Protein Adductomics: Analytical Developments and Applications in Human Biomonitoring
Abstract
1. The Exposome and Adductomics
2. Approaches to Protein Adductomics
2.1. Hb as A Target of Electrophiles
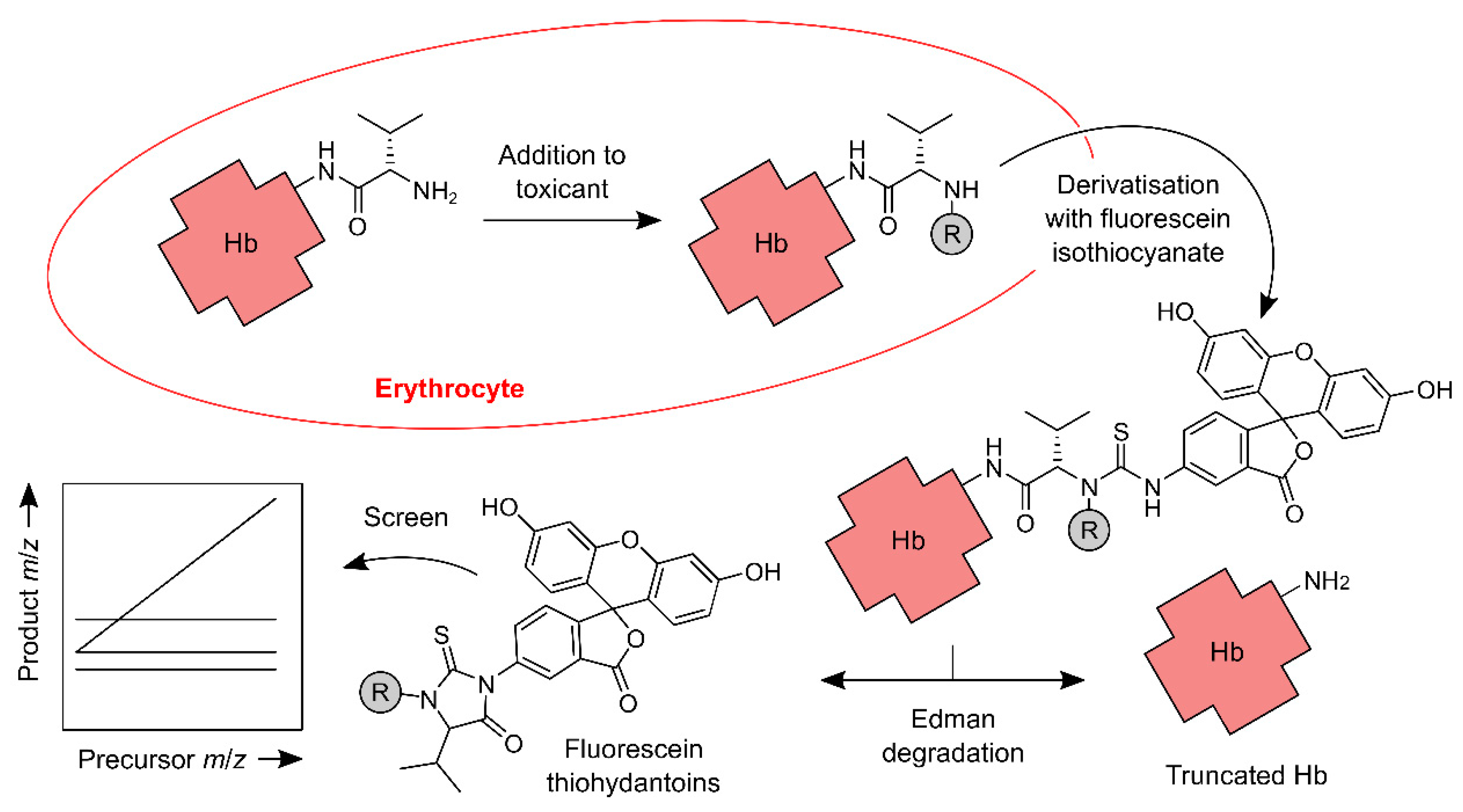
2.2. The N-alkyl Edman Method
2.3. The Role of Tandem Mass Spectrometry in Protein Adductomics
2.4. Stepped MS/MS Methods
2.5. The FIRE Screening Procedure
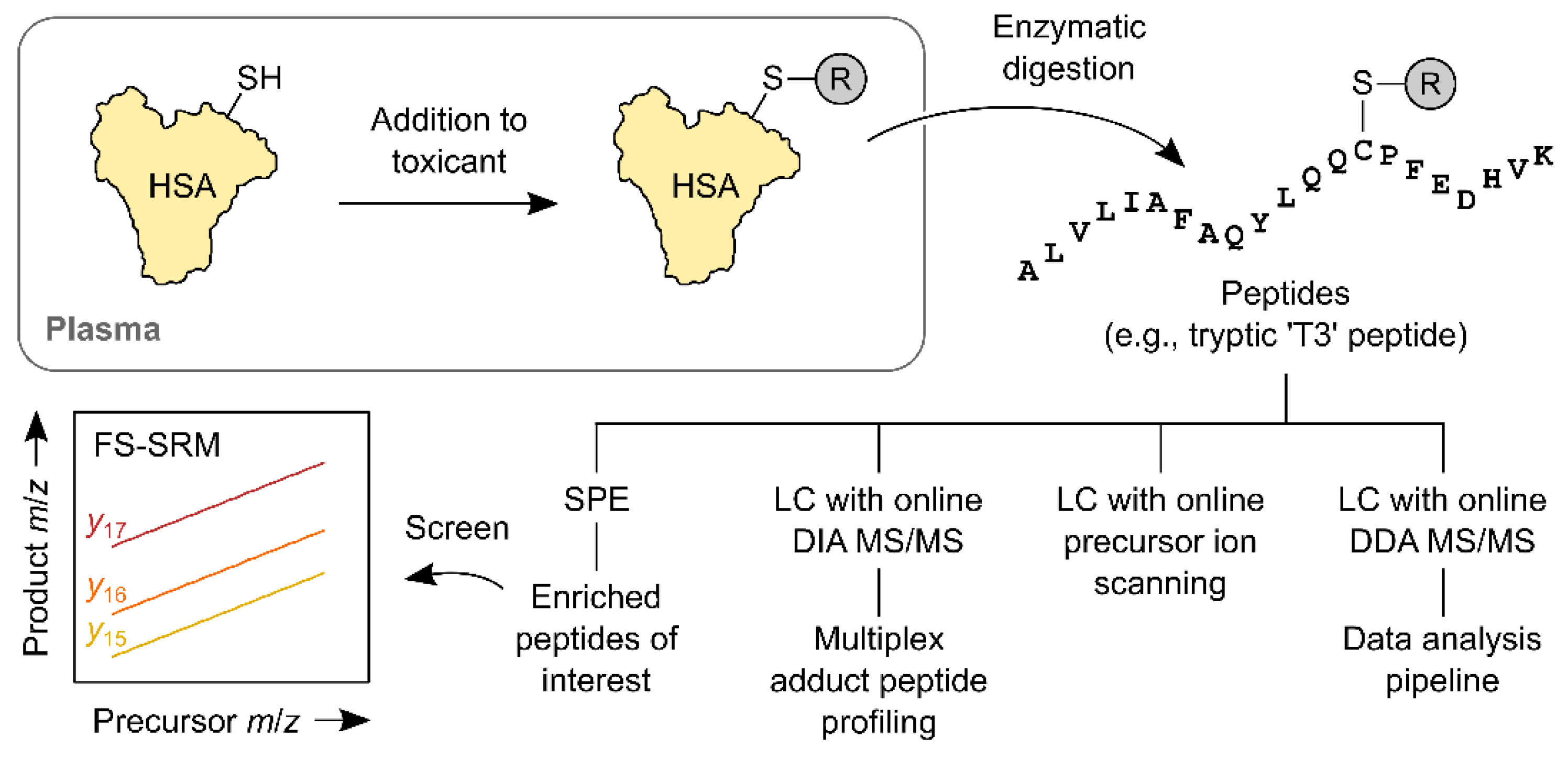
2.6. HSA as A Target of Electrophiles
2.7. HSA Cys-34 Adductomics
2.8. Fixed-Step SRM of HSA Adducts
2.9. Multiplex Adduct Peptide Profiling
2.10. Hb and HSA Compared
2.11. Other Target Proteins
2.12. Adduct Enrichment
3. Human Biomonitoring
3.1. Methodological Considerations
3.2. Human Biomonitoring of Hb Adducts
3.3. Human Biomonitoring of HSA Adducts
4. Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Rappaport, S.M. Genetic Factors Are Not the Major Causes of Chronic Diseases. PLoS ONE 2016, 11, e0154387. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Smith, M.T. Epidemiology. Environment and disease risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Barupal, D.K.; Wishart, D.; Vineis, P.; Scalbert, A. The blood exposome and its role in discovering causes of disease. Environ. Health Perspect. 2014, 122, 769–774. [Google Scholar] [CrossRef]
- Rappaport, S.M. Biomarkers intersect with the exposome. Biomarkers 2012, 17, 483–489. [Google Scholar] [CrossRef]
- Dennis, K.K.; Marder, E.; Balshaw, D.M.; Cui, Y.; Lynes, M.A.; Patti, G.J.; Rappaport, S.M.; Shaughnessy, D.T.; Vrijheid, M.; Barr, D.B. Biomonitoring in the Era of the Exposome. Environ. Health Perspect. 2017, 125, 502–510. [Google Scholar] [CrossRef]
- Stewart, B.W.; Bray, F.; Forman, D.; Ohgaki, H.; Straif, K.; Ullrich, A.; Wild, C.P. Cancer prevention as part of precision medicine: ‘Plenty to be done’. Carcinogenesis 2016, 37, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Scalbert, A.; Herceg, Z. Measuring the exposome: A powerful basis for evaluating environmental exposures and cancer risk. Environ. Mol. Mutagen. 2013, 54, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Chadeau-Hyam, M.; Gmuender, H.; Gulliver, J.; Herceg, Z.; Kleinjans, J.; Kogevinas, M.; Kyrtopoulos, S.; Nieuwenhuijsen, M.; Phillips, D.H.; et al. The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 2017, 220, 142–151. [Google Scholar] [CrossRef]
- Vrijheid, M.; Slama, R.; Robinson, O.; Chatzi, L.; Coen, M.; van den Hazel, P.; Thomsen, C.; Wright, J.; Athersuch, T.J.; Avellana, N.; et al. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. 2014, 122, 535–544. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Hackermüller, J.; Polte, T.; Scholz, S.; Aigner, A.; Altenburger, R.; Böhme, A.; Bopp, S.K.; Brack, W.; Busch, W.; et al. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ. Int. 2017, 99, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, M.K.; Richards, K.A.M.; Wernke, G.R.; Shyr, Y.; Liebler, D.C. Cytosolic and Nuclear Protein Targets of Thiol-Reactive Electrophiles. Chem. Res. Toxicol. 2006, 19, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Weerapana, E.; Simon, G.M.; Cravatt, B.F. Disparate proteome reactivity profiles of carbon electrophiles. Nat. Chem. Biol. 2008, 4, 405–407. [Google Scholar] [CrossRef]
- Medina-Cleghorn, D.; Bateman, L.A.; Ford, B.; Heslin, A.; Fisher, K.J.; Dalvie, E.D.; Nomura, D.K. Mapping Proteome-Wide Targets of Environmental Chemicals Using Reactivity-Based Chemoproteomic Platforms. Chem. Biol. 2015, 22, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Li, H.; Grigoryan, H.; Funk, W.E.; Williams, E.R. Adductomics: Characterizing exposures to reactive electrophiles. Toxicol. Lett. 2012, 213, 83–90. [Google Scholar] [CrossRef]
- Carlsson, H.; Rappaport, S.M.; Tornqvist, M. Protein Adductomics: Methodologies for Untargeted Screening of Adducts to Serum Albumin and Hemoglobin in Human Blood Samples. High Throughput 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.J. The use of biomarkers for improved retrospective exposure assessment in epidemiological studies: Summary of an ECETOC workshop. Biomarkers 2008, 13, 734–748. [Google Scholar] [CrossRef]
- Dickerson, R.E.; Geis, I. Hemoglobin; Benjamin Cummings: Menlo Park, CA, USA, 1983. [Google Scholar]
- Rubino, F.M.; Pitton, M.; Di Fabio, D.; Colombi, A. Toward an “omic” physiopathology of reactive chemicals: Thirty years of mass spectrometric study of the protein adducts with endogenous and xenobiotic compounds. Mass Spectrom. Rev. 2009, 28, 725–784. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Jack, R.; Opheim, K.E.; Toivola, B.; Lyon, A.W. Clinical Chemistry; Williams & Wilkins: Malvern, UK, 1995. [Google Scholar]
- Ueno, H.; Pospischil, M.A.; Manning, J.M.; Kluger, R. Site-specific modification of hemoglobin by methyl acetyl phosphate. Arch. Biochem. Biophys. 1986, 244, 795–800. [Google Scholar] [CrossRef]
- Bryant, M.S.; Vineis, P.; Skipper, P.L.; Tannenbaum, S.R. Hemoglobin adducts of aromatic amines: Associations with smoking status and type of tobacco. Proc. Natl. Acad. Sci. USA 1988, 85, 9788. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J.; Le Marchand, L. Metabolism and Biomarkers of Heterocyclic Aromatic Amines in Molecular Epidemiology Studies: Lessons Learned from Aromatic Amines. Chem. Res. Toxicol. 2011, 24, 1169–1214. [Google Scholar] [CrossRef] [PubMed]
- Ringe, D.; Turesky, R.J.; Skipper, P.L.; Tannenbaum, S.R. Structure of the single stable hemoglobin adduct formed by 4-aminobiphenyl in vivo. Chem. Res. Toxicol. 1988, 1, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, M.; Mowrer, J.; Jensen, S.; Ehrenberg, L. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Anal. Biochem. 1986, 154, 255–266. [Google Scholar] [CrossRef]
- Carlsson, H.; von Stedingk, H.; Nilsson, U.; Tornqvist, M. LC-MS/MS screening strategy for unknown adducts to N-terminal valine in hemoglobin applied to smokers and nonsmokers. Chem. Res. Toxicol. 2014, 27, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Tornqvist, M. Epoxide adducts to N-terminal valine of hemoglobin. Methods Enzymol. 1994, 231, 650–657. [Google Scholar] [CrossRef]
- Edman, P. Method for Determination of the Amino Acid Sequence in Peptides. Acta Chem. Scand. 1950, 4, 283–293. [Google Scholar] [CrossRef]
- Price, N.C.; Stevens, L. Fundamentals of Enzymology, 3rd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Von Stedingk, H.; Rydberg, P.; Törnqvist, M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC–MS/MS. J. Chromatogr. B 2010, 878, 2483–2490. [Google Scholar] [CrossRef]
- Preston, G.W.; Plusquin, M.; Sozeri, O.; van Veldhoven, K.; Bastian, L.; Nawrot, T.S.; Chadeau-Hyam, M.; Phillips, D.H. Refinement of a Methodology for Untargeted Detection of Serum Albumin Adducts in Human Populations. Chem. Res. Toxicol. 2017, 30, 2120–2129. [Google Scholar] [CrossRef]
- Kanaly, R.A.; Hanaoka, T.; Sugimura, H.; Toda, H.; Matsui, S.; Matsuda, T. Development of the adductome approach to detect DNA damage in humans. Antioxid. Redox Signal. 2006, 8, 993–1001. [Google Scholar] [CrossRef]
- Shibata, T.; Shimizu, K.; Hirano, K.; Nakashima, F.; Kikuchi, R.; Matsushita, T.; Uchida, K. Adductome-based identification of biomarkers for lipid peroxidation. J. Biol. Chem. 2017, 292, 8223–8235. [Google Scholar] [CrossRef]
- Sabbioni, G.; Turesky, R.J. Biomonitoring Human Albumin Adducts: The Past, the Present, and the Future. Chem. Res. Toxicol. 2017, 30, 332–366. [Google Scholar] [CrossRef] [PubMed]
- Day, B.W.; Skipper, P.L.; Zaia, J.; Tannenbaum, S.R. Benzo[a]pyrene anti-diol epoxide covalently modifies human serum albumin carboxylate side chains and imidazole side chain of histidine(146). J. Am. Chem. Soc. 1991, 113, 8505–8509. [Google Scholar] [CrossRef]
- Lindh, C.H.; Kristiansson, M.H.; Berg-Andersson, U.A.; Cohen, A.S. Characterization of adducts formed between human serum albumin and the butadiene metabolite epoxybutanediol. Rapid Commun. Mass Spectrom. 2005, 19, 2488–2496. [Google Scholar] [CrossRef]
- Sabbioni, G. Chemical and physical properties of the major serum albumin adduct of aflatoxin B1 and their implications for the quantification in biological samples. Chemico-Biol. Interact. 1990, 75, 1–15. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Arneson, K.O.; Williams, K.M.; Deng, Z.; Harris, T.M. Reaction of Aflatoxin B1 Oxidation Products with Lysine. Chem. Res. Toxicol. 2002, 15, 780–792. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Aldini, G.; Regazzoni, L.; Orioli, M.; Rimoldi, I.; Facino, R.M.; Carini, M. A tandem MS precursor-ion scan approach to identify variable covalent modification of albumin Cys34: A new tool for studying vascular carbonylation. J. Mass Spectrom. 2008, 43, 1470–1481. [Google Scholar] [CrossRef]
- Noort, D.; Hulst, A.G.; de Jong, L.P.; Benschop, H.P. Alkylation of human serum albumin by sulfur mustard in vitro and in vivo: Mass spectrometric analysis of a cysteine adduct as a sensitive biomarker of exposure. Chem. Res. Toxicol. 1999, 12, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Dasari, S.; Tabb, D.L.; Turesky, R.J. Mapping Serum Albumin Adducts of the Food-Borne Carcinogen 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by Data-Dependent Tandem Mass Spectrometry. Chem. Res. Toxicol. 2012, 25, 2179–2193. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.V.; Bellamri, M.; Wang, Y.; Langouët, S.; Turesky, R.J. 2-Amino-9H-pyrido[2,3-b]indole (AαC) Adducts and Thiol Oxidation of Serum Albumin as Potential Biomarkers of Tobacco Smoke. J. Biol. Chem. 2015, 290, 16304–16318. [Google Scholar] [CrossRef]
- Grigoryan, H.; Li, H.; Iavarone, A.T.; Williams, E.R.; Rappaport, S.M. Cys34 adducts of reactive oxygen species in human serum albumin. Chem. Res. Toxicol. 2012, 25, 1633–1642. [Google Scholar] [CrossRef]
- Dong, Q.; Yan, X.; Kilpatrick, L.E.; Liang, Y.; Mirokhin, Y.A.; Roth, J.S.; Rudnick, P.A.; Stein, S.E. Tandem Mass Spectral Libraries of Peptides in Digests of Individual Proteins: Human Serum Albumin (HSA). Mol. Cell. Proteom. 2014, 13, 2435. [Google Scholar] [CrossRef]
- Li, H.; Grigoryan, H.; Funk, W.E.; Lu, S.S.; Rose, S.; Williams, E.R.; Rappaport, S.M. Profiling Cys34 adducts of human serum albumin by fixed-step selected reaction monitoring. Mol. Cell. Proteom. 2011, 10, M110.004606. [Google Scholar] [CrossRef]
- Grigoryan, H.; Edmands, W.; Lu, S.S.; Yano, Y.; Regazzoni, L.; Iavarone, A.T.; Williams, E.R.; Rappaport, S.M. Adductomics Pipeline for Untargeted Analysis of Modifications to Cys34 of Human Serum Albumin. Anal. Chem. 2016, 88, 10504–10512. [Google Scholar] [CrossRef]
- Porter, C.J.; Bereman, M.S. Data-independent-acquisition mass spectrometry for identification of targeted-peptide site-specific modifications. Anal. Bioanal. Chem. 2015, 407, 6627–6635. [Google Scholar] [CrossRef][Green Version]
- Noort, D.; Fidder, A.; Hulst, A.G. Modification of human serum albumin by acrylamide at cysteine-34: A basis for a rapid biomonitoring procedure. Arch. Toxicol. 2003, 77, 543–545. [Google Scholar] [CrossRef]
- Goto, T.; Kojima, S.; Shitamichi, S.; Lee, S.H.; Oe, T. Chemical modificomics: A novel strategy for efficient biomarker discovery through chemical modifications on a target peptide. Anal. Methods 2012, 4, 1945–1952. [Google Scholar] [CrossRef]
- Todd, J.F.J. Recommendations for Nomenclature and Symbolism for Mass Spectroscopy. Pure Appl. Chem. 1991, 63, 1541–1566. [Google Scholar] [CrossRef]
- Kanaly, R.A.; Matsui, S.; Hanaoka, T.; Matsuda, T. Application of the adductome approach to assess intertissue DNA damage variations in human lung and esophagus. Mutat. Res. 2007, 625, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Scholz, K.; Donegan, M.; Burton, L.; Wingate, J.; Völkel, W. Metabonomics and Biomarker Discovery: LC−MS Metabolic Profiling and Constant Neutral Loss Scanning Combined with Multivariate Data Analysis for Mercapturic Acid Analysis. Anal. Chem. 2006, 78, 1296–1305. [Google Scholar] [CrossRef]
- Mann, M.; Wilm, M. Error-Tolerant Identification of Peptides in Sequence Databases by Peptide Sequence Tags. Anal. Chem. 1994, 66, 4390–4399. [Google Scholar] [CrossRef]
- Yeowell-O’Connell, K.; Rothman, N.; Smith, M.T.; Hayes, R.B.; Li, G.; Waidyanatha, S.; Dosemeci, M.; Zhang, L.; Yin, S.; Titenko-Holland, N.; et al. Hemoglobin and albumin adducts of benzene oxide among workers exposed to high levels of benzene. Carcinogenesis 1998, 19, 1565–1571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dingley, K.H.; Curtis, K.D.; Nowell, S.; Felton, J.S.; Lang, N.P.; Turteltaub, K.W. DNA and protein adduct formation in the colon and blood of humans after exposure to a dietary-relevant dose of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Epidemiol. Biomark. Prev. 1999, 8, 507–512. [Google Scholar]
- Tannenbaum, S.R.; Skipper, P.L. Biological aspects to the evaluation of risk: dosimetry of carcinogens in man. Fundam. Appl. Toxicol. 1984, 4, S367–S373. [Google Scholar] [CrossRef]
- Sabbioni, G.; Skipper, P.L.; Buchi, G.; Tannenbaum, S.R. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis 1987, 8, 819–824. [Google Scholar] [CrossRef]
- Wild, C.P.; Jiang, Y.Z.; Sabbioni, G.; Chapot, B.; Montesano, R. Evaluation of methods for quantitation of aflatoxin-albumin adducts and their application to human exposure assessment. Cancer Res. 1990, 50, 245–251. [Google Scholar]
- Noort, D.; Fidder, A.; Degenhardt-Langelaan, C.E.; Hulst, A.G. Retrospective detection of sulfur mustard exposure by mass spectrometric analysis of adducts to albumin and hemoglobin: An in vivo study. J. Anal. Toxicol. 2008, 32, 25–30. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Baynes, J.W.; TeKoppele, J.M. Effect of Collagen Turnover on the Accumulation of Advanced Glycation End Products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, B.A.G.; Wishnok, J.S.; Skipper, P.L.; Stillwell, W.G.; Tannenbaum, S.R. Lysine Adducts Between Methyltetrahydrophthalic Anhydride and Collagen in Guinea Pig Lung. Toxicol. Appl. Pharmacol. 1995, 135, 156–162. [Google Scholar] [CrossRef]
- Miller, E.J.; Gay, S. Collagen: An overview. Methods Enzymol. 1982, 82, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.J.; Kent Rhodes, R. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982, 82, 33–64. [Google Scholar] [CrossRef] [PubMed]
- SooHoo, C.K.; Singh, K.; Skipper, P.L.; Tannenbaum, S.R.; Dasari, R.R. Characterization of benzo[a]pyrene anti-diol epoxide adducts to human histones. Chem. Res. Toxicol. 1994, 7, 134–138. [Google Scholar] [CrossRef]
- Fabrizi, L.; Taylor, G.W.; Cañas, B.; Boobis, A.R.; Edwards, R.J. Adduction of the Chloroform Metabolite Phosgene to Lysine Residues of Human Histone H2B. Chem. Res. Toxicol. 2003, 16, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.E.; Li, H.; Iavarone, A.T.; Williams, E.R.; Riby, J.; Rappaport, S.M. Enrichment of cysteinyl adducts of human serum albumin. Anal. Biochem. 2010, 400, 61–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, M.K.; Grigoryan, H.; Iavarone, A.T.; Rappaport, S.M. Antibody enrichment and mass spectrometry of albumin-Cys34 adducts. Chem. Res. Toxicol. 2014, 27, 400–407. [Google Scholar] [CrossRef]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef]
- Carlsson, H.; Tornqvist, M. Strategy for identifying unknown hemoglobin adducts using adductome LC-MS/MS data: Identification of adducts corresponding to acrylic acid, glyoxal, methylglyoxal, and 1-octen-3-one. Food Chem. Toxicol. 2016, 92, 94–103. [Google Scholar] [CrossRef]
- Carlsson, H.; Motwani, H.V.; Osterman Golkar, S.; Tornqvist, M. Characterization of a Hemoglobin Adduct from Ethyl Vinyl Ketone Detected in Human Blood Samples. Chem. Res. Toxicol. 2015, 28, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Degner, A.; Carlsson, H.; Karlsson, I.; Eriksson, J.; Pujari, S.S.; Tretyakova, N.Y.; Törnqvist, M. Discovery of Novel N-(4-Hydroxybenzyl)valine Hemoglobin Adducts in Human Blood. Chem. Res. Toxicol. 2018, 31, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, H.; Aasa, J.; Kotova, N.; Vare, D.; Sousa, P.F.M.; Rydberg, P.; Abramsson-Zetterberg, L.; Tornqvist, M. Adductomic Screening of Hemoglobin Adducts and Monitoring of Micronuclei in School-Age Children. Chem. Res. Toxicol. 2017, 30, 1157–1167. [Google Scholar] [CrossRef]
- Lu, S.S.; Grigoryan, H.; Edmands, W.M.; Hu, W.; Iavarone, A.T.; Hubbard, A.; Rothman, N.; Vermeulen, R.; Lan, Q.; Rappaport, S.M. Profiling the Serum Albumin Cys34 Adductome of Solid Fuel Users in Xuanwei and Fuyuan, China. Environ. Sci. Technol. 2017, 51, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study. Lancet 2018, 391, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Grigoryan, H.; Edmands, W.M.B.; Dagnino, S.; Sinharay, R.; Cullinan, P.; Collins, P.; Chung, K.F.; Barratt, B.; Kelly, F.J.; et al. Cys34 Adductomes Differ between Patients with Chronic Lung or Heart Disease and Healthy Controls in Central London. Environ. Sci. Technol. 2018, 52, 2307–2313. [Google Scholar] [CrossRef]
- Preston, G.W.; Dagnino, S.; Ponzi, E.; Sozeri, O.; van Veldhoven, K.; Barratt, B.; Liu, S.; Grigoryan, H.; Lu, S.S.; Rappaport, S.; et al. Relationships between airborne pollutants, serum albumin adducts and short-term health outcomes in an experimental crossover study. 2019; submitted. [Google Scholar]
- Bellamri, M.; Wang, Y.; Yonemori, K.; White, K.K.; Wilkens, L.R.; Le Marchand, L.; Turesky, R.J. Biomonitoring an albumin adduct of the cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in humans. Carcinogenesis 2018, 39, 1455–1462. [Google Scholar] [CrossRef]
- Funk, W.E.; Waidyanatha, S.; Chaing, S.H.; Rappaport, S.M. Hemoglobin adducts of benzene oxide in neonatal and adult dried blood spots. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1896–1901. [Google Scholar] [CrossRef]
- Yano, Y.; Grigoryan, H.; Schiffman, C.; Edmands, W.; Petrick, L.; Hall, K.; Whitehead, T.; Metayer, C.; Dudoit, S.; Rappaport, S. Untargeted adductomics of Cys34 modifications to human serum albumin in newborn dried blood spots. Anal. Bioanal. Chem. 2019. [Google Scholar] [CrossRef]
| 1 | A note regarding language. For the reaction of a nucleophile with an electrophile, the view of the chemist is that the nucleophile is the active participant, providing electrons for the chemical bond (‘nucleophilic addition’, ‘nucleophilic attack’, and so on). Toxicologists, on the other hand, tend to speak of the toxicant as active (toxicant ‘binds’ to target), and since the toxicant is usually an electrophile the roles would seem to switch. This second interpretation is equally logical because the nucleophilic targets are often endogenous and less mobile (e.g., DNA or protein) and therefore seem to be passive entities. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preston, G.W.; Phillips, D.H. Protein Adductomics: Analytical Developments and Applications in Human Biomonitoring. Toxics 2019, 7, 29. https://doi.org/10.3390/toxics7020029
Preston GW, Phillips DH. Protein Adductomics: Analytical Developments and Applications in Human Biomonitoring. Toxics. 2019; 7(2):29. https://doi.org/10.3390/toxics7020029
Chicago/Turabian StylePreston, George W., and David H. Phillips. 2019. "Protein Adductomics: Analytical Developments and Applications in Human Biomonitoring" Toxics 7, no. 2: 29. https://doi.org/10.3390/toxics7020029
APA StylePreston, G. W., & Phillips, D. H. (2019). Protein Adductomics: Analytical Developments and Applications in Human Biomonitoring. Toxics, 7(2), 29. https://doi.org/10.3390/toxics7020029




