Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Test Treatment
2.3. Measurement Indicators and Methods
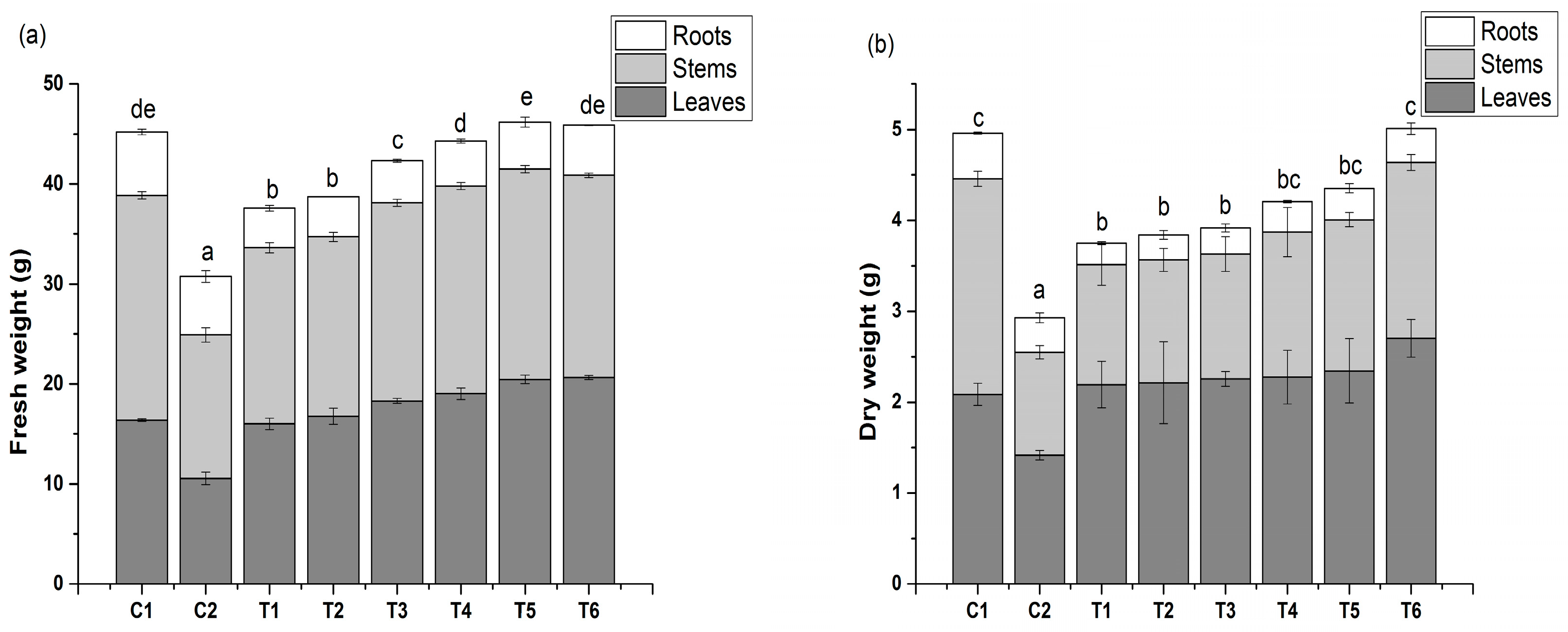
2.3.1. Determination of Biomass
2.3.2. Cu Content Measurements
2.3.3. Biochemical Indicators Measurements
2.3.4. Leaf N, NO3− and NH4+ Contents Measurement
2.3.5. Amino Acids and Protein Concentration Measurements
2.3.6. N Metabolizing Enzymes Activity Measurement
2.4. Statistical Analysis
3. Results
3.1. Effect of IAA Treatment on Total Biomass Accumulation
3.2. Effect of IAA Treatment on Cu Accumulation in the Leaves and Roots
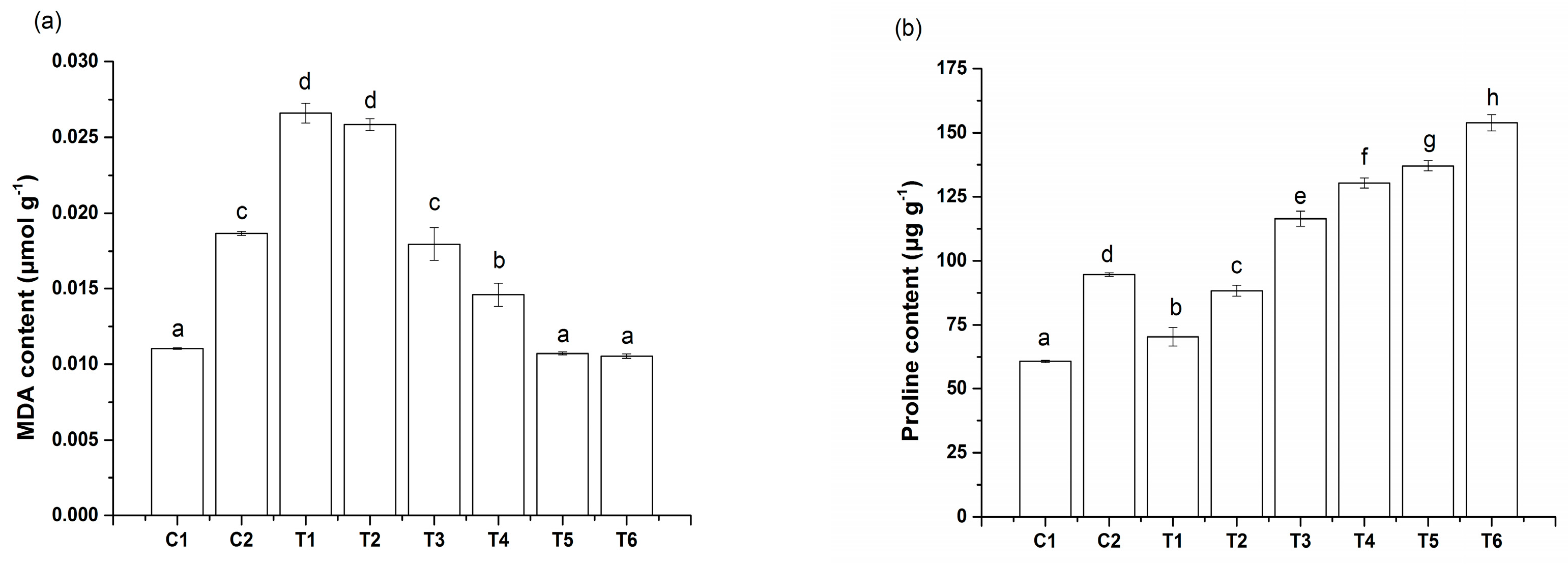
3.3. Effect of IAA Treatment on MDA and Proline Concentrations
3.4. Effect of IAA Treatment on Antioxidant Enzyme Activities
3.5. Effect of IAA Treatment on Total N Content and N Assimilation Compounds
3.6. Effect of IAA Treatment on N Metabolizing Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.C.; Zhang, T.; Stoffella, P.J. Heavy metal contamination of soils: Sources, indicators and assessment. J. Environ. Indic. 2015, 3, 109–112. [Google Scholar]
- Saez, C.A.; Roncarati, F.; Moenne, A.; Moody, A.J.; Brown, M.T. Copper-induced intra-specific oxidative damage and antioxidant responses in strains of the brown alga Ectocarpus siliculosus with different pollution histories. Aquat. Toxicol. 2015, 159, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravet, K.; Pilon, M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.; Maillet, J.; Hinsinger, P.; Pepin, M. Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef]
- Nielsen, H.D.; Brownlee, C.; Coelho, S.M.; Brown, M. Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. New Phytol. 2003, 160, 157–165. [Google Scholar] [CrossRef]
- Massoud, M.B.; Sakouhi, L.; Karmous, I.; Zhu, Y.; Ferjani, E.E.; Sheehan, D.; Chaoui, A. Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef]
- Agami, R.A. Pre-soaking in indole-3-acetic acid or spermidine enhances copper tolerance in wheat seedlings. S. Afr. J. Bot. 2016, 104, 167–174. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Prasad, S.M.; Maurya, J.N. Differential responses of pea seedlings to indole acetic acid under manganese toxicity. Acta Physiol. Plant. 2010, 33, 451–625. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Ilias, I. Hormone-induced protection of sunflower photosynthetic apparatus against copper toxicity. Biol. Plant. 2005, 49, 223–226. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P. Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: Implication of oxidative stress. Sci. Hortic. 2011, 129, 321–328. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol. Biochem. 2016, 109, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Sklodowska, M. Nickel-induced changes in nitrogen metabolism in wheat shoots. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Lisiewska, Z.; Kmiecik, W.; Gębczyński, P.; Sobczyńska, L. Amino acid profile of raw and as-eaten products of spinach (Spinacia oleracea L.). Food Chem. 2011, 126, 460–465. [Google Scholar] [CrossRef]
- Becker, C.; Klaering, H.P.; Kroh, L.W.; Krumbein, A. Cool-cultivated red leaf lettuce accumulates cyanidin-3-O-(6″-O-malonyl)-glucoside and caffeoylmalic acid. Food Chem. 2014, 146, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Anjum, M.A.; Akhtar, S. Monitoring of Growth, Yield, Biomass and Heavy Metals Accumulation in Spinach Grown under Different Irrigation Sources. Int. J. Agric. Biol. 2016, 18, 689–697. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, L.; Dai, T.; Zhou, J.; Kang, Q.; Chen, H.; Li, Z.H. Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol. Environ. Saf. 2019, 171, 771–780. [Google Scholar] [CrossRef]
- Naz, A.; Khan, S.; Qasim, M.; Khalid, S.; Muhammad, S.; Tariq, M. Metals toxicity and its bioaccumulation in purslane seedlings grown in controlled environment. Nat. Sci. 2013, 5, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239–240, 302–307. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N.; Bisht, S.S. Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci. 2002, 162, 381–388. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Kohli, R.K. Effect of 2-benzoxazolinone (BOA) on seedling growth and associated biochemical changes in mung bean (Phaseolus aureus). Z. Naturforsch. C J. Biosci. 2006, 61, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Asgher, M.; Khan, N.A.; Khan, M.I.; Fatma, M.; Masood, A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol. Environ. Saf. 2014, 106, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.; Mulvaney, C. Nitrogen–total. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Xiong, Z.T.; Liu, C.; Geng, B. Phytotoxic effects of copper on nitrogen metabolism and plant growth in Brassica pekinensis Rupr. Ecotoxicol. Environ. Saf. 2006, 64, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Singh, K.; Pandey, S.N. Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce, Pistia stratiotes L. Environ. Biol. 2011, 32, 391–394. [Google Scholar]
- Yadav, P.; Kaur, R.; Kanwar, M.K.; Sharma, A.; Verma, V.; Sirhindi, G.; Bhardwaj, R. Castasterone confers copper stress tolerance by regulating antioxidant enzyme responses, antioxidants, and amino acid balance in B. juncea seedlings. Ecotoxicol. Environ. Saf. 2018, 147, 725–734. [Google Scholar] [CrossRef]
- Chaudhry, N.Y.; Rasheed, S. Study of the external and internal morphology of Pisum sativum L., with growth hormones ie, indole-3-acetic acid and kinetin and heavy metal ie, lead nitrate. Pak. Biol. Sci. 2003, 6, 407–412. [Google Scholar]
- Ali, B.; Rani, I.; Hayat, S.; Ahmad, A. Effect of 4-Cl-indole acetic acid on seed germination of Cicer arietinum exposed to cadmium. Acta Bot. Croat. 2007, 66, 57–65. [Google Scholar]
- Wang, H.; Shan, X.; Wen, B.; Owens, G.; Fang, J.; Zhang, S. Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environ. Exp. Bot. 2007, 61, 246–253. [Google Scholar] [CrossRef]
- Fassler, E.; Evangelou, M.W.; Robinson, B.H.; Schulin, R. Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 2010, 80, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martinez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Perez-Alforcea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato ( Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Rucinska-Sobkowiak, R.; Pukacki, P.M. Antioxidative defense system in lupin roots exposed to increasing concentrations of lead. Acta Physiol. Plant. 2006, 28, 357–364. [Google Scholar] [CrossRef]
- Upadhyay, R.; Panda, S.K. Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J. Hazard. Mater. 2010, 175, 1081–1084. [Google Scholar] [CrossRef]
- Bajguz, A. Effect of brassinosteroids on nucleic acid and protein content in cultured cell of Chlorella vulgaris. Plant Physiol. Biochem. 2000, 38, 209–215. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Karmous, I.; El Ferjani, E.; Chaoui, A. Alleviation of copper toxicity in germinating pea seeds by IAA, GA3, Ca and citric acid. J. Plant Interact. 2017, 13, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Choudharya, S.P.; Bhardwajb, R.; Guptac, B.D.; Duttc, P.; Guptac, R.K.; Biondid, S.; Kanwarb, M. Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol. Plant. 2010, 140, 280–296. [Google Scholar] [CrossRef]
- Llorens, N.; Arola, L.; Bladé, C.; Mas, A. Effects of copper exposure upon nitrogen metabolism in tissue cultured Vitis vinifera. Plant Sci. 2000, 160, 159–163. [Google Scholar] [CrossRef]
- Hippler, F.W.R.; Mattos, D.J.; Boaretto, R.M.; Williams, L.E. Copper excess reduces nitrate uptake by Arabidopsis roots with specific effects on gene expression. J. Plant Physiol. 2018, 228, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Soutome, R.; Hiroyama, K.; Hagio, T.; Ida, S.; Nakagawa, H. Co-regulation of nitrate reductase and nitrite reductase in cultured spinach cells. Plant Physiol. 2000, 157, 299–306. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Pageau, K.; Lelandais, M.; Grandjean, O.; Kronenberger, J.; Valadier, M.H.; Feraud, M.; Jouglet, T.; Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006, 140, 444–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhou, Q.; Ding, L.; Sun, Y. Effect of cadmium toxicity on nitrogen metabolism in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. J. Hazard. Mater. 2008, 154, 818–825. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. cv. Strike) under high NH4NO3 application rates. Sci. Hortic. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- Feng, J.; Volk, R.J.; Jackson, W.A. Source and magnitude of ammonium generation in maize roots. Plant Physiol. 1998, 118, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Gouia, H.; Suzuki, A.; Brulfert, J.; Ghorbal, M.H. Effects of cadmium on the co-ordination of nitrogen and carbon metabolism in bean seedlings. J. Plant Physiol. 2003, 160, 367–376. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. Jexpbot 2002, 53, 979–982. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shi, G.; Xu, Q.; Hu, J. Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J. Plant Physiol. 2007, 164, 1062–1070. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sechley, K.; Yamaya, T.; Oaks, A. Compartmentation of nitrogen assimilation in higher plants. Int. Rev. Cytol. 1992, 134, 85–163. [Google Scholar]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2006, 58, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Cao, X.; Bai, Z.; Zhang, J.; Zhu, L.; Huang, J.; Jin, Q. Nitrogen metabolism correlates with the acclimation of photosynthesis to short-term water stress in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 125, 52–62. [Google Scholar] [CrossRef]
- Hoai, N.T.T.; Shim, I.S.; Kobayashi, K.; Kenji, U. Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2003, 41, 159–164. [Google Scholar] [CrossRef]
- Zhong, C.; Bai, Z.G.; Zhu, L.F.; Zhang, J.H.; Zhu, C.Q.; Huang, J.L.; Jin, Q.Y.; Cao, X.C. Nitrogen-mediated alleviation of photosynthetic inhibition under moderate water deficit stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2019, 157, 269–282. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, I. Physiological Effects of Some Uriede Compounds on Cultured Phaseolus Vulgaris Seedlings In Vivo and In Vitro. Master’s Thesis, Fac Sci Mans University, Mansoura, Egypt, 1999; pp. 34–36. [Google Scholar]

| Treatment | Leaf Concentration | Root Concentration |
|---|---|---|
| (μg g−1) | (μg g−1) | |
| C1 | 19.72 ± 0.14 a | 7.97 ± 0.06 a |
| C2 | 2875.00 ± 2.16 h | 194.15 ± 1.06 d |
| T1 | 1777.83 ± 15.46 g | 232.02 ± 0.78 e |
| T2 | 1616.50 ± 13.08 f | 601.50 ± 8.98 g |
| T3 | 1475.50 ± 16.57 e | 616.17 ± 2.49 h |
| T4 | 1319.17 ± 1.25 d | 566.33 ± 9.80 f |
| T5 | 122.03 ± 2.62 c | 137.55 ± 4.71 c |
| T6 | 72.58 ± 5.03 b | 25.87 ± 0.06 b |
| Treatment | Total N Content (g kg−1) | NO3− Content (g kg−1) | NH4+ Content (g kg−1) | Free Amino Acid Content (g kg−1) | Soluble Protein Content (g kg−1) |
|---|---|---|---|---|---|
| C1 | 83.24 ± 0.91 e | 1.24 ± 0.016 f | 0.07 ± 0.0018 a | 0.76 ± 0.010 f | 18.82 ± 0.60 cd |
| C2 | 80.54 ± 0.66 cde | 0.70 ± 0.039 a | 0.17 ± 0.0001 h | 0.39 ± 0.004 b | 12.03 ± 0.14 a |
| T1 | 77.47 ± 1.92 ab | 0.75 ± 0.006 b | 0.16 ± 0.0002 g | 0.51 ± 0.014 d | 14.83 ± 1.18 b |
| T2 | 78.06 ± 1.64 abc | 1.10 ± 0.018 d | 0.12 ± 0.0003 f | 0.49 ± 0.012 d | 13.72 ± 1.18 ab |
| T3 | 76.02 ± 1.48 a | 1.01 ± 0.0041 c | 0.11 ± 0.0004 d | 0.41 ± 0.020 b | 13.74 ± 0.70 ab |
| T4 | 78.91 ± 1.58 bcd | 1.19 ± 0.0039 e | 0.12 ± 0.0006 e | 0.67 ± 0.016 e | 14.88 ± 1.28 b |
| T5 | 81.14 ± 0.35 de | 1.23 ± 0.0084 ef | 0.09 ± 0.0006 b | 0.30 ± 0.011 a | 17.70 ± 0.05 c |
| T6 | 81.24 ± 0.34 de | 1.44 ± 0.039 g | 0.10 ± 0.0005 c | 0.44 ± 0.014 c | 20.51 ± 0.77 d |
| Treatment | NR (U g−1) | NiR (U g−1) | GS (U g−1) | GOGAT (U g−1) | GDH (U g−1) |
|---|---|---|---|---|---|
| C1 | 1224.75 ± 18.71 e | 2537.13 ± 15.71 f | 0.48 ± 0.004 f | 1.78 ± 0.028 d | 0.044 ± 0.0003 a |
| C2 | 517.03 ± 14.06 a | 1759.71 ± 3.93 b | 0.37 ± 0.003 c | 1.56 ± 0.019 c | 0.061 ± 0.0014 c |
| T1 | 659.59 ± 8.2 5b | 1582.01 ± 61.21 a | 0.23 ± 0.006 a | 1.30 ± 0.026 b | 0.051 ± 0.0009 b |
| T2 | 803.43 ± 34.34 c | 1848.56 ± 37.87 c | 0.29 ± 0.006 b | 1.24 ± 0.016 a | 0.072 ± 0.0010 d |
| T3 | 658.32 ± 10.95 b | 2128.98 ± 21.86 d | 0.37 ± 0.005 c | 1.81 ± 0.016 d | 0.088 ± 0.0003 f |
| T4 | 781.79 ± 12.47 c | 2495.48 ± 48.25 ef | 0.41 ± 0.007 d | 2.09 ± 0.013 g | 0.088 ± 0.0013 f |
| T5 | 819.98 ± 8.25 c | 2437.17 ± 34.23 e | 0.41 ± 0.004 d | 1.99 ± 0.006 f | 0.079 ± 0.0019 e |
| T6 | 1045.28 ± 22.48 d | 2792.57 ± 23.89 g | 0.42 ± 0.003 e | 1.92 ± 0.005 e | 0.072 ± 0.0015 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Q.; Li, Z.; Wang, L.; Dai, T.; Kang, Q.; Niu, D. Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics 2020, 8, 1. https://doi.org/10.3390/toxics8010001
Gong Q, Li Z, Wang L, Dai T, Kang Q, Niu D. Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics. 2020; 8(1):1. https://doi.org/10.3390/toxics8010001
Chicago/Turabian StyleGong, Qin, Zhaohua Li, Ling Wang, Tongwei Dai, Qun Kang, and Duandan Niu. 2020. "Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism" Toxics 8, no. 1: 1. https://doi.org/10.3390/toxics8010001
APA StyleGong, Q., Li, Z., Wang, L., Dai, T., Kang, Q., & Niu, D. (2020). Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics, 8(1), 1. https://doi.org/10.3390/toxics8010001




