Toxicity and Organ Distribution of Mercury in Freshwater Fish (Oreochromis niloticus) after Exposure to Water Contaminated Mercury (HgII)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Toxicity Test
2.3. Ethical Approval
2.4. Mercury Accumulation Test
2.5. Mercury Analysis in Fish Tissue
2.6. Histopathology Preparations
2.7. Data Analysis
3. Results and Discussion
3.1. Toxicity of Oreochromis niloticus against Hg(II)
3.2. Mercury Accumulation and Its Organ Distribution
3.3. Pathology Conditions
3.4. Overview of Histopathology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Washburn, S.J.; Blum, J.D.; Kurz, A.Y.; Pizzuto, J.E. Spatial and temporal variation in the isotopic composition of mercury in the South River, VA. Chem. Geol. 2018, 494, 96–108. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci. Total Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.X. Inter-species differences of total mercury and methylmercury in farmed fish in Southern China: Does feed matter? Sci. Total Environ. 2019, 651, 1857–1866. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Crowley, S.M.; Hodder, D.P.; Johnson, C.J.; Yates, D. Wildlife health indicators and mercury exposure: A case study of river otters (Lontra canadensis) in central British Columbia, Canada. Ecol. Indic. 2018, 89, 63–73. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, L.; Wang, Y.; Xie, Q.; Yin, D.; Feng, X.; Wang, D. Bioaccumulation characteristics of mercury in fish in the Three Gorges Reservoir, China. Environ. Pollut. 2018, 243, 115–126. [Google Scholar] [CrossRef]
- André, J.M.; Ribeyre, F.; Boudou, A. Mercury contamination levels and distribution in tissues and organs of delphinids (Stenella attenuata) from the eastern tropical Pacific, in relation to biological and ecological factors. Mar. Environ. Res. 1990, 30, 43–72. [Google Scholar] [CrossRef]
- Costa, M.F.; Landing, W.M.; Kehrig, H.A.; Barletta, M.; Holmes, C.D.; Barrocas, P.R.G.; Evers, D.C.; Buck, D.G.; Vasconcellos, A.C.; Hacon, S.S. Mercury in tropical and subtropical coastal environments. Environ. Res. 2012, 119, 88–100. [Google Scholar] [CrossRef]
- Horvat, M.; Covelli, S.; Faganeli, J.; Logar, M.; Mandić, V.; Rajar, R.; Širca, A.; Žagar, D. Mercury in contaminated coastal environments; a case study: The Gulf of Trieste. Sci. Total Environ. 1999, 237, 43–56. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Garcıa-Rosales, S.B.; Páez-Osuna, F. Distribution of mercury in adult penaeid shrimps from Altata-Ensenada del Pabellón lagoon (SE Gulf of California). Chemosphere 2004, 57, 1657–1661. [Google Scholar] [CrossRef]
- Liu, M.; Kakade, A.; Liu, P.; Wang, P.; Tang, Y.; Li, X. Science of the Total Environment Hg 2 + -binding peptide decreases mercury ion accumulation in fi sh through a cell surface display system. Sci. Total Environ. 2019, 659, 540–547. [Google Scholar] [CrossRef]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, R.M.; Bhalke, S.; Shukla, V.K.; Puranik, V.D. Determination of methylmercury and mercury (II) in a marine ecosystem using solid-phase microextraction gas chromatography-mass spectrometry. Anal. Chim. Acta 2005, 551, 192–198. [Google Scholar] [CrossRef]
- Limbong, D.; Kumampung, J.; Rimper, J.; Arai, T.; Miyazaki, N. Emissions and environmental implications of mercury from artisanal gold mining in north Sulawesi, Indonesia. Sci. Total Environ. 2003, 302, 227–236. [Google Scholar] [CrossRef]
- Suhendrayatna, G. Mercury Levels and Distribution in Organs of Freshwater Organisms from Krueng Sabe River, Aceh Jaya, Indonesia. In Proceedings of the 6th Annual International Workshop & Expo on Sumatra Tsunami Disaster & Recovery in Conjuction with 4th South China Sea Tsunami Workshop, Banda Aceh, Aceh, Indonesia, 22–24 November 2011; pp. 2086–3195. [Google Scholar]
- Suhendrayatna, S.; Elvitriana, E. Mercury in Sediment and Freshwater Organisms From Kr. Sikulat River Around the Artisanal Gold Mining Plants in Sawang, Aceh Province, Indonesia. In Proceedings of the 2nd Syiah Kuala University Annual International Conference, Syiah Kuala University, Banda Aceh, Indonesia, 22–24 November 2012; pp. 159–164. [Google Scholar]
- Male, Y.T.; Reichelt-Brushett, A.J.; Pocock, M.; Nanlohy, A. Recent mercury contamination from artisanal gold mining on Buru Island, Indonesia–Potential future risks to environmental health and food safety. Mar. Pollut. Bull. 2013, 77, 428–433. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105. [Google Scholar] [CrossRef]
- Teh, S.J.; Adams, S.M.; Hinton, D.E. Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquat. Toxicol. 1997, 37, 51–70. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Comparative Biochemistry and Physiology, Part C Is the fish embryo toxicity test ( FET ) with the zebra fish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C 2009, 149, 196–209. [Google Scholar]
- Rahimibashar, M.R.; Alipoor, V. The determination of LC50 and bioconcentration of mercury chloride (HgCl2) in (Esox lucius). World Appl. Sci. J. 2012, 17, 735–738. [Google Scholar]
- Jezierska, B.; Witeska, M. The metal uptake and accumulation in fish living in polluted waters. In Soil and Water Pollution Monitoring, Protection and Remediation; Springer: Dordrecht, The Netherlands, 2006; pp. 107–114. [Google Scholar]
- Giguère, A.; Campbell, P.G.C.; Hare, L.; McDonald, D.G.; Rasmussen, J.B. Influence of lake chemistry and fish age on cadmium, copper, and zinc concentrations in various organs of indigenous yellow perch (Perca flavescens). Can. J. Fish. Aquat. Sci. 2004, 61, 1702–1716. [Google Scholar] [CrossRef]
- Al-Mohanna, F.A.; Caddy, K.W.T.; Bolsover, S.R. The nucleus is insulated from large cytosolic calcium ion changes. Nature 1994, 367, 745. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Tayama, M.; Inouye, M.; Yasutake, A. Distribution and chemical form of mercury in commercial fish tissues. J. Toxicol. Sci. 2012, 37, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.; Voegborlo, R.B.; Agorku, E.S. Total mercury distribution in different tissues of six species of freshwater fish from the Kpong hydroelectric reservoir in Ghana. Environ. Monit. Assess. 2012, 184, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Suhendrayatna, O.A.; Nakajima, T.; Maeda, S. Metabolism and organ distribution of arsenic in the freshwater fish Tilapia mossambica. Appl. Organomet. Chem. 2001, 15, 566–571. [Google Scholar] [CrossRef]
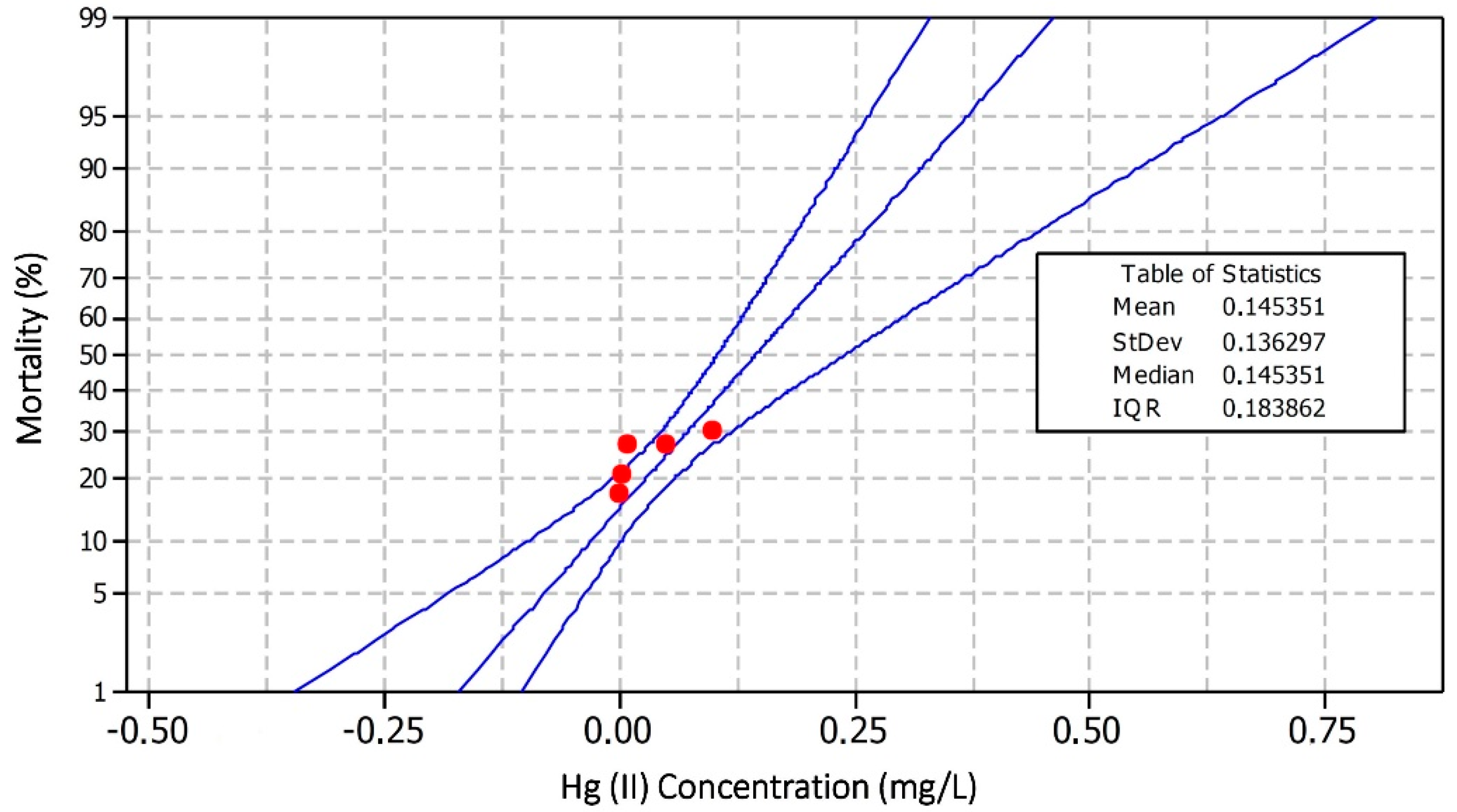
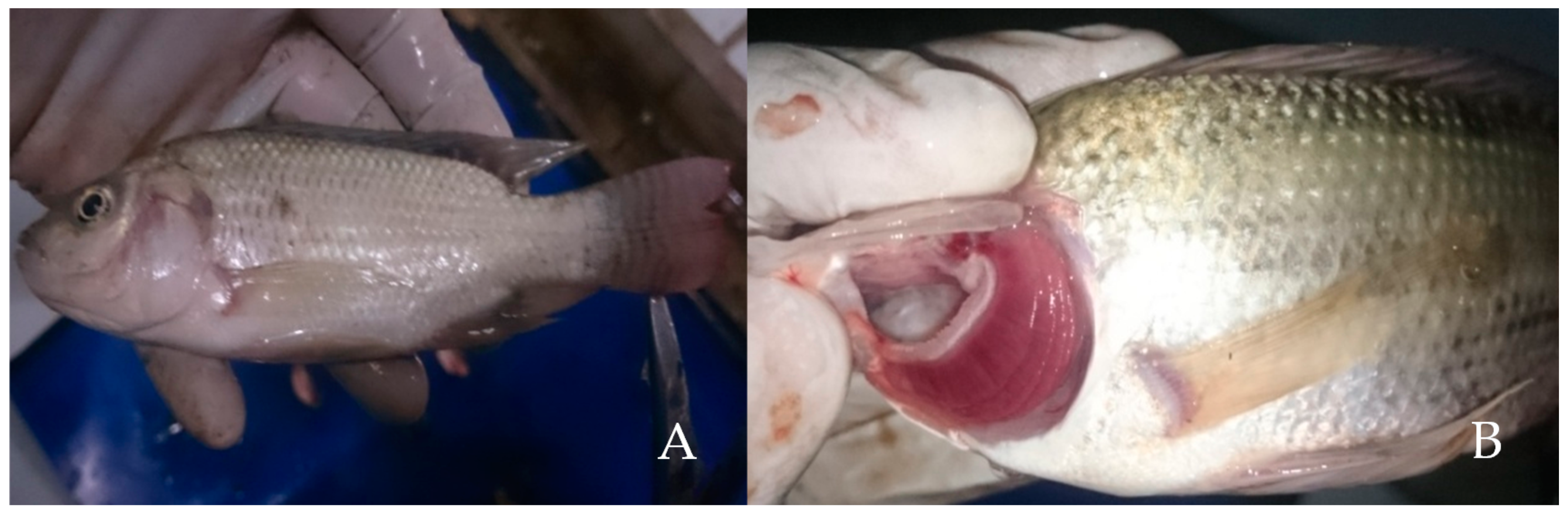
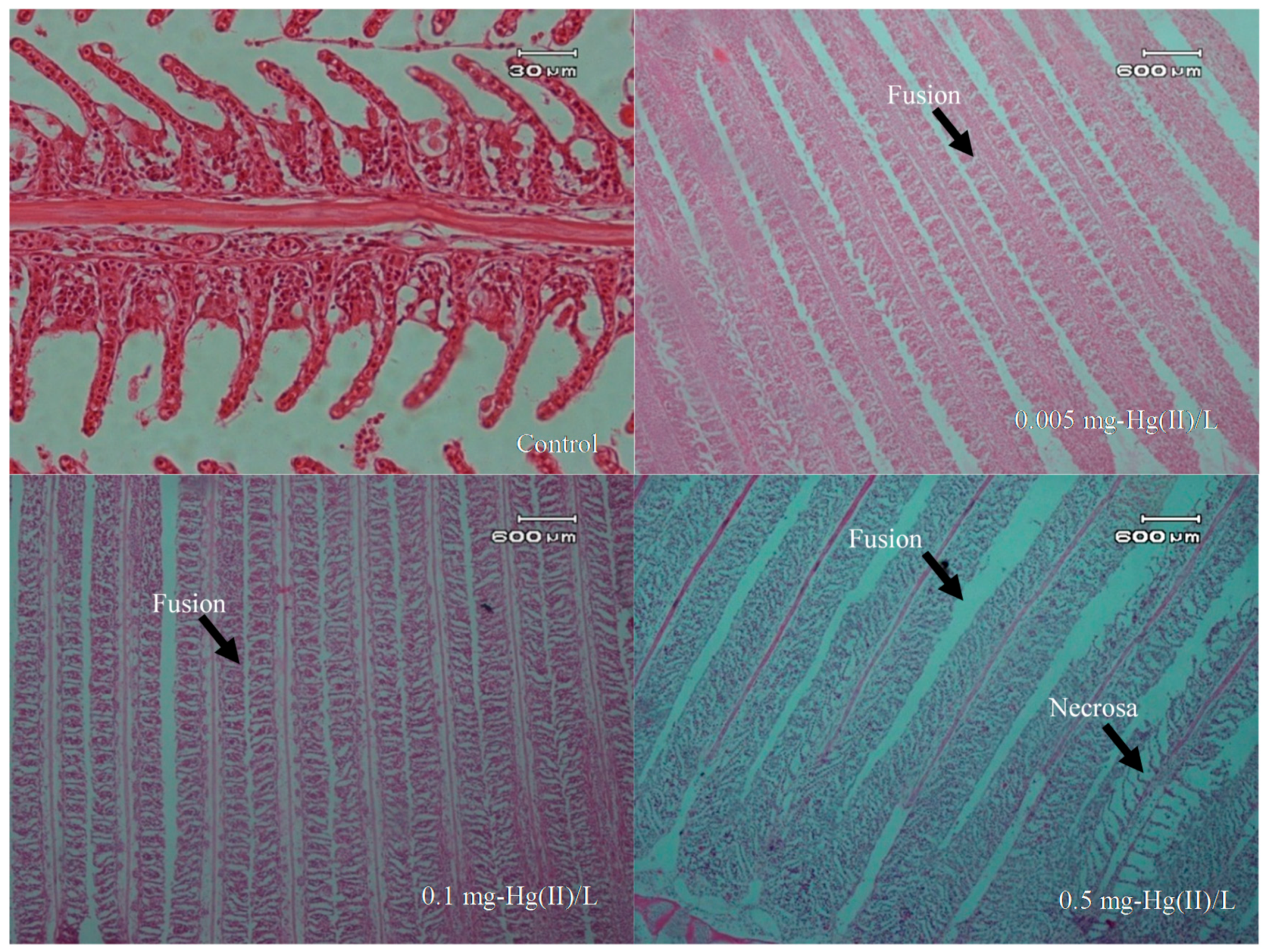
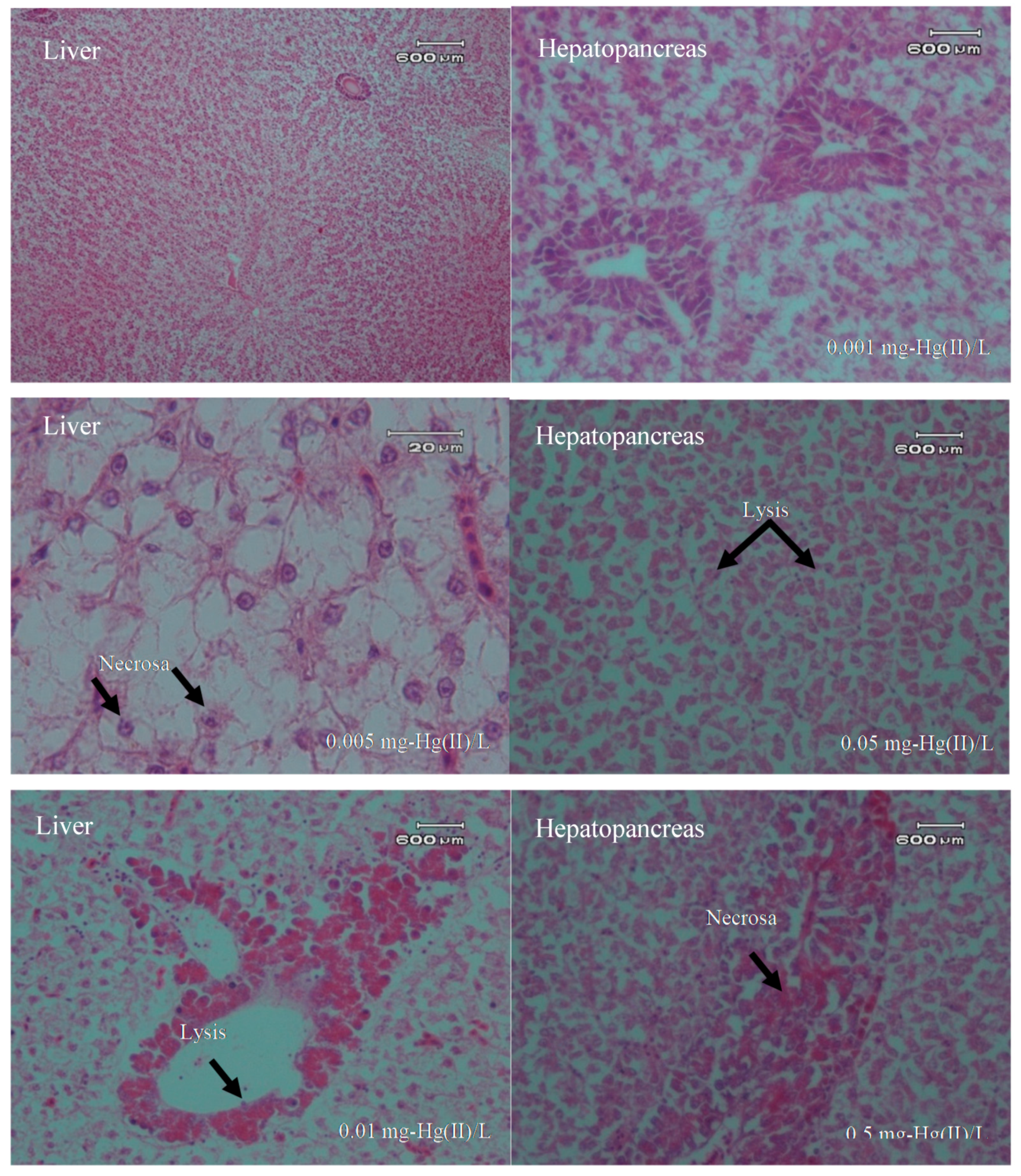
| Hg Concentration in the Water Phase (mg/L) | Repetition | No. of Fish | Mortality | Average Mortality |
|---|---|---|---|---|
| 0 (control) | 1 | 10 | 0 | 0.0 |
| 2 | 10 | 0 | ||
| 3 | 10 | 0 | ||
| 0.0012 | 1 | 10 | 2 | 1.67 |
| 2 | 10 | 1 | ||
| 3 | 10 | 2 | ||
| 0.0049 | 1 | 10 | 2 | 2.0 |
| 2 | 10 | 2 | ||
| 3 | 10 | 2 | ||
| 0.0141 | 1 | 10 | 3 | 2.33 |
| 2 | 10 | 2 | ||
| 3 | 10 | 2 | ||
| 0.0524 | 1 | 10 | 3 | 2.67 |
| 2 | 10 | 3 | ||
| 3 | 10 | 2 | ||
| 0.1126 | 1 | 10 | 3 | 3.0 |
| 2 | 10 | 3 | ||
| 3 | 10 | 3 | ||
| 0.5110 | 1 | 10 | 10 | 10 |
| 2 | 10 | 10 | ||
| 3 | 10 | 10 |
| Hg(II) in the Water Phase (mg-Hg)/L | Hg Concentration in An Organ (µg-Hg/g Dry Cells) | ||||
|---|---|---|---|---|---|
| Head | Muscle | Eye | Bone | Gill | |
| 0 (Control) | 0.26 ± 0.37 ** | 0.48 ± 0.22 ** | 0.37 ± 0.04 ** | 0.42 ± 0.04 ** | 0.35 ± 0.08 ** |
| 0.511 | 0.376 | 5.718 | 4.310 | 3.960 | tr * |
| Organs | Histopathology | Damage Level | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Gill | Lamela fusion | +++ | +++ | +++ |
| Necrosa | − | − | ++ | |
| Liver | Lysis | +++ | ++ | +++ |
| Necrosa | + | + | + | |
| Hepatopancreas | Lysis | ++ | ++ | +++ |
| Necrosa | + | + | ++ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhendrayatna, S.; Arahman, N.; Sipahutar, L.W.; Rinidar, R.; Elvitriana, E. Toxicity and Organ Distribution of Mercury in Freshwater Fish (Oreochromis niloticus) after Exposure to Water Contaminated Mercury (HgII). Toxics 2019, 7, 58. https://doi.org/10.3390/toxics7040058
Suhendrayatna S, Arahman N, Sipahutar LW, Rinidar R, Elvitriana E. Toxicity and Organ Distribution of Mercury in Freshwater Fish (Oreochromis niloticus) after Exposure to Water Contaminated Mercury (HgII). Toxics. 2019; 7(4):58. https://doi.org/10.3390/toxics7040058
Chicago/Turabian StyleSuhendrayatna, Suhendrayatna, Nasrul Arahman, Luky Wahyu Sipahutar, Rinidar Rinidar, and Elvitriana Elvitriana. 2019. "Toxicity and Organ Distribution of Mercury in Freshwater Fish (Oreochromis niloticus) after Exposure to Water Contaminated Mercury (HgII)" Toxics 7, no. 4: 58. https://doi.org/10.3390/toxics7040058
APA StyleSuhendrayatna, S., Arahman, N., Sipahutar, L. W., Rinidar, R., & Elvitriana, E. (2019). Toxicity and Organ Distribution of Mercury in Freshwater Fish (Oreochromis niloticus) after Exposure to Water Contaminated Mercury (HgII). Toxics, 7(4), 58. https://doi.org/10.3390/toxics7040058






