A Model-to-Monitor Evaluation of 2011 National-Scale Air Toxics Assessment (NATA)
Abstract
1. Introduction
2. Materials and Methods
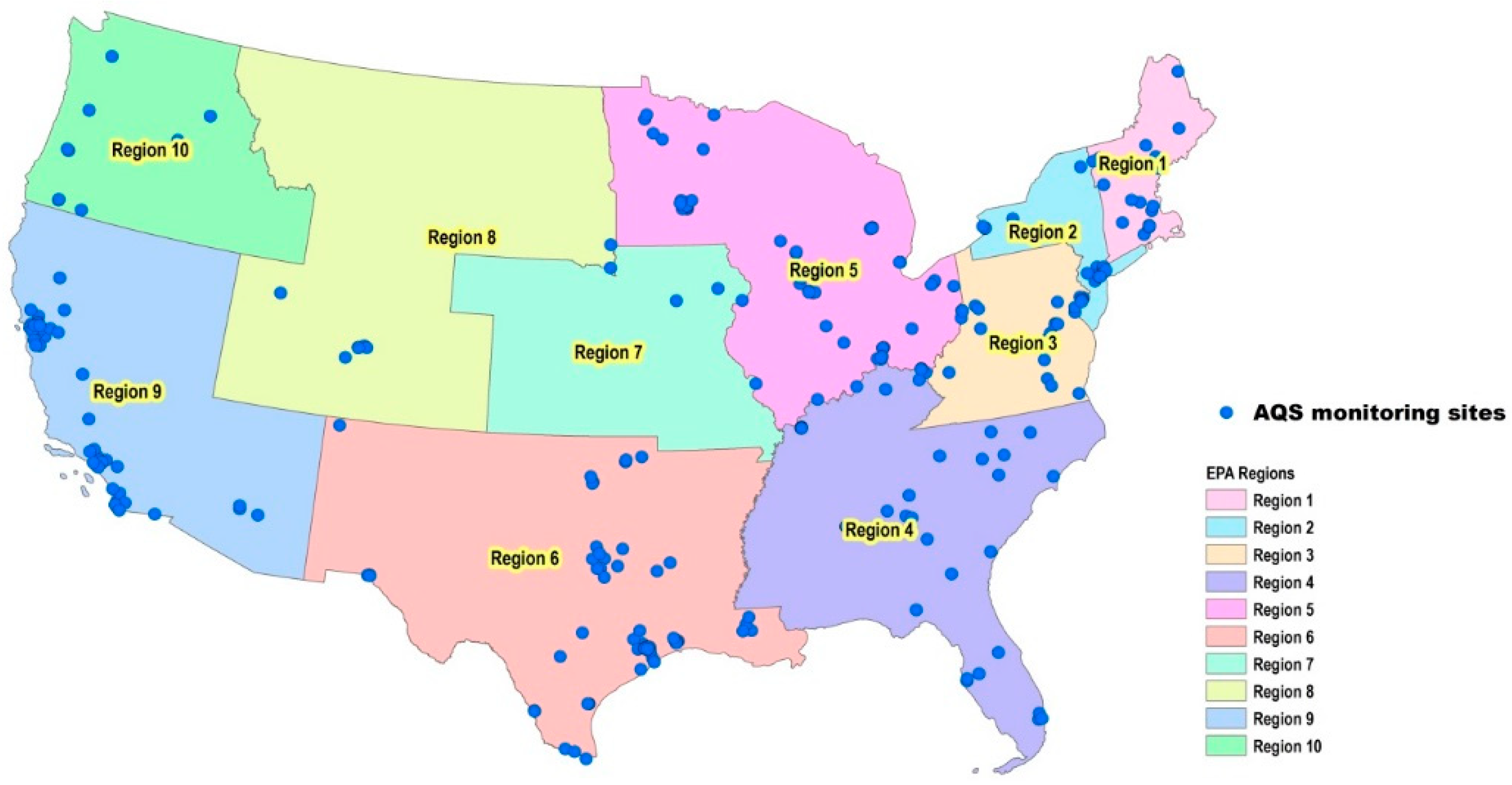
2.1. Data Sources and Compilation
2.2. Hazardous Air Pollutants (HAPs) of Interest
2.3. Model-to-Monitor Comparison Methods
3. Results
3.1. Comparison of National Statistics
3.2. National Model-to-Monitor Agreement
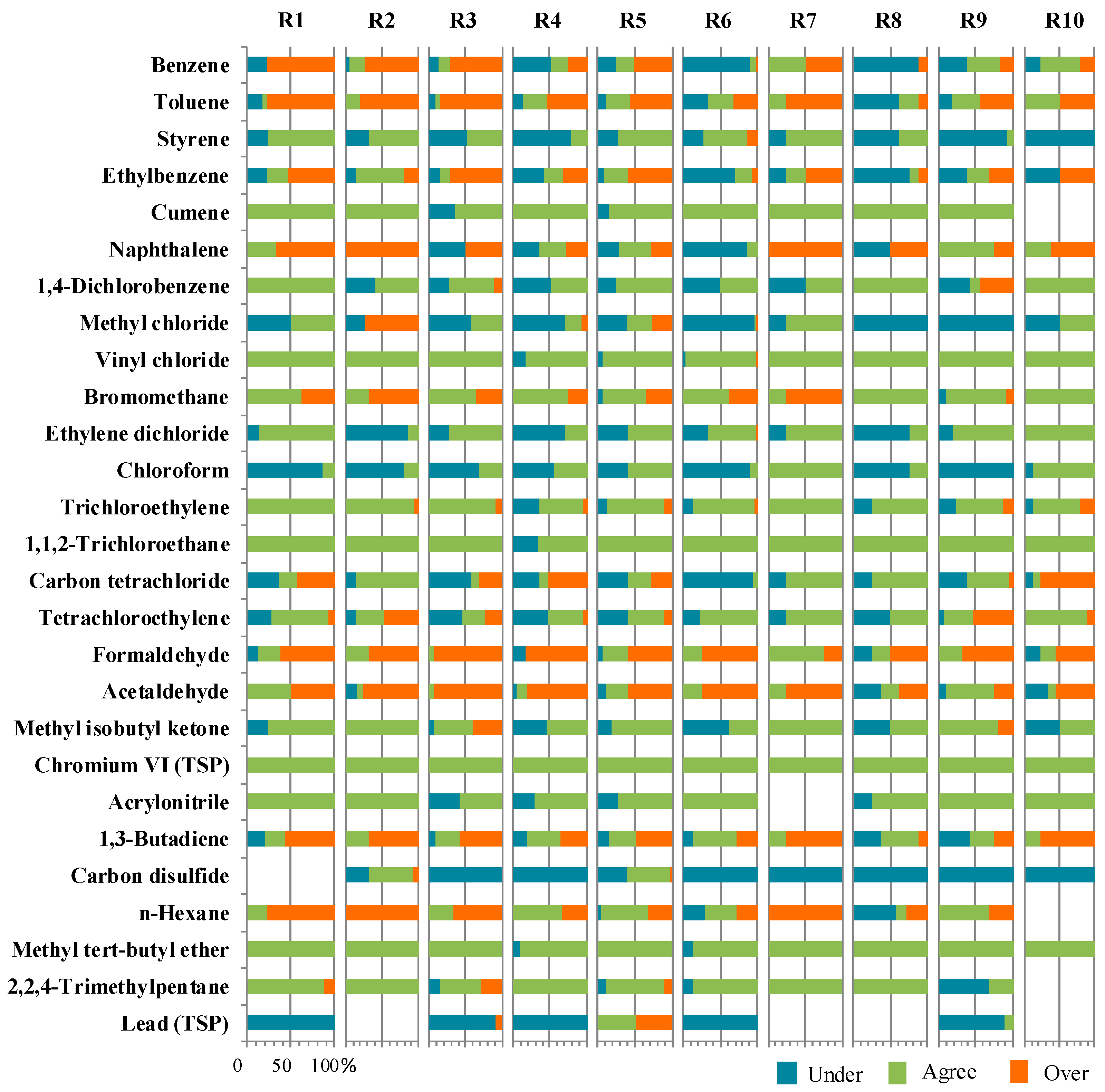
3.3. Regional Model-to-Monitor Agreement
4. Discussion
4.1. Similar Findings from National and Local Studies
4.2. Impacts of Comparison Methods and Metrics
4.3. Study Limitations
4.4. Implications for Environmental Health Disparity Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Salam, M.T.; Eckel, S.P.; Breton, C.V.; Gilliland, F.D. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. J. Thorac. Dis. 2015, 7, 46–58. [Google Scholar] [PubMed]
- Sunyer, J.; Esnaola, M.; Alvarez-Pedrerol, M.; Forns, J.; Rivas, I.; Lopez-Vicente, M.; Suades-Gonzalez, E.; Foraster, M.; Garcia-Esteban, R.; Basagana, X.; et al. Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study. PLoS Med. 2015, 12, e1001792. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.; Saldiva, P.H.; Matsuda, M.; Macchione, M.; Rangel, M.P.; Kara-Jose, N.; Berra, A. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ. Res. 2010, 110, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Lewtas, J. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res.-Rev. Mutat. 2007, 636, 95–133. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.; McDonald-Hyman, C.; Noth, E.M.; Pratt, B.; Hammond, S.K.; Balmes, J.; Tager, I. Ambient air pollution impairs regulatory T-cell function in asthma. J. Allergy Clin. Immun. 2010, 126, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Poursafa, P. Obesity and Air Pollution: Global Risk Factors for Pediatric Non-alcoholic Fatty Liver Disease. Hepat. Mon. 2011, 11, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L. Hazards of heavy metal contamination. Brit. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Dallmann, T.R.; Gu, P.S.; Presto, A.A. Application of mobile sampling to investigate spatial variation in fine particle composition. Atmos. Environ. 2016, 142, 71–82. [Google Scholar] [CrossRef]
- Liu, B.; Xue, Z.Q.; Zhu, X.L.; Jia, C.R. Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the US. Environ. Pollut. 2017, 220, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.S.; Benson, A.F.; Buckley, B.; Chan, E.A.W. Concentrations of individual fine particulate matter components in the USA around July 4th. Air Qual. Atmos. Health 2017, 10, 349–358. [Google Scholar] [CrossRef]
- Li, H.Z.; Gu, P.; Ye, Q.; Zimmerman, N.; Robinson, E.S.; Subramanian, R.; Apte, J.S.; Robinson, A.L.; Presto, A.A. Spatially dense air pollutant sampling: Implications of spatial variability on the representativeness of stationary air pollutant monitors. Atmos. Environ. 2019, 2, 100012. [Google Scholar] [CrossRef]
- Jerrett, M.; Arain, A.; Kanaroglou, P.; Beckerman, B.; Potoglou, D.; Sahsuvaroglu, T.; Morrison, J.; Giovis, C. A review and evaluation of intraurban air pollution exposure models. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 185–204. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. NATA Overview. Available online: https://www.epa.gov/national-air-toxics-assessment/nata-overview (accessed on 24 May 2018).
- De Castro, B.R. Acrolein and Asthma Attack Prevalence in a Representative Sample of the United States Adult Population 2000–2009. PLoS ONE 2014, 9, e96926. [Google Scholar] [CrossRef] [PubMed]
- Stoner, A.M.; Anderson, S.E.; Buckley, T.J. Ambient Air Toxics and Asthma Prevalence among a Representative Sample of US Kindergarten-Age Children. PLoS ONE 2013, 8, e75176. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.L.; Lyall, K.; Hart, J.E.; Laden, F.; Just, A.C.; Bobb, J.F.; Koenen, K.C.; Ascherio, A.; Weisskopf, M.G. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ. Health Perspect. 2013, 121, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Grineski, S.E.; Clark-Reyna, S.E.; Collins, T.W. School-based exposure to hazardous air pollutants and grade point average: A multi-level study. Environ. Res. 2016, 147, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Clark-Reyna, S.E.; Grineski, S.E.; Collins, T.W. Residential exposure to air toxics is linked to lower grade point averages among school children in El Paso, Texas, USA. Popul. Environ. 2016, 37, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Burwell-Naney, K.; Jiang, C.S.; Zhang, H.M.; Samantapudi, A.; Murray, R.; Dalemarre, L.; Rice, L.; Williams, E. Assessment of sociodemographic and geographic disparities in cancer risk from air toxics in South Carolina. Environ. Res. 2015, 140, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.J.; Jiang, C.S.; Wilson, S.M.; Burwell-Naney, K.; Samantapudi, A.; Zhang, H.M. Use of Segregation Indices, Townsend Index, and Air Toxics Data to Assess Lifetime Cancer Risk Disparities in Metropolitan Charleston, South Carolina, USA. Int. J. Environ. Res. Pub. Health 2014, 11, 5510–5526. [Google Scholar] [CrossRef] [PubMed]
- Grineski, S.E.; Collins, T.W.; Chakraborty, J. Hispanic heterogeneity and environmental injustice: Intra-ethnic patterns of exposure to cancer risks from traffic-related air pollution in Miami. Popul. Environ. 2013, 35, 26–44. [Google Scholar] [CrossRef] [PubMed]
- James, W.; Jia, C.R.; Kedia, S. Uneven Magnitude of Disparities in Cancer Risks from Air Toxics. Int. J. Environ. Res. Pub. Health 2012, 9, 4365–4385. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.H.; Marko, D.; Sexton, K. Cumulative cancer risk from air pollution in Houston: Disparities in risk burden and social disadvantage. Environ. Sci. Technol. 2008, 42, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Apelberg, B.J.; Buckley, T.J.; White, R.H. Socioeconomic and racial disparities in cancer risk from air toxics in Maryland. Environ. Health Perspect. 2005, 113, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J. Cancer risk from exposure to hazardous air pollutants: spatial and social inequities in Tampa Bay, Florida. Int. J. Environ. Health Res. 2012, 22, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.; Morello-Frosch, R.; Sadd, J.L. The air is always cleaner on the other side: Race, space, and ambient air toxics exposures in California. J. Urban Aff. 2005, 27, 127–148. [Google Scholar] [CrossRef]
- Ozkaynak, H.; Palma, T.; Touma, J.S.; Thurman, J. Modeling population exposures to outdoor sources of hazardous air pollutants. J. Expo. Sci. Env. Epid. 2008, 18, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Strum, M.; Touma, J.S.; Palma, T.; Thurman, J.; Ensley, D.; Smith, R. Inhalation exposure and risk from mobile source air toxics in future years. J. Expo. Sci. Env. Epid. 2007, 17, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Wells, E.M.; Holt, E.W.; Burgin, D.E.; Axelrad, D.A. Estimating risk from ambient concentrations of acrolein across the United States. Environ. Health Perspect. 2007, 115, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Teoh, S.K.; Nazaroff, W.W. Intake fraction of nonreactive vehicle emissions in US urban areas. Atmos. Environ. 2005, 39, 1363–1371. [Google Scholar] [CrossRef]
- Logue, J.M.; Small, M.J.; Robinson, A.L. Evaluating the national air toxics assessment (NATA): Comparison of predicted and measured air toxics concentrations, risks, and sources in Pittsburgh, Pennsylvania. Atmos. Environ. 2011, 45, 476–484. [Google Scholar] [CrossRef]
- Lupo, P.J.; Symanski, E. A Comparative Analysis of Modeled and Monitored Ambient Hazardous Air Pollutants in Texas: A Novel Approach Using Concordance Correlation. J. Air Waste Manag. Assoc. 2009, 59, 1278–1286. [Google Scholar] [CrossRef]
- Garcia, E.; Hurley, S.; Nelson, D.O.; Gunier, R.B.; Hertz, A.; Reynolds, P. Evaluation of the agreement between modeled and monitored ambient hazardous air pollutants in California. Int. J. Environ. Health Res. 2014, 24, 363–377. [Google Scholar] [CrossRef] [PubMed]
- George, B.J.; Schultz, B.D.; Palma, T.; Vette, A.F.; Whitaker, D.A.; Williams, R.W. An evaluation of EPA’s National-Scale Air Toxics Assessment (NATA): Comparison with benzene measurements in Detroit, Michigan. Atmos. Environ. 2011, 45, 3301–3308. [Google Scholar] [CrossRef]
- Payne-Sturges, D.C.; Burke, T.A.; Breysse, P.; Diener-West, M.; Buckley, T.J. Personal exposure meets risk assessment: A comparison of measured and modeled exposures and risks in an urban community. Environ. Health Perspect. 2004, 112, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.S.; Axelrad, D.A.; Woodruff, T.J.; Wei, Y.H.; Ligocki, M.P.; Cohen, J.P. National estimates of outdoor air toxics concentrations. J. Air Waste Manag. Assoc. 1999, 49, 1138–1152. [Google Scholar] [CrossRef]
- U.S. EPA. 2011 NATA: Assessment Results. Available online: https://www.epa.gov/national-air-toxics-assessment/2011-nata-assessment-results (accessed on 14 June 2016).
- U.S. EPA. Technical Support Document EPA 2011 National-scale Air Toxics Assessment. Available online: https://www.epa.gov/national-air-toxics-assessment/2011-nata-technical-support-document (accessed on 14 April 2016).
- U.S. EPA. Air Toxics—Monitoring Methods. Available online: https://www3.epa.gov/ttnamti1/airtox.html (accessed on 18 June 2017).
- Little, T.A. Method Validation Essentials, Limit of Blank, Limit of Detection, and Limit of Quantitation. Biopharm. Int. 2015, 28, 48–51. [Google Scholar]
- U.S. EPA. Support for the Epa National Contract for Lead Analysis. Available online: https://www3.epa.gov/ttnamti1/files/ambient/pb/nationalcontractforleadanalysis.pdf (accessed on 18 June 2017).
- U.S. EPA. Annual Summary Data. Available online: http://aqsdr1.epa.gov/aqsweb/aqstmp/airdata/download_files.html (accessed on 14 June 2016).
- U.S. EPA. EPA Region 3 Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments. Available online: https://www.epa.gov/risk/epa-region-3-guidance-handling-chemical-concentration-data-near-detection-limit-risk (accessed on 26 April 2017).
- Zhou, X.H.; Gao, S. Confidence intervals for the log-normal mean. Stat. Med. 1997, 16, 783–790. [Google Scholar] [CrossRef]
- U.S. EPA. Protocol for Determining the Best Performing Model (EPA-454/R-92-025); U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 1992.
- Eastern Research Group Inc. Results of the 2005 NATA Model-to-Monitor Comparison, Final Report. Available online: http://www.epa.gov/ttn/atw/nata2005/05pdf/nata2005_model2monitor.pdf (accessed on 17 September 2016).
- U.S. EPA. Comparison of 2002 Model-Predicted Concentrations to Monitored Data. Available online: https://archive.epa.gov/nata2002/web/pdf/2002compare.pdf (accessed on 13 April 2016).
- Wang, S.W.; Tang, X.G.; Fan, Z.H.; Wu, X.M.; Lioy, P.J.; Georgopoulos, P.G. Modeling of Personal Exposures to Ambient Air Toxics in Camden, New Jersey: An Evaluation Study. J. Air Waste Manag. Assoc. 2009, 59, 733–746. [Google Scholar] [CrossRef]
- Scheffe, R.D.; Strum, M.; Phillips, S.B.; Thurman, J.; Eyth, A.; Fudge, S.; Morris, M.; Palma, T.; Cook, R. Hybrid Modeling Approach to Estimate Exposures of Hazardous Air Pollutants (HAPs) for the National Air Toxics Assessment (NATA). Environ. Sci. Technol. 2016, 50, 12356–12364. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Comparison of ASPEN Modeling System Results to Monitored Concentrations. Available online: https://archive.epa.gov/airtoxics/nata/web/html/draft6.html#other_gases (accessed on 19 December 2017).
- Vennam, L.P.; Vizuete, W.; Arunachalam, S. Evaluation of model-predicted hazardous air pollutants (HAPs) near a mid-sized US airport. Atmos. Environ. 2015, 119, 107–117. [Google Scholar] [CrossRef]
- Stroud, C.A.; Zaganescu, C.; Chen, J.; McLinden, C.A.; Zhang, J.H.; Wang, D. Toxic volatile organic air pollutants across Canada: Multi-year concentration trends, regional air quality modelling and source apportionment. J. Atmos. Chem. 2016, 73, 137–164. [Google Scholar] [CrossRef]
- Yu, S.C.; Eder, B.; Dennis, R.; Chu, S.H.; Schwartz, S.E. New unbiased symmetric metrics for evaluation of air quality models. Atmos. Sci. Lett. 2006, 7, 26–34. [Google Scholar] [CrossRef]
- ODPHP. Determinants of Health. Available online: https://www.healthypeople.gov/2020/about/foundation-health-measures/Determinants-of-Health (accessed on 3 January 2018).
- Gee, G.C.; Payne-Sturges, D.C. Environmental health disparities: A framework integrating psychosocial and environmental concepts (vol 112, pg 1645, 2004). Environ. Health Persp. 2005, 113, A18. [Google Scholar]
- Farley, R. Racial and ethnic residential segregation in the United States: 1980 to 2000. Contemp. Sociol. 2004, 33, 21–23. [Google Scholar] [CrossRef]
- Chakraborty, J.; Collins, T.W.; Grineski, S.E.; Montgomery, M.C.; Hernandez, M. Comparing Disproportionate Exposure to Acute and Chronic Pollution Risks: A Case Study in Houston, Texas. Risk Anal. 2014, 34, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
| Hazardous Air Pollutants (HAPs) | NATA-AQS | NATA-All | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Med | Max | N | Mean | SD | Min | Med | Max | |
| Aromatic compounds | ||||||||||||
| Benzene | 274 | 0.79 | 0.47 | 0.09 | 0.69 | 3.02 | 74,034 | 0.70 | 0.43 | 0 | 0.63 | 7.50 |
| Toluene | 257 | 2.62 | 3.01 | 0.09 | 2.08 | 30.37 | 74,034 | 2.61 | 3.46 | 0 | 1.84 | 39.67 |
| Styrene | 236 | 0.05 | 0.16 | 1.1 × 10−3 | 0.02 | 1.57 | 75,023 | 0.02 | 0.06 | 0 | 0.02 | 6.72 |
| Ethylbenzene | 252 | 0.28 | 0.21 | 0.02 | 0.25 | 1.66 | 75,025 | 0.23 | 0.19 | 0 | 0.20 | 3.09 |
| Cumene | 113 | 0.01 | 0.03 | 3.8 × 10−4 | 1.3 × 10−3 | 0.2 | 75,013 | 1.9 × 10−3 | 0.01 | 0 | 8.5 × 10−4 | 1.98 |
| Naphthalene | 52 | 0.05 | 0.04 | 4.2 × 10−3 | 0.04 | 0.22 | 74,034 | 0.04 | 0.03 | 0 | 0.03 | 0.94 |
| 1,4-Dichlorobenzene | 153 | 0.01 | 0.04 | 2.6 × 10−5 | 6.4 × 10−4 | 0.27 | 74,034 | 0.02 | 0.05 | 0 | 5.1 × 10−4 | 1.13 |
| Halogenated compounds | ||||||||||||
| Methyl chloride | 203 | 1.10 | 0.07 | 1.09 | 1.09 | 2.06 | 74,591 | 1.07 | 0.13 | 0 | 1.09 | 2.06 |
| Vinyl chloride | 240 | 4.6 × 10−3 | 0.02 | 1.5 × 10−5 | 3.2 × 10−4 | 0.15 | 74,034 | 6.2 × 10−4 | 3.7 × 10−3 | 0 | 2.2 × 10−4 | 0.73 |
| Bromomethane | 200 | 0.04 | 0.09 | 0.03 | 0.03 | 1.34 | 74,438 | 0.04 | 0.05 | 0 | 0.03 | 2.00 |
| Ethylene dichloride | 230 | 3.4 × 10−3 | 0.01 | 3.2 × 10−5 | 5.3 × 10−4 | 0.08 | 74,034 | 7.5 × 10−4 | 3.7 × 10−3 | 0 | 3.8 × 10−4 | 0.31 |
| Chloroform | 252 | 0.01 | 0.03 | 1.7 × 10−7 | 1.2 × 10−3 | 0.23 | 74,034 | 3.8 × 10−3 | 0.01 | 0 | 8.6 × 10−4 | 1.57 |
| Trichloroethylene | 247 | 0.02 | 0.04 | 4.0 × 10−4 | 0.01 | 0.44 | 74,034 | 0.02 | 0.05 | 0 | 0.01 | 5.49 |
| 1,1,2-Trichloroethane | 185 | 4.1 × 10−4 | 1.2 × 10−4 | 3.9 × 10−4 | 3.9 × 10−4 | 0 | 74,871 | 3.9 × 10−4 | 1.7 × 10−4 | 0 | 3.9 × 10−4 | 0.01 |
| Carbon tetrachloride | 247 | 0.55 | 7.1 × 10−4 | 0.55 | 0.55 | 0.55 | 74,917 | 0.54 | 0.08 | 0 | 0.55 | 0.64 |
| Tetrachloroethylene | 252 | 0.09 | 0.17 | 1.5 × 10−3 | 0.03 | 1.12 | 74,034 | 0.11 | 0.24 | 0 | 0.03 | 5.07 |
| Carbonyl compounds | ||||||||||||
| Formaldehyde | 128 | 1.66 | 0.52 | 0.55 | 1.61 | 2.94 | 74,034 | 1.59 | 0.55 | 0 | 1.57 | 5.56 |
| Acetaldehyde | 133 | 1.95 | 0.55 | 0.91 | 1.85 | 3.28 | 74,034 | 1.94 | 0.66 | 0 | 1.88 | 4.15 |
| Methyl isobutyl ketone | 93 | 0.1 | 0.15 | 4.8 × 10−3 | 0.07 | 1.27 | 74,968 | 0.07 | 0.08 | 0 | 0.05 | 2.12 |
| Other compounds | ||||||||||||
| Chromium VI (TSP) | 27 | 3.4 × 10−5 | 3.6 × 10−5 | 2.3 × 10−6 | 2.1 × 10−5 | 1.4 × 10−4 | 74,034 | 3.9 × 10−5 | 8.0 × 10−5 | 0 | 2.1 × 10−5 | 4.4 × 10−3 |
| Acrylonitrile | 81 | 2.6 × 10−3 | 0.01 | 4.9 × 10−6 | 3.5 × 10−4 | 0.09 | 74,034 | 7.9 × 10−4 | 0.01 | 0 | 3.0 × 10−4 | 1.24 |
| 1,3-Butadiene | 258 | 0.08 | 0.06 | 3.9 × 10−3 | 0.06 | 0.44 | 74,034 | 0.06 | 0.05 | 0 | 0.05 | 0.79 |
| Carbon disulfide | 93 | 0.05 | 0.34 | 0.01 | 0.01 | 3.27 | 74,906 | 0.01 | 0.05 | 0 | 0.01 | 4.91 |
| n-Hexane | 159 | 0.91 | 0.6 | 0.12 | 0.83 | 2.87 | 75,020 | 0.76 | 0.66 | 0 | 0.60 | 16.05 |
| Methyl tert-butyl ether | 127 | 1.1 × 10−3 | 0.01 | 3.6 × 10−9 | 2.0 × 10−5 | 0.12 | 73,815 | 1.5 × 10−3 | 0.01 | 0 | 7.4 × 10−6 | 0.31 |
| 2,2,4-Trimethylpentane | 105 | 0.36 | 0.19 | 0.1 | 0.34 | 0.92 | 75,009 | 0.37 | 0.23 | 0 | 0.33 | 3.59 |
| Lead (TSP) | 51 | 1.5 × 10−3 | 1.5 × 10−3 | 9.5 × 10−5 | 1.0 × 10−3 | 0.01 | 74,034 | 1.0 × 10−3 | 1.5 × 10−3 | 0 | 6.8 × 10−4 | 0.10 |
| HAPs | N | LOQ µg/m3 | ∆M < LOQ? Y/N | ΔM < LOQ % of Sites | AQS | NATA | Wilcoxon Rank Test p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean µg/m3 | SD µg/m3 | Med µg/m3 | Max µg/m3 | <LOQ % | Mean µg/m3 | SD µg/m3 | Med µg/m3 | Max µg/m3 | <LOQ % | ||||||
| Aromatic compounds | |||||||||||||||
| Benzene | 274 | 0.06 | Y | 12.0 | 0.95 | 0.78 | 0.73 | 5.57 | 0.7 | 0.79 | 0.47 | 0.69 | 3.02 | 0.0 | 0.01 |
| Toluene | 257 | 0.10 | N | 8.2 | 1.75 | 1.27 | 1.41 | 6.98 | 0.4 | 2.62 | 3.01 | 2.08 | 30.37 | 0.4 | <0.001 |
| Styrene | 236 | 0.04 | N | 45.3 | 0.14 | 0.19 | 0.07 | 1.45 | 35.6 | 0.05 | 0.16 | 0.02 | 1.57 | 81.4 | <0.001 |
| Ethylbenzene | 252 | 0.05 | Y | 18.3 | 0.32 | 0.80 | 0.21 | 12.57 | 9.1 | 0.28 | 0.21 | 0.25 | 1.66 | 10.7 | 0.60 * |
| Cumene | 113 | 0.50 | Y | 94.7 | 0.11 | 0.40 | 0.03 | 3.79 | 93.8 | 0.01 | 0.03 | 1.3 × 10−3 | 0.20 | 100 | <0.001 |
| Naphthalene | 52 | 7.8 × 10−4 | N | 3.9 | 0.07 | 0.11 | 0.04 | 0.56 | 0.0 | 0.05 | 0.04 | 0.04 | 0.22 | 0.0 | 0.89 * |
| 1,4-Dichlorobenzene | 153 | 0.07 | Y | 62.8 | 0.18 | 0.68 | 0.05 | 7.40 | 63.4 | 0.01 | 0.04 | 6.4 × 10−4 | 0.27 | 94.1 | <0.001 |
| Halogenated compounds | |||||||||||||||
| Methyl chloride | 203 | 0.04 | N | 12.3 | 1.23 | 0.25 | 1.23 | 2.5 | 0.5 | 1.10 | 0.07 | 1.09 | 2.06 | 0.0 | <0.001 |
| Vinyl chloride | 240 | 0.01 | Y | 91.7 | 0.01 | 0.04 | 0.00 | 0.51 | 89.6 | 4.6 × 10−3 | 0.02 | 3.2 × 10−4 | 0.15 | 95.0 | 0.01 |
| Bromomethane | 200 | 0.02 | Y | 59.5 | 0.02 | 0.04 | 0.01 | 0.46 | 69.5 | 0.04 | 0.09 | 0.03 | 1.34 | 0.0 | <0.001 |
| Ethylene dichloride | 230 | 0.02 | Y | 54.8 | 0.07 | 0.34 | 0.02 | 4.73 | 53.0 | 3.4 × 10−3 | 0.01 | 5.3 × 10−4 | 0.08 | 95.7 | <0.001 |
| Chloroform | 252 | 0.04 | N | 26.2 | 0.13 | 0.39 | 0.09 | 6.05 | 25.0 | 0.01 | 0.03 | 1.2 × 10−3 | 0.23 | 93.7 | <0.001 |
| Trichloroethylene | 247 | 0.03 | Y | 72.1 | 0.11 | 1.07 | 0.01 | 16.85 | 62.8 | 0.02 | 0.04 | 0.01 | 0.44 | 76.9 | <0.001 |
| 1,1,2-Trichloroethane | 185 | 0.05 | Y | 93.5 | 0.01 | 0.02 | 0.00 | 0.15 | 93.5 | 4.1 × 10−4 | 1.2 × 10−4 | 3.9 × 10−4 | 1.8 × 10−3 | 100 | 0.03 |
| Carbon tetrachloride | 247 | 0.04 | N | 25.1 | 0.54 | 0.19 | 0.59 | 1.68 | 3.6 | 0.55 | 7.1 × 10−4 | 0.55 | 0.55 | 0.0 | <0.001 |
| Tetrachloroethylene | 252 | 0.06 | N | 39.7 | 0.13 | 0.14 | 0.09 | 1.10 | 29.0 | 0.09 | 0.17 | 0.03 | 1.12 | 67.1 | <0.001 |
| Carbonyl compounds | |||||||||||||||
| Formaldehyde | 128 | 0.03 | N | 4.7 | 1.29 | 0.71 | 1.16 | 6.77 | 0.0 | 1.66 | 0.52 | 1.61 | 2.94 | 0.0 | <0.001 |
| Acetaldehyde | 133 | 0.03 | N | 5.3 | 1.56 | 0.63 | 1.44 | 3.73 | 0.0 | 1.95 | 0.55 | 1.85 | 3.28 | 0.0 | <0.001 |
| Methyl isobutyl ketone | 93 | 0.08 | Y | 65.6 | 0.12 | 0.10 | 0.10 | 0.60 | 38.7 | 0.10 | 0.15 | 0.07 | 1.27 | 53.8 | 0.00 |
| Other compounds | |||||||||||||||
| ChromiumVI (TSP) | 27 | 2.7 × 10−3 | Y | 100.0 | 1.3 × 10−5 | 1.3 × 10−5 | 1.2 × 10−5 | 4.7 × 10−5 | 100 | 3.4 × 10−5 | 3.6 × 10−5 | 2.1 × 10−5 | 1.4 × 10−4 | 100 | 0.00 |
| Acrylonitrile | 81 | 0.10 | Y | 79.0 | 0.11 | 0.24 | 0.01 | 1.06 | 79.0 | 2.6 × 10−3 | 0.01 | 3.5 × 10−4 | 0.09 | 100 | <0.001 |
| 1,3-Butadiene | 258 | 0.02 | Y | 29.5 | 0.08 | 0.14 | 0.05 | 1.74 | 34.1 | 0.08 | 0.06 | 0.06 | 0.44 | 12.0 | <0.001 |
| Carbon disulfide | 93 | 0.02 | N | 20.4 | 0.92 | 3.44 | 0.07 | 29.44 | 19.4 | 0.05 | 0.34 | 0.01 | 3.27 | 95.7 | <0.001 |
| n-Hexane | 159 | 0.31 | Y | 43.4 | 0.81 | 0.75 | 0.57 | 4.39 | 19.5 | 0.91 | 0.60 | 0.83 | 2.87 | 16.4 | <0.001 |
| Methyl tert-butyl ether | 127 | 0.16 | Y | 98.4 | 0.01 | 0.07 | 0.00 | 0.72 | 98.4 | 1.1 × 10−3 | 0.01 | 2.0 × 10−5 | 0.12 | 100 | 0.08 * |
| 2,2,4-Trimethylpentane | 105 | 0.28 | Y | 79.1 | 0.50 | 0.53 | 0.34 | 3.1 | 39.0 | 0.36 | 0.19 | 0.34 | 0.92 | 38.1 | 0.16 * |
| Lead (TSP) | 51 | 2.6 × 10−4 | N | 7.8 | 0.01 | 0.01 | 3.4 × 10−3 | 0.07 | 7.8 | 1.5 × 10−3 | 1.5 × 10−3 | 1.0 × 10−3 | 0.01 | 5.9 | <0.001 |
| HAPs | N | ΔM < LOQ | Within 95% CL | Total Agreement | Less than 95% LCL (Under) | Greater than 95% UCL (Over) |
|---|---|---|---|---|---|---|
| Aromatic compounds | ||||||
| Benzene | 274 | 12.0 | 9.9 | 21.9 | 43.8 | 34.3 |
| Toluene | 257 | 8.2 | 20.6 | 28.8 | 18.7 | 52.5 * |
| Styrene | 236 | 45.3 | 5.9 | 51.3 * | 45.3 | 3.4 |
| Ethylbenzene | 252 | 18.3 | 9.5 | 27.8 | 36.5 | 35.7 |
| Cumene | 113 | 94.7 | 0.0 | 94.7 * | 5.3 | 0.0 |
| Naphthalene | 52 | 3.8 | 26.9 | 30.8 | 30.8 | 38.5 |
| 1,4-Dichlorobenzene | 153 | 62.7 | 3.3 | 66.0 * | 30.7 | 3.3 |
| Halogenated compounds | ||||||
| Methyl chloride | 203 | 12.3 | 10.3 | 22.7 | 63.1 * | 14.3 |
| Vinyl chloride | 240 | 91.7 | 2.9 | 94.6 * | 5.0 | 0.4 |
| Bromomethane | 200 | 59.5 | 3.5 | 63.0 * | 2.5 | 34.5 |
| Ethylene dichloride | 230 | 54.8 | 3.5 | 58.3 * | 41.3 | 0.4 |
| Chloroform | 252 | 26.2 | 5.2 | 31.3 | 68.7 * | 0.0 |
| Trichloroethylene | 247 | 72.1 | 4.9 | 76.9 * | 15.0 | 8.1 |
| 1,1,2-Trichloroethane | 185 | 93.5 | 1.6 | 95.1 * | 4.9 | 0.0 |
| Carbon tetrachloride | 247 | 25.1 | 2.8 | 27.9 | 49.8 * | 22.3 |
| Tetrachloroethylene | 252 | 39.7 | 13.9 | 53.6 * | 29.8 | 16.7 |
| Carbonyl compounds | ||||||
| Formaldehyde | 128 | 4.7 | 21.1 | 25.8 | 7.0 | 67.2 * |
| Acetaldehyde | 133 | 5.3 | 21.8 | 27.1 | 10.5 | 62.4 * |
| Methyl isobutyl ketone | 93 | 65.6 | 3.2 | 68.8 | 23.7 | 7.5 |
| Other compounds | ||||||
| Chromium VI (TSP) | 27 | 100.0 | 0.0 | 100.0 * | 0.0 | 0.0 |
| Acrylonitrile | 81 | 79.0 | 1.2 | 80.2 * | 19.8 | 0.0 |
| 1,3-Butadiene | 258 | 29.5 | 10.5 | 39.9 | 18.2 | 41.9 |
| Carbon disulfide | 93 | 20.4 | 17.2 | 37.6 | 60.2 * | 2.2 |
| n-Hexane | 159 | 43.4 | 5.0 | 48.4 | 13.2 | 38.4 |
| Methyl tert-butyl ether | 127 | 98.4 | 0.0 | 98.4 * | 1.6 | 0.0 |
| 2,2,4-Trimethylpentane | 105 | 79.0 | 0.0 | 79.0 * | 17.1 | 3.8 |
| Lead (TSP) | 51 | 7.8 | 5.9 | 13.7 | 74.5 * | 11.8 |
| HAPs | N | Fractional Bias | Total Agreement | |||
|---|---|---|---|---|---|---|
| ΔM < LOQ | (−2, −0.67) | (−0.67, 0.67) | (0.67, 2) | |||
| % | ||||||
| Aromatic compounds | ||||||
| Benzene | 274 | 12.0 | 20.1 | 55.8 | 12.0 | 67.9 * |
| Toluene | 257 | 8.2 | 11.7 | 48.2 | 31.9 | 56.4 * |
| Styrene | 236 | 45.3 | 49.2 | 2.5 | 3.0 | 47.9 |
| Ethylbenzene | 252 | 18.3 | 19.4 | 40.1 | 22.2 | 58.3 * |
| Cumene | 113 | 94.7 | 5.3 | 0 | 0 | 94.7 * |
| Naphthalene | 52 | 3.8 | 21.2 | 55.8 | 19.2 | 59.6 * |
| 1,4-Dichlorobenzene | 153 | 62.7 | 34.0 | 1.3 | 2.0 | 64.1 * |
| Halogenated compounds | ||||||
| Methyl chloride | 203 | 12.3 | 0.5 | 85.7 | 1.5 | 98.0 * |
| Vinyl chloride | 240 | 91.7 | 6.3 | 2.1 | 0 | 93.8 * |
| Bromomethane | 200 | 59.5 | 4.0 | 2.0 | 34.5 | 61.5 * |
| Ethylene dichloride | 230 | 54.8 | 44.8 | 0.4 | 0 | 55.2 * |
| Chloroform | 252 | 26.2 | 71.8 | 2.0 | 0 | 28.2 |
| Trichloroethylene | 247 | 72.1 | 18.6 | 2.0 | 7.3 | 74.1 * |
| 1,1,2-Trichloroethane | 185 | 93.5 | 6.5 | 0 | 0 | 93.5 * |
| Carbon tetrachloride | 247 | 25.1 | 0.4 | 63.6 | 10.9 | 88.7 * |
| Tetrachloroethylene | 252 | 39.7 | 42.5 | 4.8 | 13.1 | 44.4 |
| Carbonyl compounds | ||||||
| Formaldehyde | 128 | 4.7 | 1.6 | 78.1 | 15.6 | 82.8 * |
| Acetaldehyde | 133 | 5.3 | 0 | 81.2 | 13.5 | 86.5 * |
| Methyl isobutyl ketone | 93 | 65.6 | 23.7 | 4.3 | 6.5 | 69.9 * |
| Other compounds | ||||||
| Chromium VI (TSP) | 27 | 100.0 | 0 | 0 | 0 | 100 * |
| Acrylonitrile | 81 | 79.0 | 21.0 | 0 | 0 | 79.0 * |
| 1,3-Butadiene | 258 | 29.5 | 18.6 | 16.7 | 35.3 | 46.1 |
| Carbon disulfide | 93 | 20.4 | 75.3 | 2.2 | 2.2 | 22.6 |
| n-Hexane | 159 | 43.4 | 8.8 | 20.8 | 27.0 | 64.2 * |
| Methyl tert-butyl ether | 127 | 98.4 | 1.6 | 0 | 0 | 98.4 * |
| 2,2,4-Trimethylpentane | 105 | 79.0 | 13.3 | 5.7 | 1.9 | 84.8 * |
| Lead (TSP) | 51 | 7.8 | 68.6 | 13.7 | 9.8 | 21.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Jia, C. A Model-to-Monitor Evaluation of 2011 National-Scale Air Toxics Assessment (NATA). Toxics 2019, 7, 13. https://doi.org/10.3390/toxics7010013
Xue Z, Jia C. A Model-to-Monitor Evaluation of 2011 National-Scale Air Toxics Assessment (NATA). Toxics. 2019; 7(1):13. https://doi.org/10.3390/toxics7010013
Chicago/Turabian StyleXue, Zhuqing, and Chunrong Jia. 2019. "A Model-to-Monitor Evaluation of 2011 National-Scale Air Toxics Assessment (NATA)" Toxics 7, no. 1: 13. https://doi.org/10.3390/toxics7010013
APA StyleXue, Z., & Jia, C. (2019). A Model-to-Monitor Evaluation of 2011 National-Scale Air Toxics Assessment (NATA). Toxics, 7(1), 13. https://doi.org/10.3390/toxics7010013






