Involved | Related Clinical and Biological Features | |
| Benzodiazepines | Coma, aspiration pneumonia | |
| Neuroleptics | Coma, respiratory failure, shock, aspiration pneumonia, renal failure, rhabdomyolysis | |
| Cardiotrops | Cardiac arrhythmias, shock, heart conduction dysfunction, cardiac arrest, renal failure, elevation in lactate, rhabdomyolysis, liver failure | |
| Tricyclic antidepressants | Coma, seizures, shock, heart conduction dysfunction, aspiration pneumonia, renal failure, rhabdomyolysis | |
| The Rarest Toxicants in Western Countries | Specific Features | Specific Management |
| Herbicides, paraquat | Respiratory failure (acute respiratory distress syndrome), elevation in transaminase, renal failure | No effective antidote |
| Rubigine® (Hydrofluoric acid) | Gastrointestinal disturbances (corrosion), dermal injuries, hypocalcemia, metabolic acidosis, respiratory failure, seizures, dysrhythmias, cardiovascular disorders, cardiac arrest | Calcium gluconate, analgesics, hemodialysis, |
| Pesticides Organophosphates | Excessive respiratory secretions, and bronchoconstriction, bradycardia, myosis, neuromuscular weakness, seizures, coma, cardiovascular collapse | Atropine, pralidoxime, diazepam |
| Star Fruit (Carambola) (Toxin: oxalic acid) | Hiccups, vomiting, encephalopathy, seizures, neuropsychiatric manifestations, acute kidney injury and chronic kidney disease Renal histology: oxalate crystals obstructing the tubules | No effective antidote Renal replacement therapy (hemodialysis or hemoperfusion) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resiere, D.; Kallel, H.; Oxybel, O.; Chabartier, C.; Florentin, J.; Brouste, Y.; Gueye, P.; Megarbane, B.; Mehdaoui, H. Clinical and Epidemiological Characteristics of Severe Acute Adult Poisoning Cases in Martinique: Implicated Toxic Exposures and Their Outcomes. Toxics 2020, 8, 28. https://doi.org/10.3390/toxics8020028
Resiere D, Kallel H, Oxybel O, Chabartier C, Florentin J, Brouste Y, Gueye P, Megarbane B, Mehdaoui H. Clinical and Epidemiological Characteristics of Severe Acute Adult Poisoning Cases in Martinique: Implicated Toxic Exposures and Their Outcomes. Toxics. 2020; 8(2):28. https://doi.org/10.3390/toxics8020028
Chicago/Turabian StyleResiere, Dabor, Hatem Kallel, Odile Oxybel, Cyrille Chabartier, Jonathan Florentin, Yannick Brouste, Papa Gueye, Bruno Megarbane, and Hossein Mehdaoui. 2020. "Clinical and Epidemiological Characteristics of Severe Acute Adult Poisoning Cases in Martinique: Implicated Toxic Exposures and Their Outcomes" Toxics 8, no. 2: 28. https://doi.org/10.3390/toxics8020028
APA StyleResiere, D., Kallel, H., Oxybel, O., Chabartier, C., Florentin, J., Brouste, Y., Gueye, P., Megarbane, B., & Mehdaoui, H. (2020). Clinical and Epidemiological Characteristics of Severe Acute Adult Poisoning Cases in Martinique: Implicated Toxic Exposures and Their Outcomes. Toxics, 8(2), 28. https://doi.org/10.3390/toxics8020028






