Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation
2.2. Synthesis and Characterization of Starch-Stabilized nZVI
2.3. Characterization of Bare nZVI
2.4. Biochar and Humic Acid
2.5. Pot Culture Experiment
2.6. Plant Harvesting
2.7. Plant and Soil Analysis
2.8. Data Analyses
3. Results
3.1. Cr(VI) Concentrations in Soil
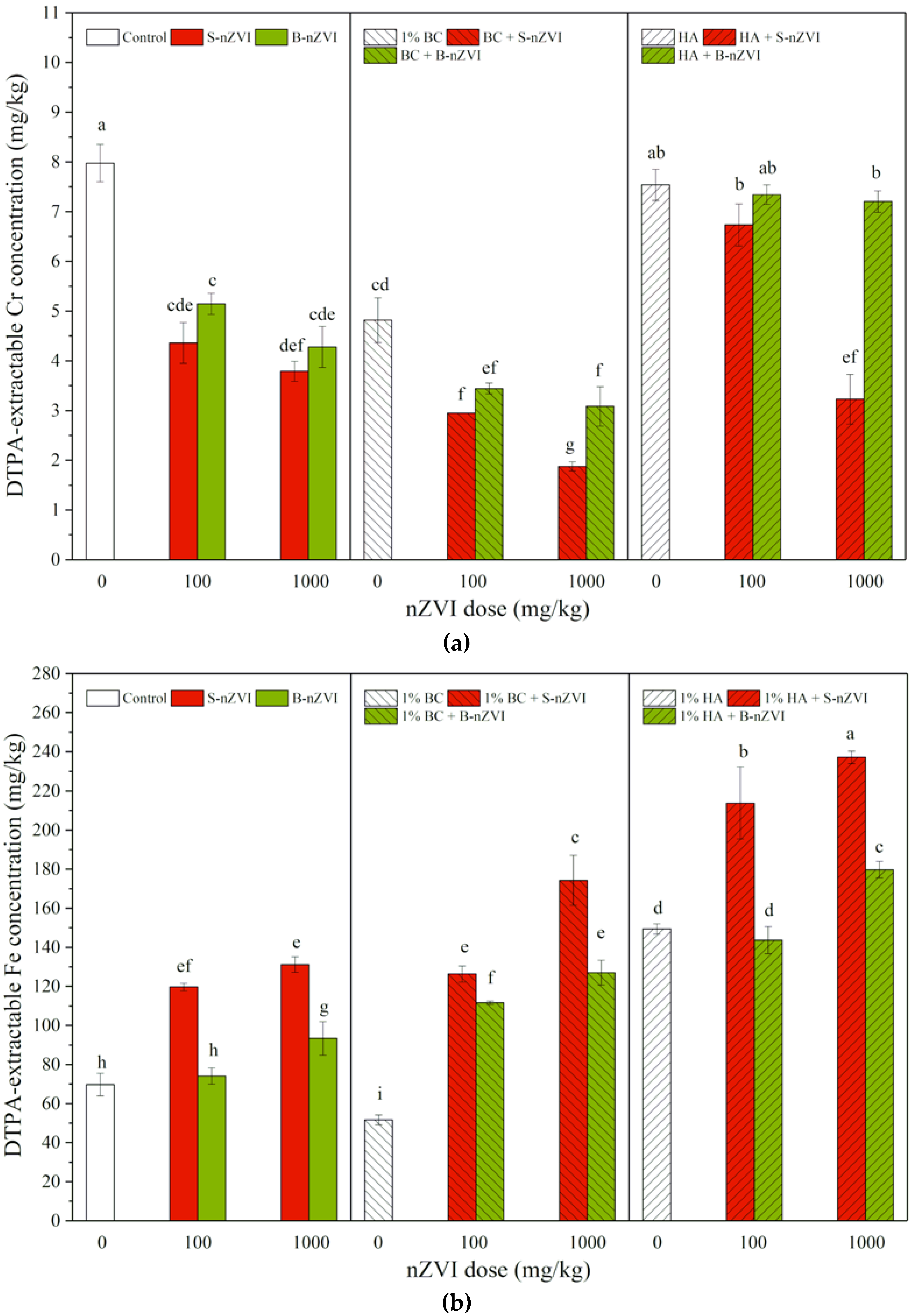
3.2. DTPA-Extractable Cr and Fe Concentrations in Soil
3.3. Soil pH
3.4. Plant Biomass
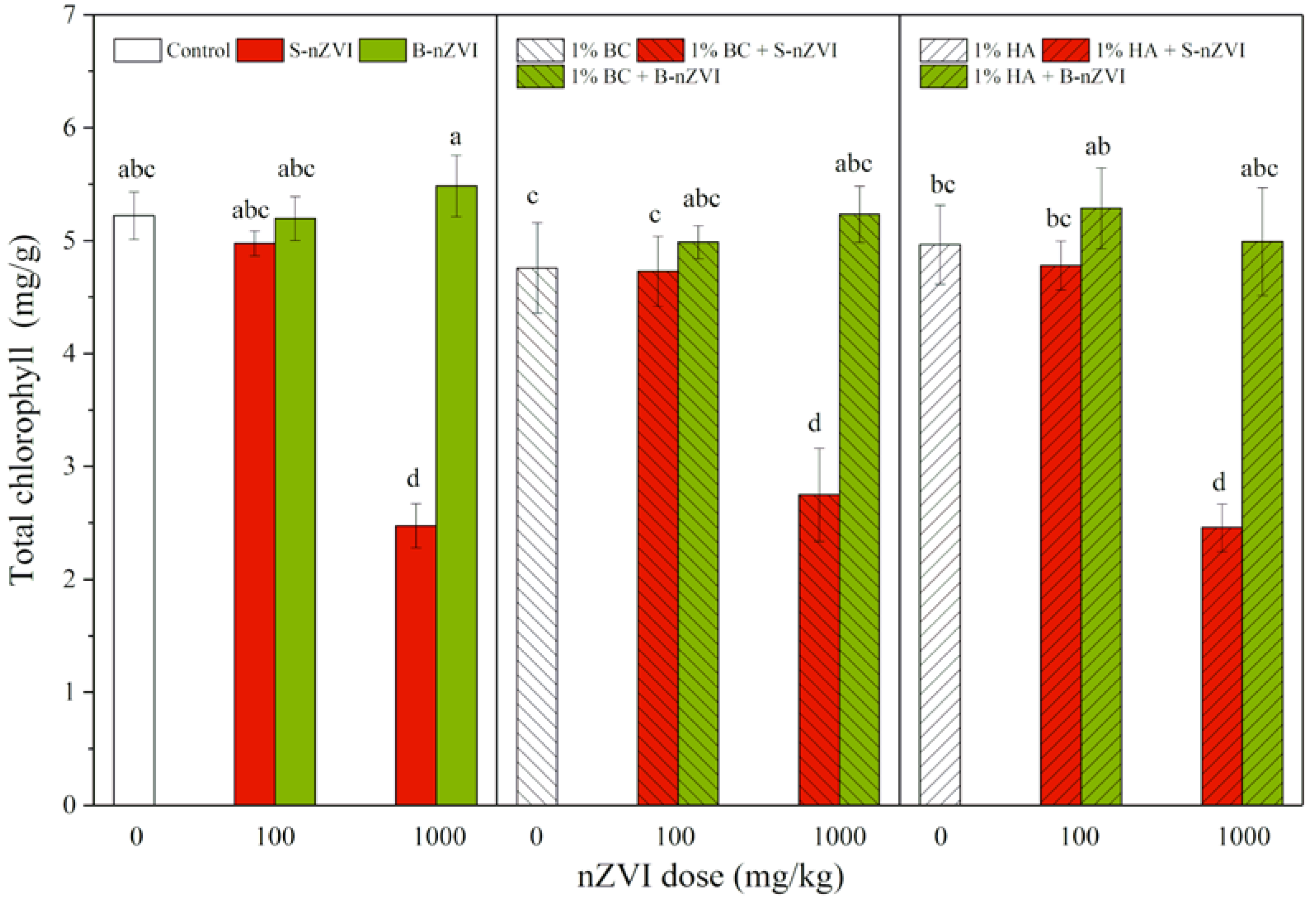
3.5. Chlorophyll Content
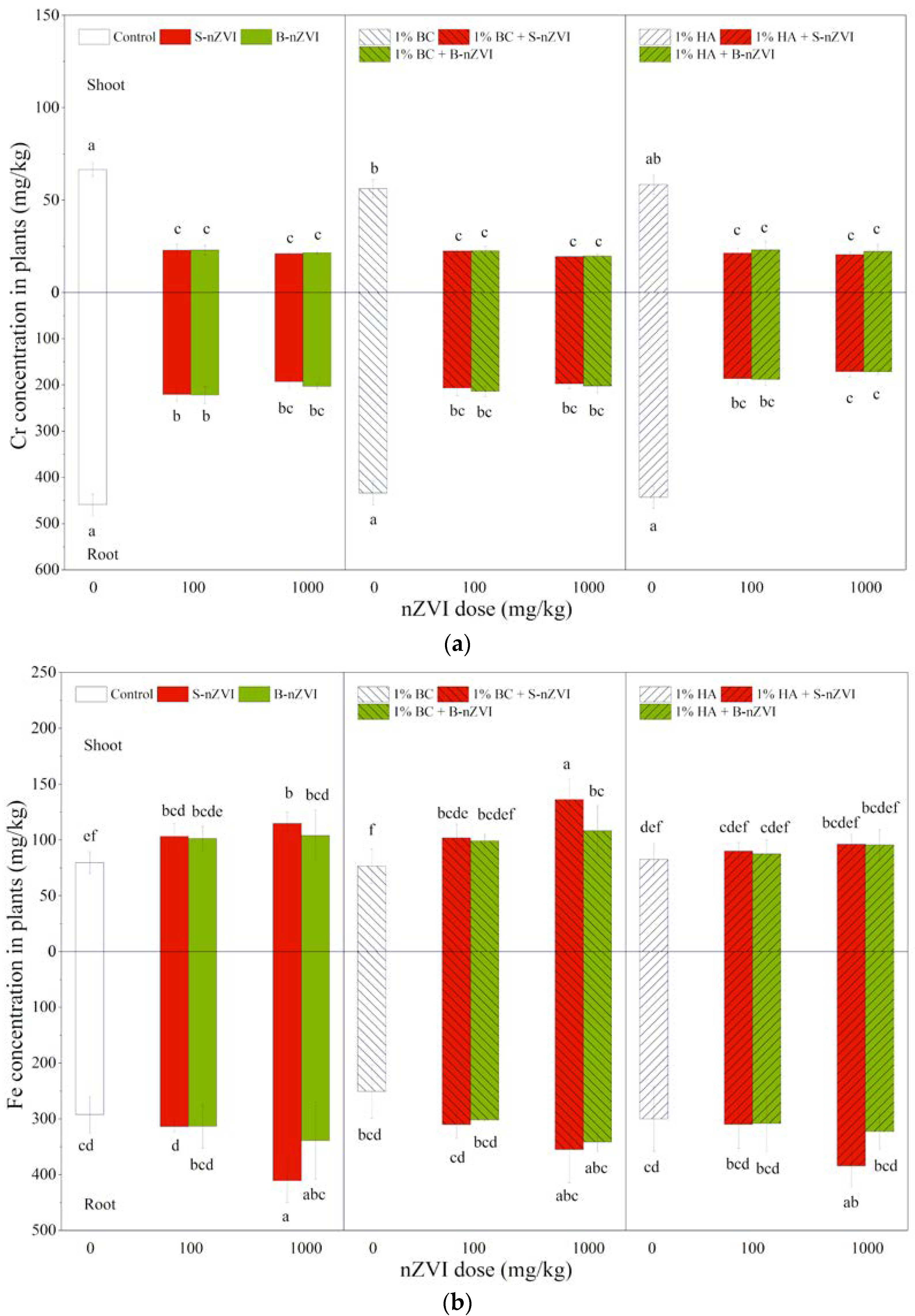
3.6. Cr and Fe Concentrations in Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Palmer, C.D.; Wittbrodt, P.R. Processes affecting the remediation of chromium-contaminated sites. Environ. Health Perspect. 1991, 92, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, B.; Jia, X. Occurrence and fractionation of Cr along the Loushan River affected by a chromium slag heap in East China. Environ. Sci. Pollut. Res. Int. 2017, 24, 15655–15666. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, W.; Zheng, F.; Sun, Y. Removal of Cr (VI) from simulated and leachate wastewaters by bentonite-supported zero-valent iron nanoparticles. Int. J. Environ. Res. Public. Health 2018, 15, 2162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gahoi, P.; Verma, N. Simultaneous scavenging of Cr (VI) from soil and facilitation of nutrient uptake in plant using a mixture of carbon microfibers and nanofibers. Chemosphere 2020, 239, 124760. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, F.; Yang, W.; Wang, F. Removal of chromium from a contaminated soil using oxalic acid, citric acid, and hydrochloric acid: Dynamics, mechanisms, and concomitant removal of non-targeted metals. Int. J. Environ. Res. Public. Health 2019, 16, 2771. [Google Scholar] [CrossRef]
- Prevot, A.B.; Ginepro, M.; Peracaciolo, E.; Zelano, V.; De Luca, D. Chemical vs bio-mediated reduction of hexavalent chromium. An in-vitro study for soil and deep waters remediation. Geoderma 2018, 312, 17–23. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef]
- Wan, J.; Feng, X.; Li, Y.; He, J.; Zhao, N.; Liu, Z.; Lin, Y.; Yang, C.; Liang, W. Effect of mesoporous silica molecular sieve coating on nZVI for 2,4-DCP degradation: Morphology and mechanism during the reaction. Chem. Engin. Process. Process Intensif. 2019, 135, 68–81. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Ma, J.; Liu, H.; Ning, P. Highly efficient immobilization of NZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere 2019, 220, 1003–1013. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Liu, F.; Tang, J.; Huang, F.; Zhang, L. Diversity in the species and fate of chlorine during TCE reduction by two nZVI with non-identical anaerobic corrosion mechanism. Chemosphere 2019, 230, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal (loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Costa, G.; Nande, M.; Martín, C.; Martín, M.; Sánchez-Fortún, S. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total Environ. 2019, 656, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Liu, F.; Yi, S.; Chen, Y.Z.; Geng, X.; Zheng, C. Simultaneous stabilization of Pb and improvement of soil strength using nZVI. Sci. Total Environ. 2019, 651, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, L.; Ren, W.; Deng, X.; Liu, T. Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr (VI) in the soil leachate by nZVI/Ni bimetal material. Environ. Pollut. 2017, 227, 444–450. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Vanzetto, G.V.; Thomé, A. Bibliometric study of the toxicology of nanoescale zero valent iron used in soil remediation. Environ. Pollut. 2019, 252, 74–83. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Ma, S.; Shang, Z. Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem. Eng. J. 2016, 302, 663–669. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.Q.; Tsang, D.C.W.; Lin, D.H. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Yirsaw, B.D.; Megharaj, M.; Chen, Z.L.; Naidu, R. Environmental application and ecological significance of nano-zero valent iron. J. Environ. Sci. 2016, 44, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, R.; Coutris, C.; Nguyen, N.H.A.; Sevcu, A.; Gallego-Urrea, J.A.; Baun, A.; Joner, E.J. Ecotoxicity testing and environmental risk assessment of iron nanomaterials for sub-surface remediation-Recommendations from the FP7 project NanoRem. Chemosphere 2017, 182, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Pangging, M.; Jang, M.H.; Hwang, Y.S.; Chang, Y.S. Impact of surface modification on the toxicity of zerovalent iron nanoparticles in aquatic and terrestrial organisms. Ecotoxicol. Environ. Saf. 2018, 163, 436–443. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619, 815–826. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Bolan, N.; Ok, Y.S.; Naidu, R. Enhancement of chromate reduction in soils by surface modified biochar. J. Environ. Manag. 2017, 186, 277–284. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Megharaj, M.; Chen, Z.; Naidu, R. The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J. Environ. Qual. 2012, 41, 1175–1184. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Enhanced mobilization of arsenic and heavy metals from mine tailings by humic acid. Chemosphere 2009, 74, 274–279. [Google Scholar] [CrossRef]
- Clemente, R.; Bernal, M.P. Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 2006, 64, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, P.R.; Palmer, C.D. Reduction of Cr(VI) by soil humic acids. Eur. J. Soil Sci. 1997, 48, 151–162. [Google Scholar] [CrossRef]
- Leita, L.; Margon, A.; Pastrello, A.; Arčon, I.; Contin, M.; Mosetti, D. Soil humic acids may favour the persistence of hexavalent chromium in soil. Environ. Pollut. 2009, 157, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Tsang, D.C.; Yang, F.; Zhao, L.; Qiu, H.; Cao, X. Participation of soil active components in the reduction of Cr (VI) by biochar: Differing effects of iron mineral alone and its combination with organic acid. J. Hazard. Mater. 2020, 384, 121455. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Tao, X.; Yao, C.; Tsang, D.C.; Cao, X. Interaction with low molecular weight organic acids affects the electron shuttling of biochar for Cr (VI) reduction. J. Hazard. Mater. 2019, 378, 120705. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.Q.; Han, B.; Qian, T.W.; Zhao, D.Y. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, R.; Zheng, F.; Zhang, S.; Jiao, W.; Wang, F. Evaluating phytotoxicity of bare and starch-stabilized zero-valent iron nanoparticles in mung bean. Chemosphere 2019, 236, 124336. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.; Cheng, W.; Tsang, P.E.; Zhao, D. Ageing decreases the phytotoxicity of zero-valent iron nanoparticles in soil cultivated with Oryza sativa. Ecotoxicology 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Demarchi, C.A.; da Silva, L.M.; Ślawska-Waniewska, A.; Nedelko, N.; Dal Magro, J.; Scapinello, J.; Rodrigues, C.A. Phytotoxicity study of Ag@Fe2O3 nanocomposites based on O-carboxymethylchitosan on Cucumis sativus. J. Environ. Chem. Eng. 2019, 7, 102890. [Google Scholar] [CrossRef]
- Ma, X.; Gurung, A.; Deng, Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci. Total Environ. 2013, 443, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Cheng, W.; Yan, X.; Tsang, P.E.; Zhao, D. Higher concentrations of nanoscale zero-valent iron (nZVI) in soil induced rice chlorosis due to inhibited active iron transportation. Environ. Pollut. 2016, 210, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Alam, M.S.; Chen, N.; Alessi, D.S.; Igalavithana, A.D.; Tsang, D.C.; Ok, Y.S. Removal of hexavalent chromium in aqueous solutions using biochar: Chemical and spectroscopic investigations. Sci. Total Environ. 2018, 625, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Fang, J.; Zhao, D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- James, B.R.; Bartlett, R.J. Behavior of chromium in soils. VI. Interactions between oxidation-reduction and organic complexation. J. Environ. Qual. 1983, 12, 173–176. [Google Scholar] [CrossRef]
- Liu, T.; Tsang, D.C.; Lo, I.M. Chromium (VI) reduction kinetics by zero-valent iron in moderately hard water with humic acid: Iron dissolution and humic acid adsorption. Environ. Sci. Technol. 2008, 42, 2092–2098. [Google Scholar] [CrossRef]
- Chuan, M.; Shu, G.; Liu, J. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gil-Díaz, M.; Pinilla, P.; Alonso, J.; Lobo, M. Viability of a nanoremediation process in single or multi-metal (loid) contaminated soils. J. Hazard. Mater. 2017, 321, 812–819. [Google Scholar] [CrossRef]
- Pei, G.; Zhu, Y.; Wen, J.; Pei, Y.; Li, H. Vinegar residue supported nanoscale zero-valent iron: Remediation of hexavalent chromium in soil. Environ. Pollut. 2020, 256, 113407. [Google Scholar] [CrossRef] [PubMed]
- Gil-Díaz, M.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.; Lobo, M.C. Comparing different commercial zero valent iron nanoparticles to immobilize As and Hg in brownfield soil. Sci. Total Environ. 2017, 584, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Misra, V.; Singh, R.P. Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. J. Nanopart. Res. 2011, 13, 4063–4073. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Vose, P.B. Iron nutrition in plants: A world overview. J. Plant Nutr. 1982, 5, 233–249. [Google Scholar] [CrossRef]



| Soil Parameters | Value |
|---|---|
| pH | 6.78 |
| Cr(VI) (mg/kg) | 50.0 |
| Total Cr (mg/kg) | 928.2 |
| Total Cd (mg/kg) | 0.15 |
| DTPA-extractable Fe (mg/kg) | 62.8 |
| DTPA-extractable Zn (mg/kg) | 0.96 |
| Cation exchange capacity (CEC) (cmol/kg) | 7.22 |
| Organic matter (g/kg) | 12.6 |
| Total N (g/kg) | 0.78 |
| Available P (mg/kg) | 289.4 |
| Available K (mg/kg) | 170.6 |
| Nitrate N (mg/kg) | 19.6 |
| Sand (%) | 68.3 |
| Silt (%) | 22.7 |
| Clay (%) | 9.0 |
| Variables | nZVI Type | nZVI Dosage | BC | HA | nZVI Type × Dosage | nZVI Type × BC | nZVI Dosage × BC | nZVI Type × Dosage × BC | nZVI Type × HA | nZVI Dosage × HA | nZVI Type × Dosage × HA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Cr(VI) | 45.2 *** | 83.0 *** | 294.3 *** | 615.3 *** | 0.0 ns | 0.0 ns | 257.9 *** | 0.1 ns | 0.4 ns | 234.5 *** | 0.3 ns |
| DTPA-Cr | 118.7 *** | 82.9 *** | 202.2 *** | 58.5 *** | 38.2 *** | 0.5 ns | 0.0 ns | 2.5 ns | 30.8 *** | 13.7 ** | 37.8 *** |
| DTPA-Fe | 429.5 *** | 153.2 *** | 40.0 *** | 1237.1 *** | 2.0 ns | 3.9 ns | 9.0 ** | 13.8 ** | 16.5 *** | 7.0 * | 0.2 ns |
| Soil pH | 1.1 ns | 11.7 ** | 46.9 *** | 1511.8 *** | 5.7 * | 0.1 ns | 1.5 ns | 0.0 ns | 11.3 ** | 4.4 * | 0.1 ns |
| Shoot FWs | 250.1 *** | 174.2 *** | 1.5 ns | 8.5 ** | 184.1 *** | 0.1 ns | 0.4 ns | 1.9 ns | 0.0 ns | 0.7 ns | 1.0 ns |
| Root FWs | 34.0 *** | 71.5 *** | 0.4 ns | 0.0 ns | 40.9 *** | 0.6 ns | 0.2 ns | 1.9 ns | 0.8 ns | 1.0 ns | 4.7 * |
| Shoot DWs | 109.4 *** | 83.9 *** | 0.4 ns | 3.0 ns | 68.2 *** | 1.0 ns | 1.3 ns | 2.9 ns | 0.0 ns | 0.8 ns | 0.1 ns |
| Root DWs | 54.9 *** | 85.7 *** | 0.1 ns | 0.0 ns | 55.9 *** | 0.5 ns | 0.7 ns | 1.3 ns | 0.1 ns | 0.0 ns | 4.3 * |
| Chlorophyll | 257.9 *** | 140.8 *** | 4.4 * | 3.5 ns | 150.3 *** | 1.1 ns | 1.1 ns | 1.5 ns | 0.2 ns | 0.8 ns | 3.0 ns |
| Shoot Cr conc. | 0.2 ns | 0.9 ns | 3.0 ns | 1.4 ns | 0.0 ns | 0.0 ns | 0.1 ns | 0.0 ns | 0.1 ns | 0.0 ns | 0.0 ns |
| Root Cr conc. | 0.1 ns | 4.7 * | 1.6 ns | 14.8 *** | 0.1 ns | 0.2 ns | 1.2 ns | 0.5 ns | 0.0 ns | 0.6 ns | 0.5 ns |
| Shoot Fe conc. | 1.6 ns | 5.6 * | 0.1 ns | 3.2 ns | 0.7 ns | 0.1 ns | 0.7 ns | 0.1 ns | 0.2 ns | 0.0 ns | 0.3 ns |
| Root Fe conc. | 3.4 ns | 8.8 ** | 3.8 ns | 0.6 ns | 2.5 ns | 0.2 ns | 0.6 ns | 0.5 ns | 0.0 ns | 0.3 ns | 0.0 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zheng, F.; Wang, W.; Zhang, S.; Wang, F. Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance. Toxics 2020, 8, 26. https://doi.org/10.3390/toxics8020026
Sun Y, Zheng F, Wang W, Zhang S, Wang F. Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance. Toxics. 2020; 8(2):26. https://doi.org/10.3390/toxics8020026
Chicago/Turabian StyleSun, Yuhuan, Fangyuan Zheng, Wenjie Wang, Shuwu Zhang, and Fayuan Wang. 2020. "Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance" Toxics 8, no. 2: 26. https://doi.org/10.3390/toxics8020026
APA StyleSun, Y., Zheng, F., Wang, W., Zhang, S., & Wang, F. (2020). Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance. Toxics, 8(2), 26. https://doi.org/10.3390/toxics8020026






