Comparative Analysis between Ecotoxicity of Nitrogen-, Phosphorus-, and Potassium-Based Fertilizers and Their Active Ingredients
Abstract
:1. Introduction
2. Materials and Methods
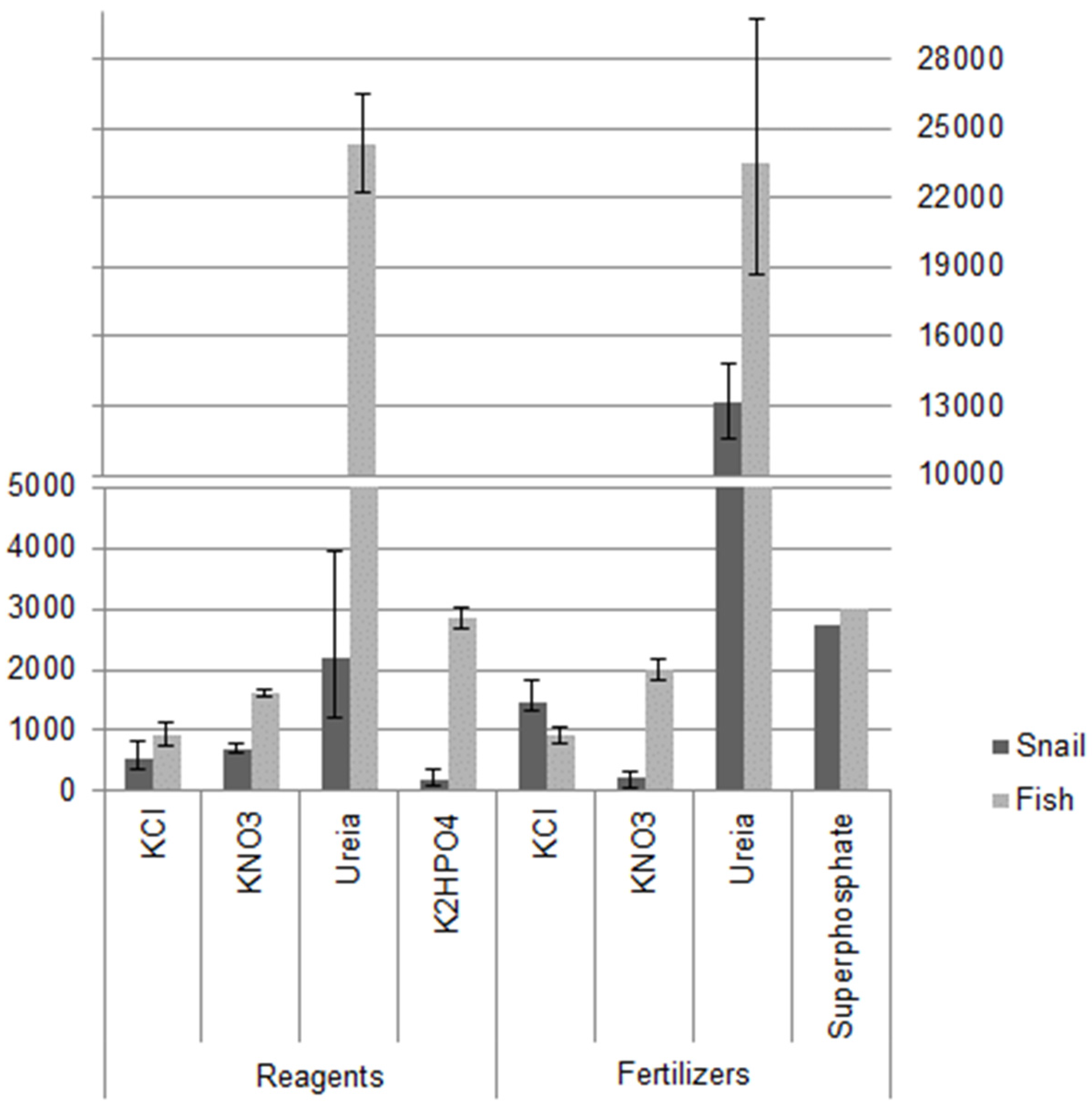
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IFA (International Fertilizer Industry Association). Assessment of Fertilizer Use by Crop at the Global Level; IFA: Paris, France, 2013; Available online: http://www.fertilizer.org (accessed on 5 September 2016).
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- ESTEVES, F.A. Fundamentos de Limnologia, 2nd ed.; Interciência: Rio de Janeiro, Brazil, 1998. [Google Scholar]
- Vilela, L.; Sousa, D.M.G.; Silva, J.E. Adubação potássica. In Cerrado: Correção do solo e adubação; Sousa, D.M.G., Lobato, E., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2004. [Google Scholar]
- Rosolem, C.A.; Vicentini, J.P.T.M.M.; Steiner, F. Suprimento de potássio em função da adubação potássica residual em um Latossolo Vermelho do Cerrado. Rev. Bras. Ciênc. Solo 2012, 36, 1507–1515. [Google Scholar] [CrossRef]
- Resende, A.V.; Martins, E.S.; Oliveira, C.G.; Sena, M.C.; Machado, C.T.T.; Kinpara, D.I.; Oliveira-Filho, E.C. Suprimentos de potássio e pesquisa de uso de rochas “in natura” na agricultura brasileira. Espaço Geogr. 2006, 9, 19–42. Available online: http://www.lsie.unb.br/espacoegeografia/index.php?journal=espacoegeografia&page=article&op=view&path%5B%5D=46&path%5B%5D=45 (accessed on 21 September 2016). [Google Scholar]
- Utz, L.R.P.; Böhrer, M.B.C. Acute and chronic toxicity of potassium chloride (KCl) and potassium acetate (KC2H3O2) to Daphnia similis and Ceriodaphnia dubia (Crustacea; Cladocera). Bull. Environ. Contam. Toxicol. 2001, 66, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Zeng, C. Acute toxicity of sodium nitrate, potassium nitrate, and potassium chloride and their effects on the hemolymph composition and gill structure of early juvenile blues swimmer crabs (Portunus pelagicus Linnaeus, 1758) (Decapoda, Brachyura, Portunidae). Environ. Toxicol. Chem. 2007, 26, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate pollution of groundwater; all right…, but nothing else? Sci. Total Environ. 2016, 539, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Zagatto, P.A. Ecotoxicologia. In Ecotoxicologia aquática: Princípios e aplicações; Zagatto, P.A., Bertoletti, E., Eds.; Rima: São Carlos, Brazil, 2006; pp. 1–13. [Google Scholar]
- Oliveira-Filho, E.C. Avaliação da toxicidade. In Princípios de Toxicologia Ambiental; Sisinno, C.L.S., Oliveira-Filho, E.C., Eds.; Interciência: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.B.R.; Azevedo, F.; Winkaler, E.U. Toxicidade e efeitos da amônia em peixes neotropicais. In Tópicos Especiais em Biologia Aquática e Aqüicultura; Cyrino, J.E.P., Urbinati, E.C., Eds.; Sociedade Brasileira Aqüicultura e Biologia aquática: Jaboticabal, Brazil, 2006; pp. 81–95. [Google Scholar]
- Barbieri, E.; Bondioli, A.C.V. Acute toxicity of ammonia in pacu fish (Piaractus mesopotamicus, Holberg, 1887) at different temperature levels. Aquac. Res. 2015, 46, 565–571. [Google Scholar] [CrossRef]
- Penttinen, S.; Malk, V.; Väisänen, A.; Penttinen, O.P. Using the critical body residue approach to determine the acute toxicity of cadmium at varying levels of water hardness and dissolved organic carbon concentrations. Ecotoxicol. Environ. Saf. 2011, 74, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.W.; Stromberg, P.; Bruner, K.A.; Boulet, L.D. Molluscicidal activity of potassium to the zebra mussel, Dreissena polymorphia: Toxicity and mode of action. Aquat. Toxicol. 1991, 20, 219–234. [Google Scholar] [CrossRef]
- Kim, E.; Yoo, S.; Ro, H.Y.; Han, H.J.; Baek, Y.W.; Eom, I.C.; Kim, H.M.; Kim, P.; Choi, K. Aquatic toxicity assessment of phosphate compounds. Environ. Health Toxicol. 2013, 28, 1–7. [Google Scholar] [CrossRef]
- Sousa, D.M.G.; Lobato, E. Calagem e adubação para culturas anuais e semiperenes. In Cerrado: Correção do solo e adubação; Sousa, D.M.G., Lobato, E., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2004. [Google Scholar]
- Oliveira-Filho, E.C.; Geraldino, B.R.; Grisolia, C.K.; Paumgartten, F.J.R. Acute Toxicity of Endosulfan, Nonylphenol Ethoxylate and Ethanol to Different Life Stages of the Freshwater Snail Biomphalaria tenagophila (Orbigny, 1835). Bull. Environ. Contam. Toxicol. 2005, 75, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- ABNT (Associação Brasileira de Normas Técnicas). Ecotoxicologia aquática - Toxicidade aguda - Método de ensaio com peixes. NBR 15088; ABNT: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Hamilton, M.A.; Russo, R.C.; Thurston, R.V. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714–719. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwaters and Marine Organisms; EPA-821-R-02-012, 4th ed.U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Frankenberger, W.F., Jr.; Mehra, H.C.; Gjerd, D.T. Environmental applications of ion chromatography. J. Chromatogr. 1990, 504, 211–245. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria; Available online: http://www.r-project.org/ (accessed on 5 September 2016).
- Mount, D.R.; Gulley, D.D.; Hocket, T.J.R.; Garrison, T.D.; Evans, J.M. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 1997, 16, 2009–2019. [Google Scholar] [CrossRef]
- Ignácio, N.F.; Américo, J.H.P.; Silva, M.A.; Carraschi, S.P.; Ikefuti, C.V.; Cruz, C.; Machado-Neto, J.G. Classificação ecotoxicológica do inseticida Fipronil para o peixe de espécie pacu. Congresso Nacional de Meio Ambiente de Poços de Caldas 2014, 6. Available online: http://meioambientepocos.com.br/portal/anais/2014/index.php (accessed on 5 September 2016). [Google Scholar]
- Struewing, K.A.; Lazorchak, J.M.; Weaver, P.C.; Johnson, B.R.; Funk, D.H.; Buchwalter, D.B. Part 2: Sensitivity comparisons of the mayfly Centroptilum triangulifer to Ceriodaphnia dubia and Daphnia magna using standard reference toxicants; NaCl, KCl and CuSO4. Chemosphere 2015, 149, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Dowden, B.F.; Bennet, H.J. Toxicity of selected chemicals to certain animals. J. Water Poll. Control Fed. 1965, 37, 1308–1316. Available online: http://www.jstor.org/stable/25035374 (accessed on 22 September 2016). [Google Scholar]
- Rubin, A.J.; Elmaraghy, G.A. Studies on the toxicity of ammonia, nitrate and their mixtures to guppy fry. Water Res. 1977, 11, 927–935. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Englande, A.J., Jr.; Malek, E.A. Toxicity evaluation of ammonium sulphate and urea to three developmental stages of freshwater snails. Arch. Environ. Contam. Toxicol. 1991, 21, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Eshra, E.H. Toxicity of methomyl, copper hydroxide and urea fertilizer on some land snails. Ann. Agric. Sci. 2014, 59, 281–284. [Google Scholar] [CrossRef]
- Sangeetha, S.; Sujatha, K.; Senthilkumaar, P.; Kalyanaraman, V.; Eswari, S. Acute toxicity of some agriculture fertilizers to fingerlings of Catla catla. Indian J. Sci. Technol. 2011, 4, 770–772. Available online: http://www.indjst.org/index.php/indjst/article/view/30108 (accessed on 22 September 2016). [Google Scholar]
- Harless, M.L.; Huckins, C.J.; Grant, J.B.; Pypker, T.G. Effects of six chemical deicers on larval wood frogs (Rana sylvatica). Environ. Toxicol. Chem. 2011, 30, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Perret, P.; Egger, M.; Degremont, A.A. Essai de lutte anti-mollusque par augmentation de la biomasse planctonique et traitement molluscicide: Association Urée-N-tritylmorpholine. Acta Tropica 1972, 29, 175–181. [Google Scholar] [PubMed]
- Reish, D.L. The effects of varying concentrations of nutrient, chlorinity, and dissolved oxygen on polychaetous annelids. Water Res. 1970, 4, 721–735. [Google Scholar] [CrossRef]
- Vieira, R.F.; Ramos, M.M. Fertirrigação. In Recomendações para o uso de corretivos e fertilizantes em Minas Gerais; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; Comissão de Fertilidade do Solo do Estado de Minas Gerais: Viçosa, Brazil, 1999. [Google Scholar]
- Oliveira-Filho, E.C.; Caixeta, N.R.; Simplicio, N.C.S.; Sousa, S.R.; Aragão, T.P.; Muniz, D.H.F. Implications of water hardness in ecotoxicological assessments for water quality regulatory purposes: A case study with the snail Biomphalaria glabrata (Say, 1818). Braz. J. Biol. 2014, 74, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, E.; Ajima, M.N.O.; Keke, R.I.; Wieski, K. Effect of single superphosphate fertilizer on survival and respiratory dynamics of Nile tilapia, Oreochromis niloticus (Actinopterygii: Perciformes: Cichlidae). Acta Ichthyol. Piscat. 2009, 39, 103–110. [Google Scholar] [CrossRef]
- Kegley, S.E.; Hill, B.R.; Choi, A.H. PAN Pesticide Database; Pesticide Action Network: Oakland, CA, USA, 2014; Available online: http://www.pesticideinfo.org/ (accessed on 5 September 2016).
- USEPA. ECOTOX User Guide: ECOTOXicology Database System. Version 4.0. Available online: http:/www.epa.gov/ecotox/ (accessed on 5 September 2016).
- Ministry of Health. Vigilância e Controle de Moluscos de Importância Epidemiológica, 2nd ed.Editora do Ministério da Saúde: Brasília, Brazil, 2007.
- Freitas, E.C.; Rocha, O. Acute and chronic effects of sodium and potassium on the tropical freshwater cladoceran Pseudosida ramosa. Ecotoxicology 2011, 20, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Vijayavel, K.; Balasubramanian, M.P. Interaction of potash and decis in the ecophysiology of a freshwater fish Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2007, 66, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Camargo, J.A. Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull. Environ. Contam. Toxicol. 2003, 70, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, H.J. Nitrate toxicity in Siberian sturgeon (Acipenser baeri). Aquaculture 2006, 253, 688–693. [Google Scholar] [CrossRef]
- Scott, G.; Crunkilton, R.L. Acute and chronic toxicity of nitrate to fathead minnows (Pimephales promelas), Ceriodaphnia dubia, and Daphnia magna. Environ. Toxicol. Chem. 2000, 19, 2918–2922. [Google Scholar] [CrossRef]
- Satoh, S.; Porn-Ngam, N.; Takeuchi, T.; Watanabe, T. Effect of various types of phosphate on zinc availability to rainbow trout. Nippon Suisan Gakkaishi 1993, 59, 1395–1400. [Google Scholar] [CrossRef]
| Substance | Concentration | Ammonium | Potassium | Chloride | Nitrate | Phosphate |
|---|---|---|---|---|---|---|
| KCl | Lowest Concentration | 0.000 | 113.404 | 132.215 | 0.000 | 0.000 |
| Highest Concentration | 0.000 | 1038.781 | 1043.386 | 0.000 | 0.000 | |
| KNO3 | Lowest Concentration | 0.000 | 105.629 | 2.553 | 100.671 | 0.000 |
| Highest Concentration | 0.000 | 1343.164 | 0.000 | 1314.378 | 0.000 | |
| Urea | Lowest Concentration | 0.000 | 1.255 | 1.867 | 0.000 | 0.000 |
| Highest Concentration | 12.357 | 0.000 | 1.669 | 0.000 | 0.000 | |
| KH2PO4 | Lowest Concentration | 0.000 | 22.590 | 0.240 | 0.000 | 66.702 |
| Highest Concentration | 0.000 | 1001.143 | 0.000 | 0.000 | 2732.077 | |
| Dilution water | - | 0.000 | 2.079 | 0.649 | 0.000 | 0.000 |
| Substance | Concentration | Ammonium | Potassium | Chloride | Nitrate | Phosphate |
|---|---|---|---|---|---|---|
| KCl | Lowest Concentration | 0.000 | 101.197 | 128.476 | 0.000 | 0.000 |
| Highest Concentration | 0.000 | 1020.555 | 854.058 | 0.000 | 0.000 | |
| KNO3 | Lowest Concentration | 0.000 | 38.875 | 1.442 | 41.208 | 0.000 |
| Highest Concentration | 0.000 | 1055.488 | 0.000 | 1135.409 | 0.000 | |
| Urea | Lowest Concentration | 2.300 | 5.263 | 5.742 | 0.000 | 0.000 |
| Highest Concentration | 20.867 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Superphosphate | Lowest Concentration | 0.000 | 0.000 | 1.495 | 0.000 | 28.753 |
| Highest Concentration | 0.000 | 0.000 | 0.000 | 0.000 | 576.473 | |
| Dilution water | - | 0.000 | 2.079 | 0.649 | 0.000 | 0.000 |
| Potassium in Reagent | Chemical Reagent | |||
| 48 h | 96 h | |||
| B. glabrata | D. rerio | B. glabrata | D. rerio | |
| KCl – K | 422.27 (270.14–660.07) | 802.39 (523.53–1229.78) | 277.98 (183.58–420.92) | 527.91 (432.64–644.16) |
| KNO3 – K | 347.07 (303.49–396.89) | 786.45 (732.92–843.89) | 347.07 (303.49–396.89) | 786.45 (732.92–843.89) |
| KH2PO4 – K | 90.32 (33.11–246.34) | 811.47 (690.15–954.12) | 44.26 (19.83–98.75) | 774.16 (721.89–830.21) |
| Potassium in Fertilizer | Commercial Fertilizer | |||
| 48 h | 96 h | |||
| B. glabrata | D. rerio | B. glabrata | D. rerio | |
| KCl – K | 891.98 (801.01–993.29) | 589.25 (502.64–690.79) | 785.20 (610.17–1010.43) | 509.65 (445.97–582.42) |
| KNO3 – K | 139.90 (111.63–175.33) | 993.65 (944.33–1045.55) | 102.73 (68.40–154.29) | 993.65 (944.33–1045.55) |
| Nitrogen in Reagent | Chemical Reagent | |||
| 48 h | 96 h | |||
| B. glabrata | D. rerio | B. glabrata | D. rerio | |
| KNO3 – N | 354.09 (311.55–402.44) | 856.23 (825.64–887.95) | 354.09 (311.55–402.44) | 856.23 (825.64–887.95) |
| CO(NH2)2 – N | 0.81 (0.62–1.05) | 9.05 (8.36–9.81) | 0.10 (0.001–17.73) | 8.55 (8.08 –9.04) |
| Nitrogen in Fertilizer | Commercial Fertilizer | |||
| 48 h | 96 h | |||
| B. glabrata | D. rerio | B. glabrata | D. rerio | |
| KNO3 – N | 153.38 (125.98–186.87) | 988.01 (878.70–1110.93) | 117.40 (79.88–172.56) | 988.01 (878.70–1110.93) |
| CO(NH2)2 – N | 8.51 (5.38–13.45) | 6.28 (4.65–8.48) | 3.39 (2.89–3.99) | 6.28 (4.65–8.48) |
| Phosphate in Reagent and in Fertilizer | 48 h | 96 h | ||
|---|---|---|---|---|
| B. glabrata | D. rerio | B. glabrata | D. rerio | |
| KH2PO4 – PO4 | 260.75 (105.14–646.65) | 2171.37 (1811.21–2603.15) | 133.73 (61.00–293.16) | 2056.98 (1901.46–2225.22) |
| Ca(H2PO4)2 + CaSO4 – PO4 | >576.473 | >576.473 | 515.26 | >576.473 |
| Species Name | LC50/EC50 (95% Confidence Interval Limit) | Study |
|---|---|---|
| Ceriodaphnia dubia | 630.0 mg∙L−1 (580–670) | Mount et al. (1997) [ 26] |
| Daphnia magna | 660.0 mg∙L−1 (440–880) | Mount et al. (1997) [ 26] |
| Daphnia similis | 986.66 mg∙L−1 (293.34–1313.32) | Utz and Böhrer (2001) [ 8] |
| Pimephales promelas | 910.0 mg∙L−1 (750–1090) | Mount et al. (1997) [ 26] |
| Piaractus mesopotamicus | 1370 mg∙L−1 | Ignácio et al. (2014) [ 27] |
| Centroptilum triangulifer | 1956.7 mg∙L−1 | Struewing et al. (2015) [ 28] |
| Species Name | LC50/EC50 | Study |
|---|---|---|
| Daphnia magna | 490 mg∙L−1 | Dowden and Bennet (1965) [29] |
| Lepomis macrochirus | 5500 mg∙L−1 | Dowden and Bennet (1965) [29] |
| Poecilia reticulata | 1380 mg∙L−1 | Rubin and Elmaraghy (1977) [30] |
| Species Name | LC50/EC50 (95% Confidence Intervals) | Study |
|---|---|---|
| Biomphalaria havanensis | 21,412.08 mg∙L−1 (19,269.66–23,538.43) | Tchounwou et al. (1991) [31] |
| Helisoma trivolvis | 13,476.59 mg∙L−1 (11,957.82–14,888.23) | Tchounwou et al. (1991) [31] |
| Eobania vermiculata | 54,860 mg∙L−1 | Eshra (2014) [32] |
| Theba pisana | 48,860 mg∙L−1 | Eshra (2014) [32] |
| Catla catla | 280 mg∙L−1 (260–280) | Sangeetha et al. (2011) [33] |
| Rana sylvatica | 14,370 mg∙L−1 (12,400–16,110) | Harless et al. (2011) [34] |
| Species Name | LC50/EC50 (95% Confidence Interval Limit) | Study |
|---|---|---|
| Oreochromis niloticus | 3760 mg∙L−1 (2990–4010) | Omoregie et al. (2009) [39] |
| Chironomus yoshimatsui | 1520 mg∙L−1 | Kegley et al. (2014) [40] |
| Viviparus bengalensis | 2280 mg∙L−1 | Kegley et al. (2014) [40] |
| Radix luteola | 3000 mg∙L−1 | USEPA, (2015) [41] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simplício, N.D.C.S.; Muniz, D.H.d.F.; Rocha, F.R.M.; Martins, D.C.; Dias, Z.M.B.; Farias, B.P.d.C.; Oliveira-Filho, E.C. Comparative Analysis between Ecotoxicity of Nitrogen-, Phosphorus-, and Potassium-Based Fertilizers and Their Active Ingredients. Toxics 2017, 5, 2. https://doi.org/10.3390/toxics5010002
Simplício NDCS, Muniz DHdF, Rocha FRM, Martins DC, Dias ZMB, Farias BPdC, Oliveira-Filho EC. Comparative Analysis between Ecotoxicity of Nitrogen-, Phosphorus-, and Potassium-Based Fertilizers and Their Active Ingredients. Toxics. 2017; 5(1):2. https://doi.org/10.3390/toxics5010002
Chicago/Turabian StyleSimplício, Nathan De Castro Soares, Daphne Heloísa de Freitas Muniz, Fernanda Regina Moreira Rocha, Denis Cavalcanti Martins, Zélia Malena Barreira Dias, Bruno Pereira da Costa Farias, and Eduardo Cyrino Oliveira-Filho. 2017. "Comparative Analysis between Ecotoxicity of Nitrogen-, Phosphorus-, and Potassium-Based Fertilizers and Their Active Ingredients" Toxics 5, no. 1: 2. https://doi.org/10.3390/toxics5010002
APA StyleSimplício, N. D. C. S., Muniz, D. H. d. F., Rocha, F. R. M., Martins, D. C., Dias, Z. M. B., Farias, B. P. d. C., & Oliveira-Filho, E. C. (2017). Comparative Analysis between Ecotoxicity of Nitrogen-, Phosphorus-, and Potassium-Based Fertilizers and Their Active Ingredients. Toxics, 5(1), 2. https://doi.org/10.3390/toxics5010002








