Propensity of Tagetes erecta L., a Medicinal Plant Commonly Used in Diabetes Management, to Accumulate Perfluoroalkyl Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection: Tagetes erecta L. and River Water
2.3. Sample Pre-Treatment and Solid Phase Extraction
2.3.1. Plant Samples
2.3.2. River Water Samples
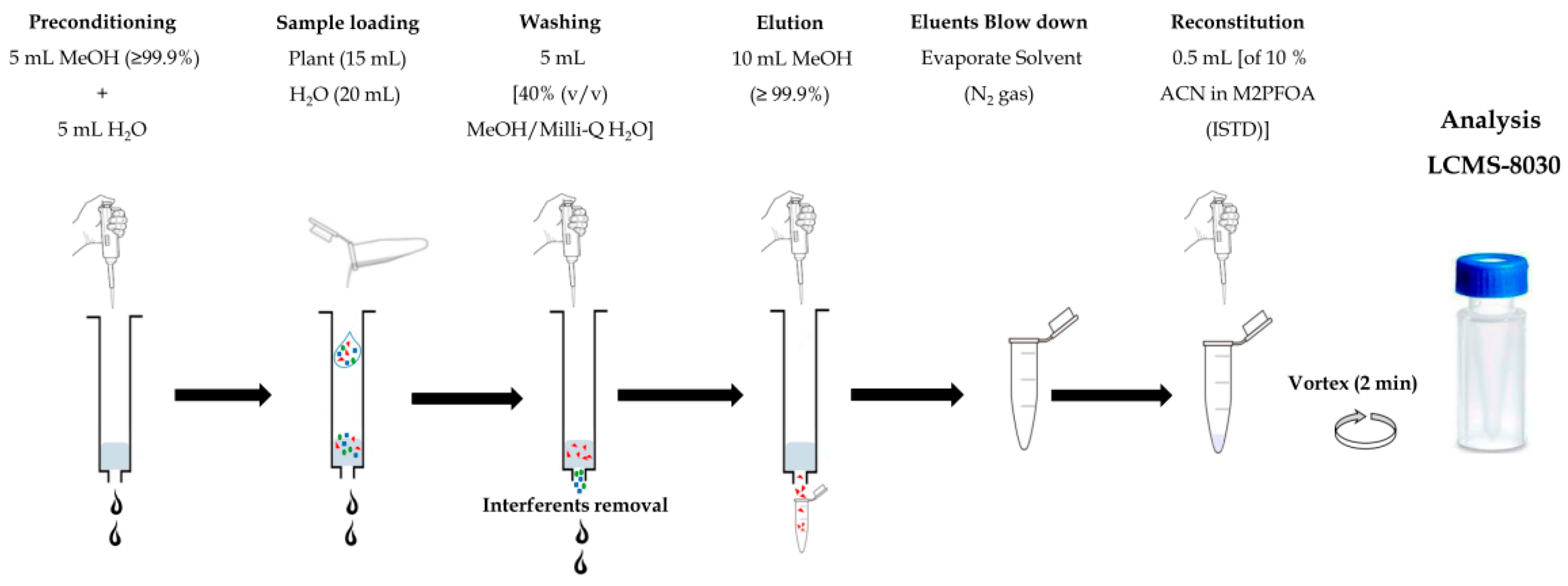
2.3.3. Solid Phase Extraction
2.4. LCMS-8030 Analysis
2.4.1. LCMS-8030 Configuration for PFOA, PFOS and PFBS Quantification
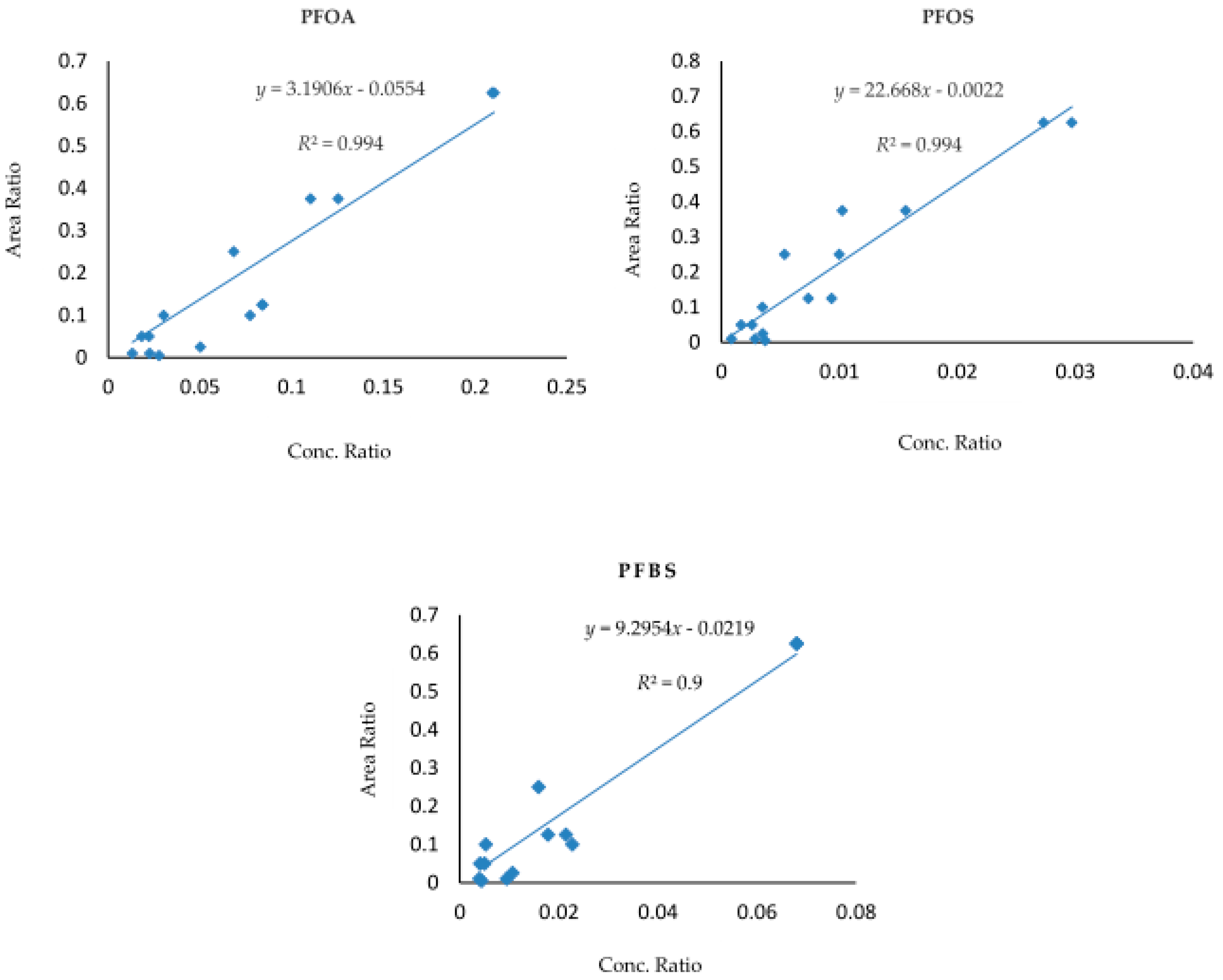
2.4.2. Validation of Method
- RRF is the relative response factor;
- ANAT is peak the area of the native compound;
- AIS is the peak area of the internal standard in the standard;
- CNAT is the concentration of the native standard;
- CIS is the internal standard concentration.
- FC is the final concentration;
- ANAT is the peak area of the target analyte;
- AIS represents the peak area of the internal standard used for that particular analyte;
- RRF is the calculated relative response factor of the specific analyte;
- VIS is the volume of the internal standard added in the sample prior to extraction (mL);
- VS is the volume of the sample (mL).
3. Results
3.1. LCMS Calibration Curves for the Detection and Quantification of PFOA, PFOS and PFBS
3.2. LCMS Chromatographs for PFOA, PFOS, and PFBS
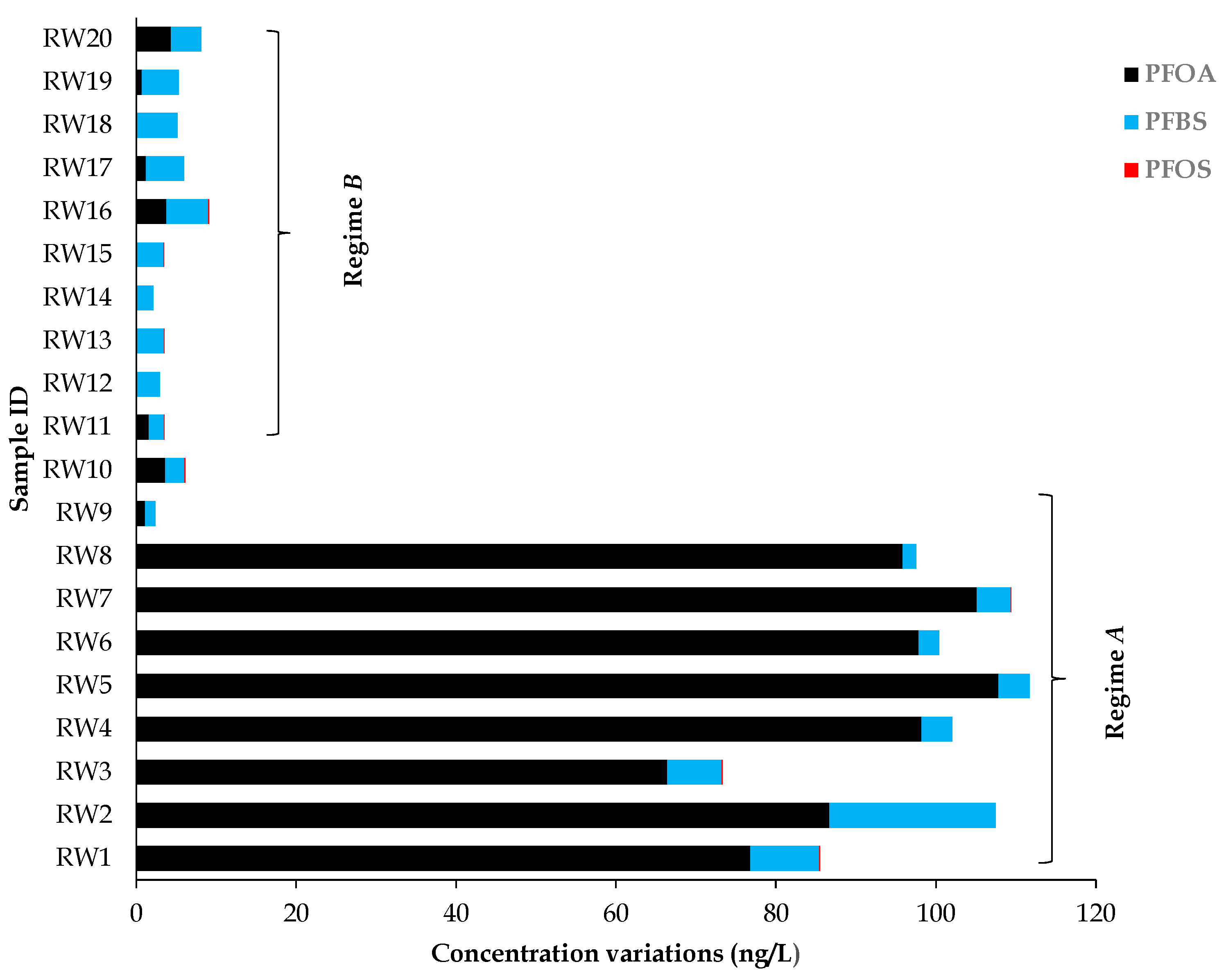
3.3. Results of Previously Known Contaminated River Water
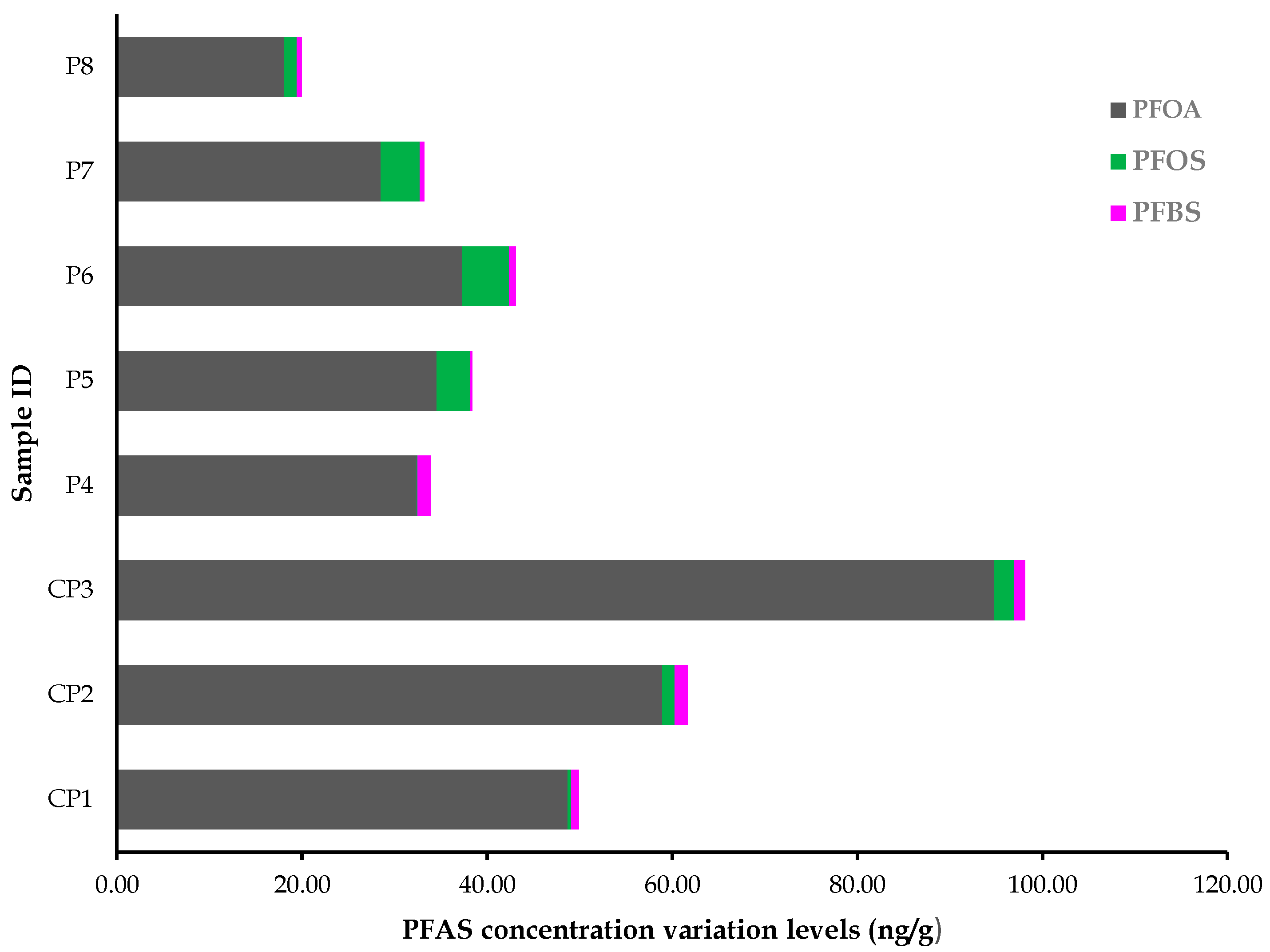
3.4. PFOA, PFOS and PFBS Accumulation in a Commonly-Used Medicinal Plant
4. Discussion
4.1. New Evidence on the Contamination of Salt River by PFASs
4.2. Traces of PFASs in the Investigated Medicinal Plant
4.3. Tagetes erecta L. Sorption Aptitude by Means of Bioconcentration Factor (BCF)
4.4. Environmental Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Vasudevan, P.; Kashyap, S.; Sharma, S. Tagetes: A multipurpose plant. Bioresour. Technol. 1997, 62, 29–35. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, Q.; Wang, W.; Zhang, C.; Cao, Z.; Bao, M.; He, Y. Transcriptomic analysis of differentially expressed genes during flower organ development in genetic male sterile and male fertile Tagetes erecta by digital gene-expression profiling. PLoS ONE 2016, 11, e0150892. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhang, C.; Sun, Y.; Wang, W.; He, Y.; Bao, M. Characterization and Functional Analysis of Five MADS-Box B Class Genes Related to Floral Organ Identification in Tagetes erecta. PloS ONE 2017, 12, e0169777. [Google Scholar] [CrossRef] [PubMed]
- Ayyadurai, N.; Valarmathy, N.; Kannan, S.; Jansirani, D.; Alsenaidy, A. Evaluation of cytotoxic properties of Curcuma longa and Tagetes erecta on cancer cell line (Hep2). Afr. J. Pharm. Pharmacol. 2013, 7, 736–739. [Google Scholar] [CrossRef]
- Bhatt, B.J. Comparative analysis of larvicidal activity of essential oils of Cymbopogon flexeous (Lemon grass) and Tagetes erecta (Marigold) against Aedes aegypti larvae. Eur. J. Exp. Biol. 2013, 3, 422–427. [Google Scholar]
- Chkhikvishvili, I.; Sanikidze, T.; Gogia, N.; Enukidze, M.; Machavariani, M.; Kipiani, N.; Vinokur, Y.; Rodov, V. Constituents of French marigold (Tagetes patula L.) Flowers Protect Jurkat T-Cells against Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Rodda, R.; Avvari, S.K.; Chidrawar, R.V.; Reddy, T.R. Pharmacological screening of synergistic antidiabetic efficacy of Tagetes erecta and Foeniculum vulgare. Int. J. Phytopharmacol. 2013, 4, 223–229. [Google Scholar]
- Hemali, P.; Sumitra, C. Evaluation of antioxidant efficacy of different fractions of Tagetes erecta L. Flowers. J. Pharm. Biol. Sci. 2014, 9, 28–37. [Google Scholar] [CrossRef]
- Shetty, L.J.; Sakr, F.M.; Al-Obaidy, K.; Patel, M.J.; Shareef, H. A brief review on medicinal plant Tagetes erecta Linn A. J. Appl. Pharm. Sci. 2015, 5, 091–095. [Google Scholar]
- Bailung, B.; Puzari, M. Traditional use of plants by the Ahoms in human health management in upper Assam, India. J. Med. Plants Stud. 2016, 4, 48–51. [Google Scholar]
- Wang, W.; Xu, H.; Chen, H.; Tai, K.; Liu, F.; Gao, Y. In vitro antioxidant, anti-diabetic and antilipemic potentials of quercetagetin extracted from marigold (Tagetes erecta L.) inflorescence residues. J. Food Sci. Technol. 2016, 53, 2614–2624. [Google Scholar] [CrossRef]
- Davids, D.; Gibson, D.; Johnson, Q. Ethnobotanical survey of medicinal plants used to manage high blood pressure and type 2 diabetes mellitus in Bitterfontein, Western Cape Province, South Africa. J. Ethnopharmacol. 2016, 194, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Mudumbi, J.B.N.; Ntwampe, S.K.O.; Matsha, T.; Mekuto, L.; Itoba-Tombo, E.F. Recent developments in polyfluoroalkyl compounds research: A focus on human/environmental health impact, suggested substitutes and removal strategies. Environ. Monit. Assess. 2017, 189, 402. [Google Scholar] [CrossRef]
- Mudumbi, J.B.N.; Ntwampe, S.K.O.; Mekuto, L.; Matsha, T.; Itoba-Tombo, E.F. The role of pollutants in type 2 diabetes mellitus (T2DM) and their prospective impact on phytomedicinal treatment strategies. Environ. Monit. Assess. 2018, 190, 262. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Al-Qahtani, K.M. Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012, 38, 31–37. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Kiran, G.S.; Gupta, M.; Sharma, N.K.; Prasad, K.S. A study on distribution of heavy metal contamination in the vegetables using GIS and analytical technique. Int. J. Ecol. Dev. 2012, 21, 89–99. [Google Scholar]
- AlKhader, A.M.F. The impact of phosphorus fertilizers on heavy metals content of soils and vegetables grown on selected farms in Jordan. Agrotechnol 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Ezigbo, V.O.; Odinma, S.C. Trace element analysis of some leafy and non-leafy vegetable samples in Anam District of Aghamelum Anambra State of Nigeria. Int. J. Sci. Technol. 2015, 4, 119–124. [Google Scholar]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Moyano, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214. [Google Scholar] [CrossRef]
- Hyland, K.C.; Blaine, A.C.; Dickenson, E.R.; Higgins, C.P. Accumulation of contaminants of emerging concern in food crops—Part 1: Edible strawberries and lettuce grown in reclaimed water. Environ. Toxicol. Chem. 2015, 34, 2213–2221. [Google Scholar] [CrossRef]
- Hyland, K.C.; Blaine, A.C.; Higgins, C.P. Accumulation of contaminants of emerging concern in food crops—Part 2: Plant distribution. Environ. Toxicol. Chem. 2015, 34, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Trejo, N.; Matus, I.; del Pozo, A.; Walter, I.; Hirzel, J. Cadmium phytoextraction capacity of white lupine (Lupinus albus L.) and narrow-leafed lupine (Lupinus angustifolius L.) in three contrasting agroclimatic conditions of Chile. Chil. J. Agric. Res. 2016, 76, 228–235. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova Panovska, T.; Ruskovska, T.; Bačeva, K.; Stafilov, T. Influence of heavy metal stress on antioxidant status and DNA damage in Urtica dioica. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova-Panovska, T.; Bačeva, K.; Stafilov, T. Content of toxic and essential metals in medicinal herbs growing in polluted and unpolluted areas of Macedonia. Arhiv za Higijenu Rada i Toksikologiju 2010, 61, 297–303. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, Y.; Chang, S.; Zhao, Z.; Zhao, Y.; Yuan, X.; Wu, F.; Sun, H. Occurrence and Phase Distribution of Neutral and Ionizable Per-and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China. Environ. Sci. Technol. 2018, 52, 1301–1310. [Google Scholar] [CrossRef]
- Mudumbi, J.B.N.; Ntwampe, S.K.; Muganza, M.; Okonkwo, J.O. Susceptibility of riparian wetland plants to perfluorooctanoic acid (PFOA) accumulation. Int. J. Phytoremed. 2014, 16, 926–936. [Google Scholar] [CrossRef]
- Mudumbi, J.B.N.; Ntwampe, S.K.O.; Muganza, F.M.; Okonkwo, J.O. Perfluorooctanoate and perfluorooctane sulfonate in South African river water. Water Sci. Technol. 2014, 69, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mudumbi, J.B.N.; Ntwampe, S.K.O.; Muganza, M.; Rand, A.; Okonkwo, O.J. Concentrations of Perfluorooctanoate and Perfluorooctane Sulfonate in Sediment of Western Cape Rivers, South Africa. Carpath. J. Earth Environ. 2014, 9, 147–158. [Google Scholar]
- Zhang, K.; Huang, J.; Yu, G.; Zhang, Q.; Deng, S.; Wang, B. Destruction of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) by ball milling. Water Sci. Technol. 2013, 47, 6471–6477. [Google Scholar] [CrossRef]
- DeWitt, J.C. (Ed.) Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; Springer International Publishing: Basel, Switzerland, 2015; Available online: https://link.springer.com/content/pdf/10.1007/978-3-319-15518-0.pdf (accessed on 14 February 2019).
- Sedlak, M.D.; Benskin, J.P.; Wong, A.; Grace, R.; Greig, D.J. Per-and polyfluoroalkyl substances (PFASs) in San Francisco Bay wildlife: Temporal trends, exposure pathways, and notable presence of precursor compounds. Chemosphere 2017, 185, 1217–1226. [Google Scholar] [CrossRef]
- Sedlak, M.; Sutton, R.; Wong, A.; Lin, D. Per and Polyfluoroalkyl Substances (PFASs) in San Francisco Bay: Synthesis and Strategy; San Francisco Estuary Institute: San Francisco, CA, USA, 2018; Available online: https://www.sfei.org/sites/default/files/biblio_files/PFAS%20Synthesis%20and%20Strategy.pdf (accessed on 14 February 2019).
- Krippner, J.; Brunn, H.; Falk, S.; Georgii, S.; Schubert, S.; Stahl, T. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in maize (Zea mays). Chemosphere 2014, 94, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Krippner, J.; Falk, S.; Brunn, H.; Georgii, S.; Schubert, S.; Stahl, T. Accumulation potentials of perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs) in maize (Zea mays). J. Agric. Food Chem. 2015, 63, 3646–3653. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Struckhoff, G.; Pugh, K.; Singh, O. Uptake and translocation of sulfamethazine by alfalfa grown under hydroponic conditions. J. Environ. Sci. 2017, 53, 217–223. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L. Uptake and metabolism of 10:2 fluorotelomer alcohol in soil-earthworm (Eisenia fetida) and soil-wheat (Triticum aestivum L.) systems. Environ. Pollut. 2017, 220, 124–131. [Google Scholar] [CrossRef]
- Sznajder-Katarzyńska, K.; Surma, M.; Cieślik, E.; Wiczkowski, W. The perfluoroalkyl substances (PFASs) contamination of fruits and vegetables. Food Addit. Contam. Part A 2018, 35, 1776–1786. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, Y.; Qu, B. PFCA uptake and translocation in dominant wheat species (Triticum aestivum L.). Int. J. Phytoremed. 2018, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, X.; Wu, Y.; Zhao, S.; Li, Y.; Ma, L.; Chen, C.; Huang, J.; Yu, G. Temporal trends and transport of perfluoroalkyl substances (PFASs) in a subtropical estuary: Jiulong River Estuary, Fujian, China. Sci. Total Environ. 2018, 639, 263–270. [Google Scholar] [CrossRef]
- Mathieu, C.; McCall, M. Survey of Per-and Poly-Fluoroalkyl Substances (PFASs) in Washington State Rivers and Lakes. 2018. Available online: https://cedar.wwu.edu/ssec/2018ssec/allsessions/63/ (accessed on 20 December 2018).
- van den Dungen, M.W.; Kok, D.E.; Polder, A.; Hoogenboom, R.L.; van Leeuwen, S.P.; Steegenga, W.T.; Kampman, E.; Murk, A.J. Accumulation of persistent organic pollutants in consumers of eel from polluted rivers compared to marketable eel. Environ. Pollut. 2016, 219, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, T.; Hassan, Y.; Rauert, C.; Harner, T. Poly- and perfluoroalkyl substances (PFASs) in indoor dust and food packaging materials in Egypt: Trends in developed and developing countries. Chemosphere 2016, 144, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Li, Q.; Xiao, F.; Song, G.L.; Lu, Y.; Yang, H.B.; Liao, C.X. The study of acute toxicity of perfluorooctanoic acid on Zebra fish. In Architectural, Energy and Information Engineering, Proceedings of the 2015 International Conference on Architectural, Energy and Information Engineering (AEIE 2015), Xiamen, China, 19–20 May 2015; Taylor & Francis Group: London, UK, 2016. [Google Scholar]
- Heydebreck, F.; Tang, J.; Xie, Z.; Ebinghaus, R. Alternative and legacy perfluoroalkyl substances: Differences between European and Chinese river/estuary systems. Environ. Sci. Technol. 2015, 49, 8386–8395. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.; Sun, Y.; Guo, Y.; Dai, J. Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol. 2018, 52, 7621–7629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, T.; Meng, J.; Wang, P.; Li, Q.; Lu, Y. Perfluoroalkyl substances in the Daling River with concentrated fluorine industries in China: Seasonal variation, mass flow, and risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Eshun, A.; Hogarh, J.N.; Bentum, J.K.; Adjei, J.K.; Negishi, J.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Masunaga, S. Perfluoroalkyl acids (PFAAs) in the Pra and Kakum River basins and associated tap water in Ghana. Sci. Total Environ. 2017, 579, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Butterfield, D.; Nizzetto, L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.; Campo, J.; Farré, M.; Pérez, F.; Picó, Y.; Barceló, D. Perfluoroalkyl substances in the Ebro and Guadalquivir river basins (Spain). Sci. Total Environ. 2016, 540, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Labadie, P.; Chevreuil, M. Partitioning behaviour of perfluorinated alkyl contaminants between water, sediment and fish in the Orge River (nearby Paris, France). Environ. Pollut. 2011, 159, 391–397. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wiberg, K.; Ribeli, E.; Josefsson, S.; Futter, M.; Gustavsson, J.; Ahrens, L. Spatial distribution and source tracing of per-and polyfluoroalkyl substances (PFASs) in surface water in Northern Europe. Environ. Pollut. 2017, 220, 1438–1446. [Google Scholar] [CrossRef]
- Wang, T.; Vestergren, R.; Herzke, D.; Yu, J.; Cousins, I.T. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese rivers. Environ. Sci. Technol. 2016, 50, 11584–11592. [Google Scholar] [CrossRef] [PubMed]
- Walters, R. Nutrient Transport to Roots. Technical Note 6; North Carolina State University, 2011. Available online: http://open-furrow.soil.ncsu.edu/Documents/DHC/nutrient%20transport%20to%20roots.pdf (accessed on 17 December 2018).
- Pagani, A.; Sawyer, E.J.; Mallarino, P.A.; Moody, L.; Davis, J.; Phillips, S. Site-Specific Nutrient Management: For Nutrient Management Planning to Improve Crop Production, Environmental Quality, and Economic Return. NRCS, NRCS 001 May 2013. Available online: http://www.agronext.iastate.edu/soilfertility/nutrienttopics/4r/Site-SpecificNutrientManagementPlanning_ver2.pdf (accessed on 11 December 2018).
- Schwartz, J. Nutrient Movement and Root Uptake. 2015. Available online: https://360yieldcenter.com/plant-health/soil-nutrient-series-part-1-nutrient-movement-and-root-uptake/ (accessed on 14 December 2016).
- Yoo, H.; Washington, J.W.; Jenkins, T.M.; Ellington, J.J. Quantitative determination of perfluorochemicals and fluorotelomer alcohols in plants from biosolid-amended fields using LC/MS/MS and GC/MS. Environ. Sci. Technol. 2011, 45, 7985–7990. [Google Scholar] [CrossRef]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; McGrath, S.P. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 2003, 249, 37–43. [Google Scholar] [CrossRef]
- Nworie, O.E.; Qin, J.; Lin, C. Trace Element Uptake by Herbaceous Plants from the Soils at a Multiple Trace Element-Contaminated Site. Toxics 2019, 7, 3. [Google Scholar] [CrossRef]
- Booi, X. Perfluorinated Compounds and Trihalomethanes in Drinking Water Sources of the Western Cape, South Africa. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2013. Available online: http://etd.cput.ac.za/handle/20.500.11838/863 (accessed on 14 December 2016).
- Zhou, Q.; Pan, G.; Zhang, J. Effective sorption of perfluorooctane sulfonate (PFOS) on hexadecyltrimethylammonium bromide immobilized mesoporous SiO2 hollow sphere. Chemosphere 2013, 90, 2461–2466. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.D.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Wu, Y.; Zhang, H.; Liu, Y.; Hu, X.; Huang, H.; Zhang, S. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environ. Pollut. 2016, 216, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Semenya, S.; Potgieter, M.; Erasmus, L. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo Province, South Africa. J. Ethnopharmacol. 2012, 141, 440–445. [Google Scholar] [CrossRef]
- Street, R.A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A review. J. Chem. 2012, 2013, 1–16. [Google Scholar] [CrossRef]
- Ayepola, O.R.; Brooks, N.L.; Oguntibeju, O.O. Kolaviron Improved Resistance to Oxidative Stress and Inflammation in the Blood (Erythrocyte, Serum, and Plasma) of Streptozotocin-Induced Diabetic Rats. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ayepola, O.R.; Cerf, M.E.; Brooks, N.L.; Oguntibeju, O.O. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomedicine 2014, 21, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Mounanga, M.B.; Mewono, L.; Angone, S.A. Toxicity studies of medicinal plants used in sub-Saharan Africa. J. Ethnopharmacol. 2015, 174, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Aremu, A.O.; Van Staden, J. Medicinal plants: An invaluable, dwindling resource in sub-Saharan Africa. J. Ethnopharmacol. 2015, 174, 595–606. [Google Scholar] [CrossRef] [PubMed]
| Compound | Acronym | Transition Qualifier (m/z) | Transition Quantifier (m/z) | Retention Time (min) |
|---|---|---|---|---|
| Targets | ||||
| Perfluorooctanoic acid | PFOA | 413.00 > 169.05 | 413.00 > 368.95 | 8.6 |
| Perfluorooctane sulfonate | PFOS | 499.00 > 98.90 | 499.00 > 80.15 | 8.9 |
| Perfluorobutane sulfonate | PFBS | 299.00 > 99.10 | 299.00 > 80.10 | 6.8 |
| ISTD | ||||
| Perfluoro-n-[1,2,3,4-13C4] octanoic acid | M2PFOA | 414.80 > 169.00 | 414.80 > 369.90 | 8.7 |
| Compounds | Regimes | |||
|---|---|---|---|---|
| Sample ID | PFOA | PFBS | PFOS | |
| RW1 | 76.79 | 8.59 | 0.08 | Regime A |
| RW2 | 86.69 | 20.75 | ND | |
| RW3 | 66.44 | 6.78 | 0.12 | |
| RW4 | 98.21 | 3.82 | ND | |
| RW5 | 107.82 | 3.88 | <LOD | |
| RW6 | 97.82 | 2.59 | ND | |
| RW7 | 105.12 | 4.26 | 0.06 | |
| RW8 | 95.81 | 1.72 | ND | |
| RW9 | 1.15 | 1.24 | <LOQ | |
| RW10 | 3.65 | 2.41 | 0.06 | |
| RW11 | 1.56 | 1.89 | 0.10 | Regime B |
| RW12 | <LOQ | 2.99 | <LOQ | |
| RW13 | <LOQ | 3.49 | 0.06 | |
| RW14 | <LOQ | 2.12 | <LOQ | |
| RW15 | <LOQ | 3.44 | 0.06 | |
| RW16 | 3.76 | 5.29 | 0.06 | |
| RW17 | 1.20 | 4.83 | <LOQ | |
| RW18 | <LOQ | 5.16 | <LOQ | |
| RW19 | 0.71 | 4.61 | <LOQ | |
| RW20 | 4.35 | 3.77 | <LOQ | |
| Average PFAS Conc./n = 20/Water (ng/L) | Plant Samples | PFOA/BCF | PFBS/BCF | PFOS/BCF | |||
|---|---|---|---|---|---|---|---|
| PFOA (37.6) | CS1 | 48.70 | 1.30 | 0.75 | 0.16 | 0.41 | 13.67 |
| CS2 | 58.96 | 1.57 | 1.44 | 0.31 | 1.29 | 43.00 | |
| CS3 | 94.83 | 2.52 | 1.15 | 0.24 | 2.17 | 72.33 | |
| PFBS (4.7) | S4 | 32.36 | 0.86 | 1.44 | 0.31 | 0.12 | 4.00 |
| S5 | 34.55 | 0.92 | 0.25 | 0.05 | 3.57 | 119.00 | |
| PFBS (4.7) | S6 | 37.34 | 0.99 | 0.74 | 0.16 | 5.03 | 167.67 |
| S7 | 28.49 | 0.76 | 0.45 | 0.10 | 4.24 | 141.33 | |
| S8 | 18.05 | 0.48 | 0.51 | 0.11 | 1.39 | 46.33 | |
| River | Country | Sampling Year | Level | PFOA | PBFS | PFOS | Reference |
|---|---|---|---|---|---|---|---|
| Salt | South Africa | 2017 | mean | 107.82 | 20.75 | 0.12 | This study |
| Salt | South Africa | 2014 | mean | 390.0 | n/a | 46.8 | [29] |
| Diep | South Africa | 2014 | mean | 314.4 | n/a | 181.8 | [29] |
| Eerste | South Africa | 2014 | mean | 145.5 | n/a | 22.5 | [29] |
| Yangtze | China | 2016 | mean | 13.5 | 1.84 | 1.83 | [47] |
| Yellow | China | 2016 | mean | 2.05 | 0.99 | 1.84 | [47] |
| Pearl | China | 2016 | mean | 7.45 | 4.49 | 11.09 | [47] |
| Kakum | Ghana | NI | mean | 167.4 | n/a | 113 | [49] |
| Tai | China | 2012 | mean | 24.7 | 3.18 | 9.78 | [50] |
| Liao | China | 2016 | mean | 8.95 | 0.94 | 3.46 | [47] |
| Ganges | India | 2014 | mean | 1.2 | n/a | 1.7 | [50] |
| Guadalquivir | Spain | NI | mean | 11.6 | 10.1 | 1.8 | [51] |
| Orge | France | 2011 | mean | 9.4 | 4.4 | 17.4 | [52] |
| Rhine | Europe | NI | mean | 4.72 | 21.28 | ND | [46] |
| Swedish | Sweden | 2013 | mean | 4.2 | n/a | 6.9 | [53] |
| Pearl | China | 2013 | mean | 3.13 | ND | 2.2 | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudumbi, J.B.N.; Daso, A.P.; Okonkwo, O.J.; Ntwampe, S.K.O.; Matsha, T.E.; Mekuto, L.; Itoba-Tombo, E.F.; Adetunji, A.T.; Sibali, L.L. Propensity of Tagetes erecta L., a Medicinal Plant Commonly Used in Diabetes Management, to Accumulate Perfluoroalkyl Substances. Toxics 2019, 7, 18. https://doi.org/10.3390/toxics7010018
Mudumbi JBN, Daso AP, Okonkwo OJ, Ntwampe SKO, Matsha TE, Mekuto L, Itoba-Tombo EF, Adetunji AT, Sibali LL. Propensity of Tagetes erecta L., a Medicinal Plant Commonly Used in Diabetes Management, to Accumulate Perfluoroalkyl Substances. Toxics. 2019; 7(1):18. https://doi.org/10.3390/toxics7010018
Chicago/Turabian StyleMudumbi, John Baptist Nzukizi, Adegbenro Peter Daso, Okechukwu Jonathan Okonkwo, Seteno Karabo Obed Ntwampe, Tandi E. Matsha, Lukhanyo Mekuto, Elie Fereche Itoba-Tombo, Adewole T. Adetunji, and Linda L. Sibali. 2019. "Propensity of Tagetes erecta L., a Medicinal Plant Commonly Used in Diabetes Management, to Accumulate Perfluoroalkyl Substances" Toxics 7, no. 1: 18. https://doi.org/10.3390/toxics7010018
APA StyleMudumbi, J. B. N., Daso, A. P., Okonkwo, O. J., Ntwampe, S. K. O., Matsha, T. E., Mekuto, L., Itoba-Tombo, E. F., Adetunji, A. T., & Sibali, L. L. (2019). Propensity of Tagetes erecta L., a Medicinal Plant Commonly Used in Diabetes Management, to Accumulate Perfluoroalkyl Substances. Toxics, 7(1), 18. https://doi.org/10.3390/toxics7010018









