Effects of Long-Term Heavy Metal Pollution on Microbial Community Structure in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sample Collection
2.3. Determination of Soil Physicochemical Indicators and Heavy Metals
2.4. High-Throughput Sequencing of Bacteria and Fungi in Soil
2.5. Statistical Analysis
3. Results
3.1. Effects of Heavy Metal Pollution Levels on Soil Physicochemical Properties
3.2. Effects of Heavy Metal Pollution Levels on the α Diversity of Bacteria and Fungi in Soil
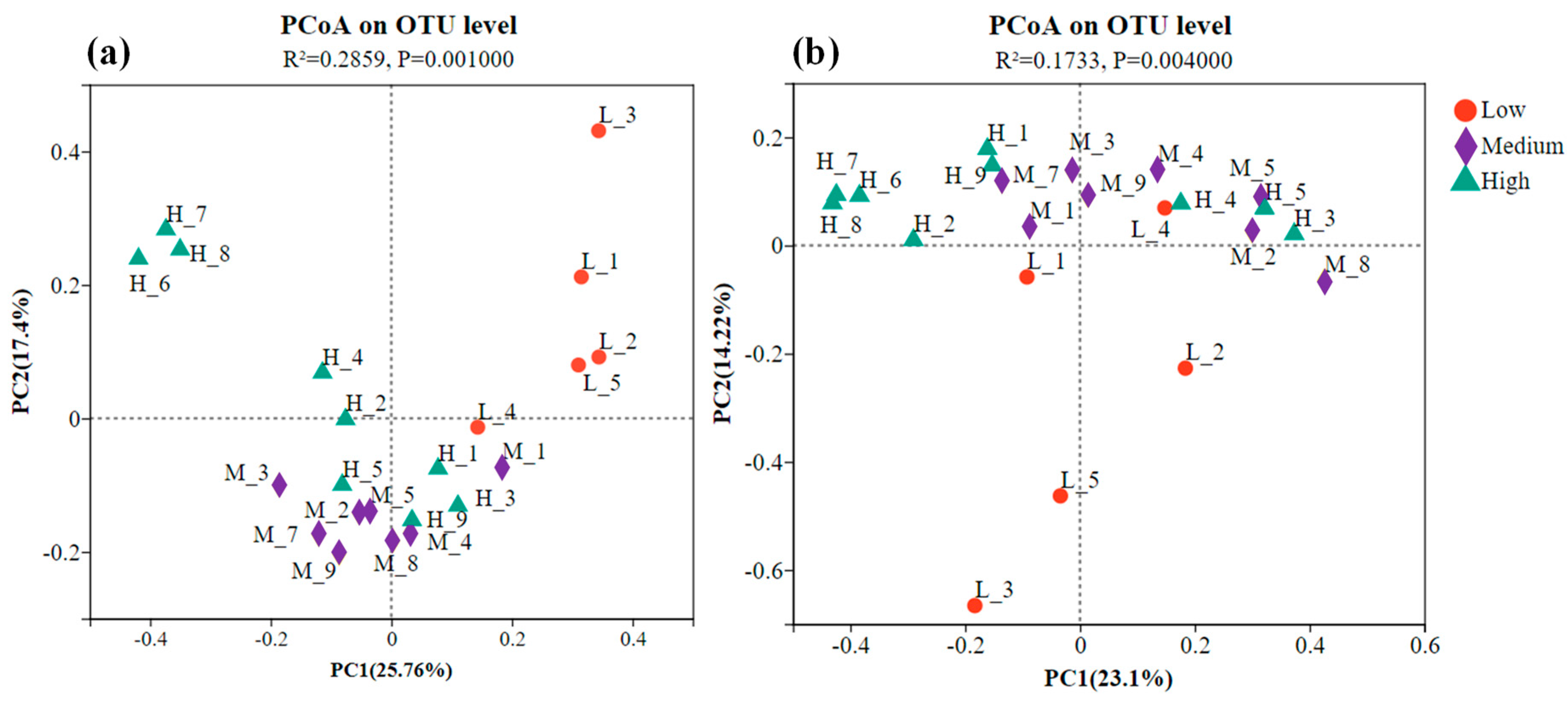
3.3. Effects of Heavy Metal Pollution Levels on the β-Diversity of Bacteria and Fungi in Soil
3.4. Effects of Heavy Metal Pollution Levels on the Composition of Bacterial and Fungal Communities
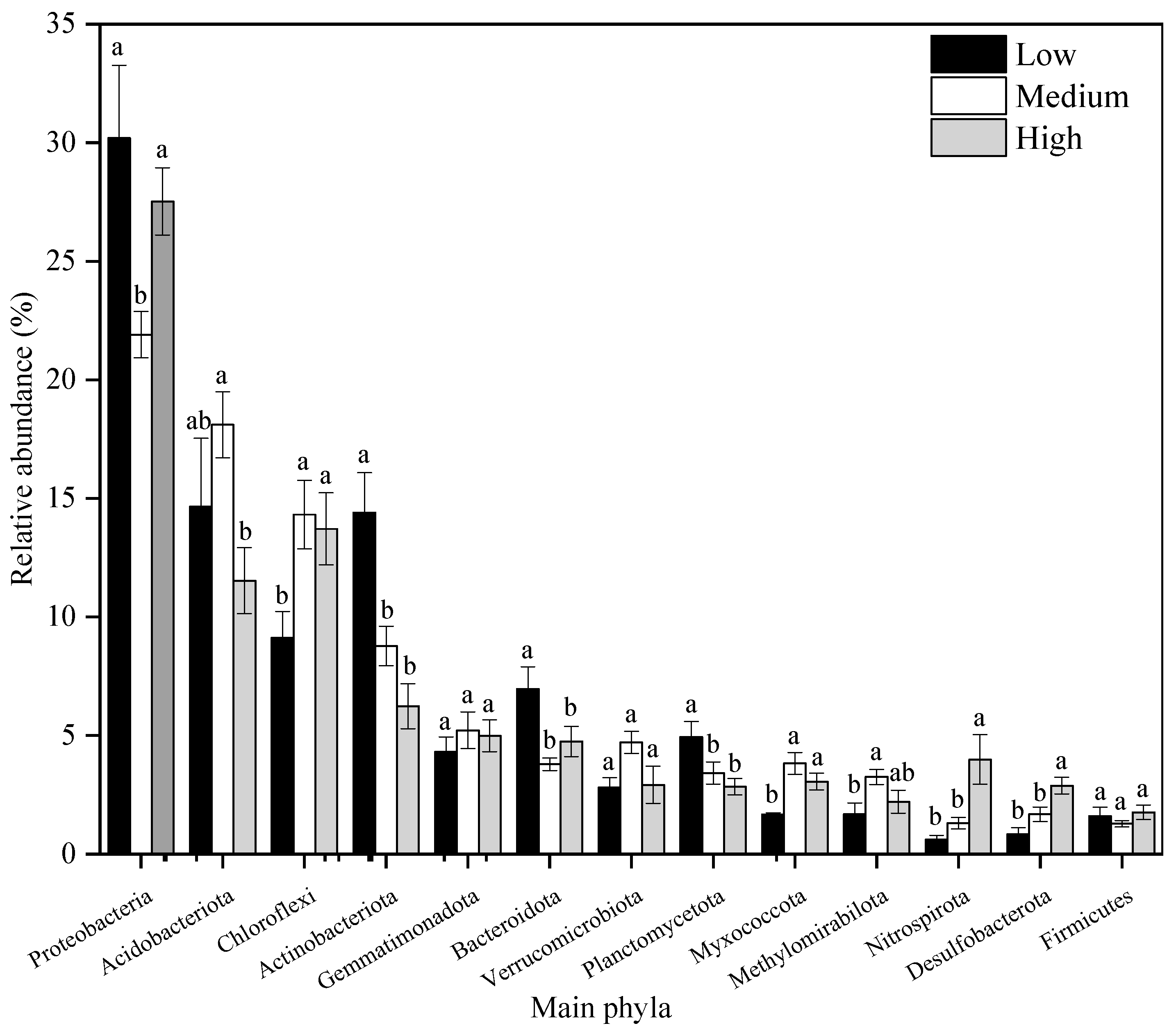
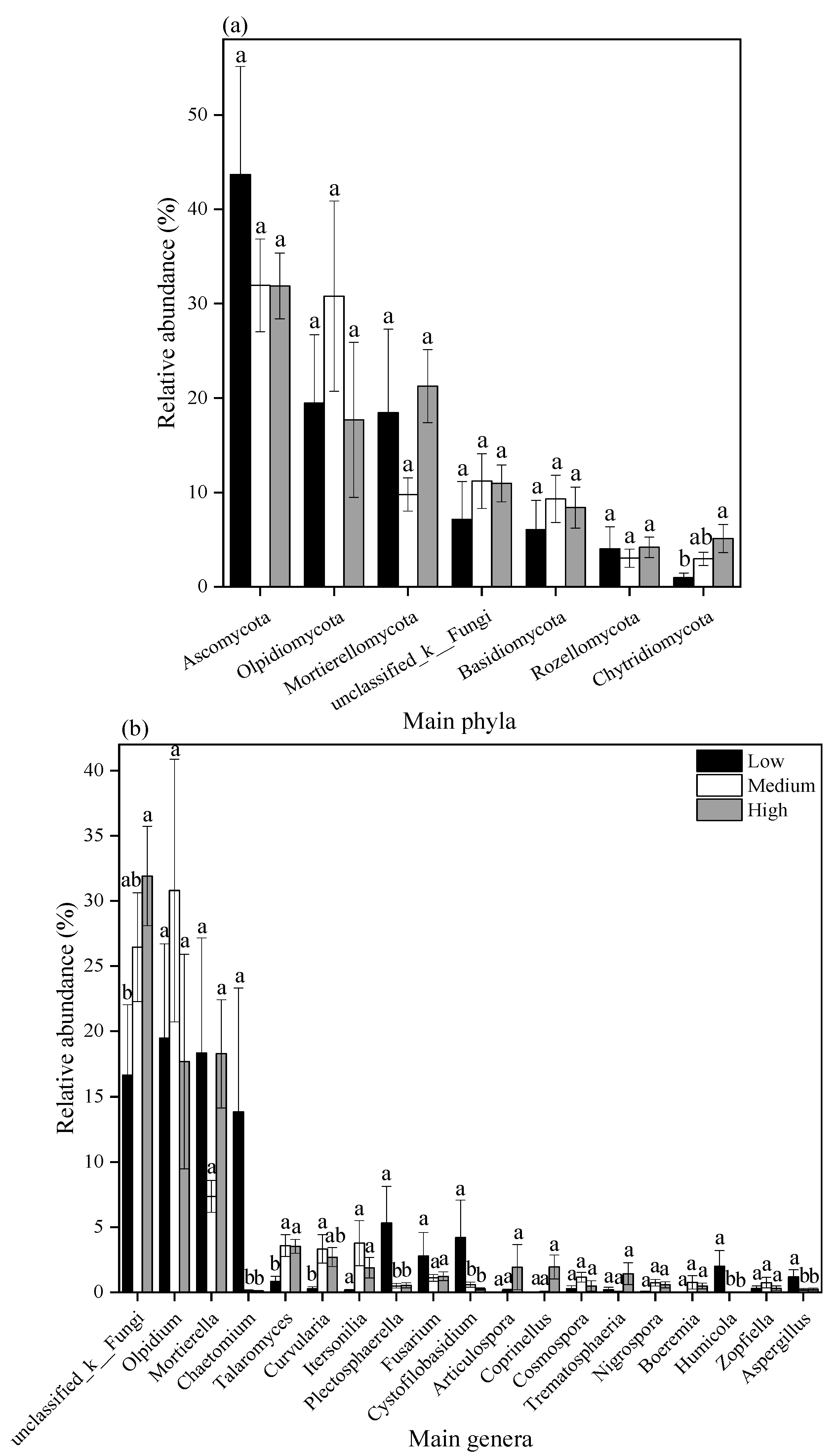
3.4.1. Relative Abundance of Bacterial and Fungal Communities Under Different Heavy Metal Pollution Levels
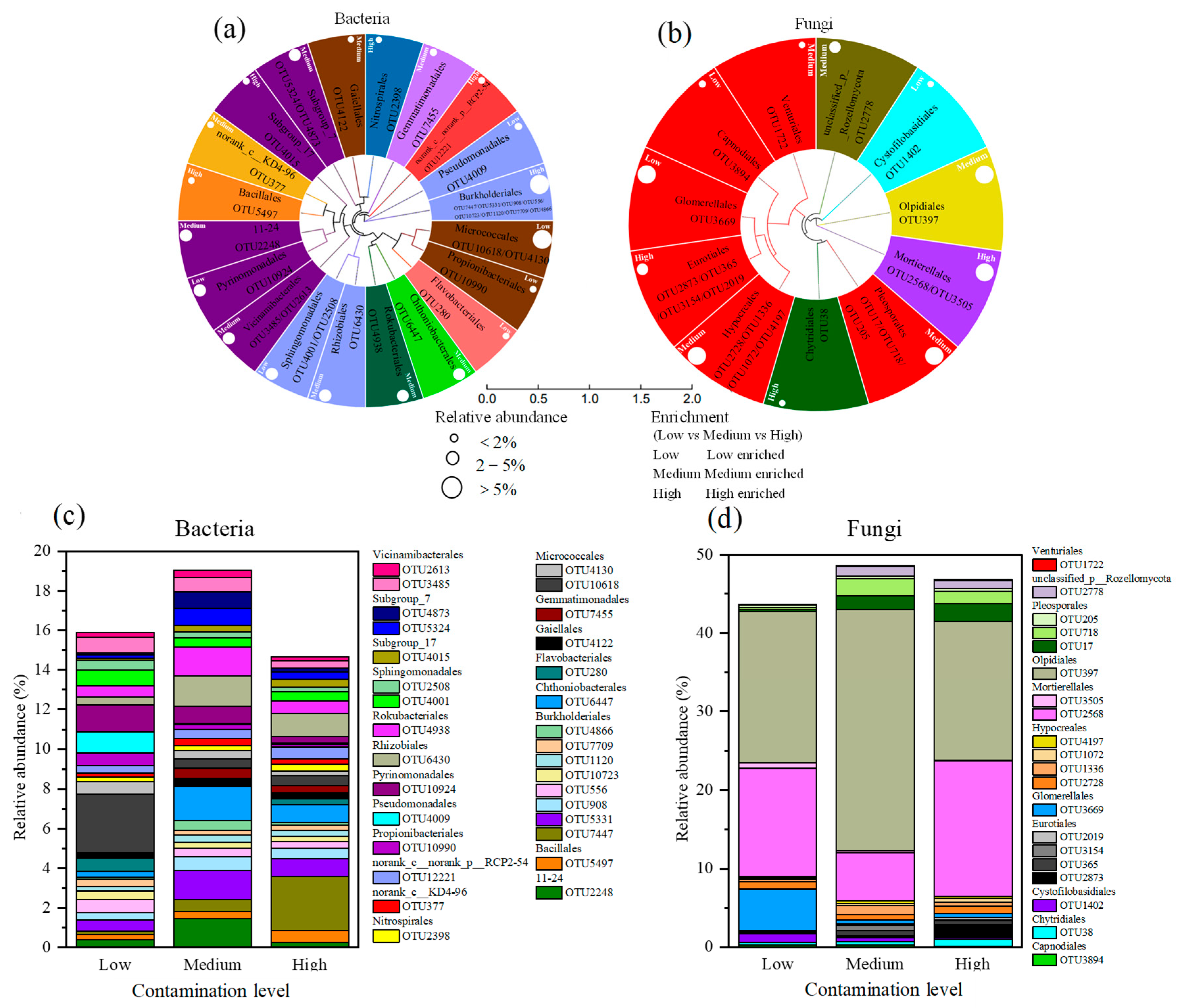
3.4.2. Relative Abundance of Major Bacterial and Fungal Communities Under Different Heavy Metal Pollution Levels
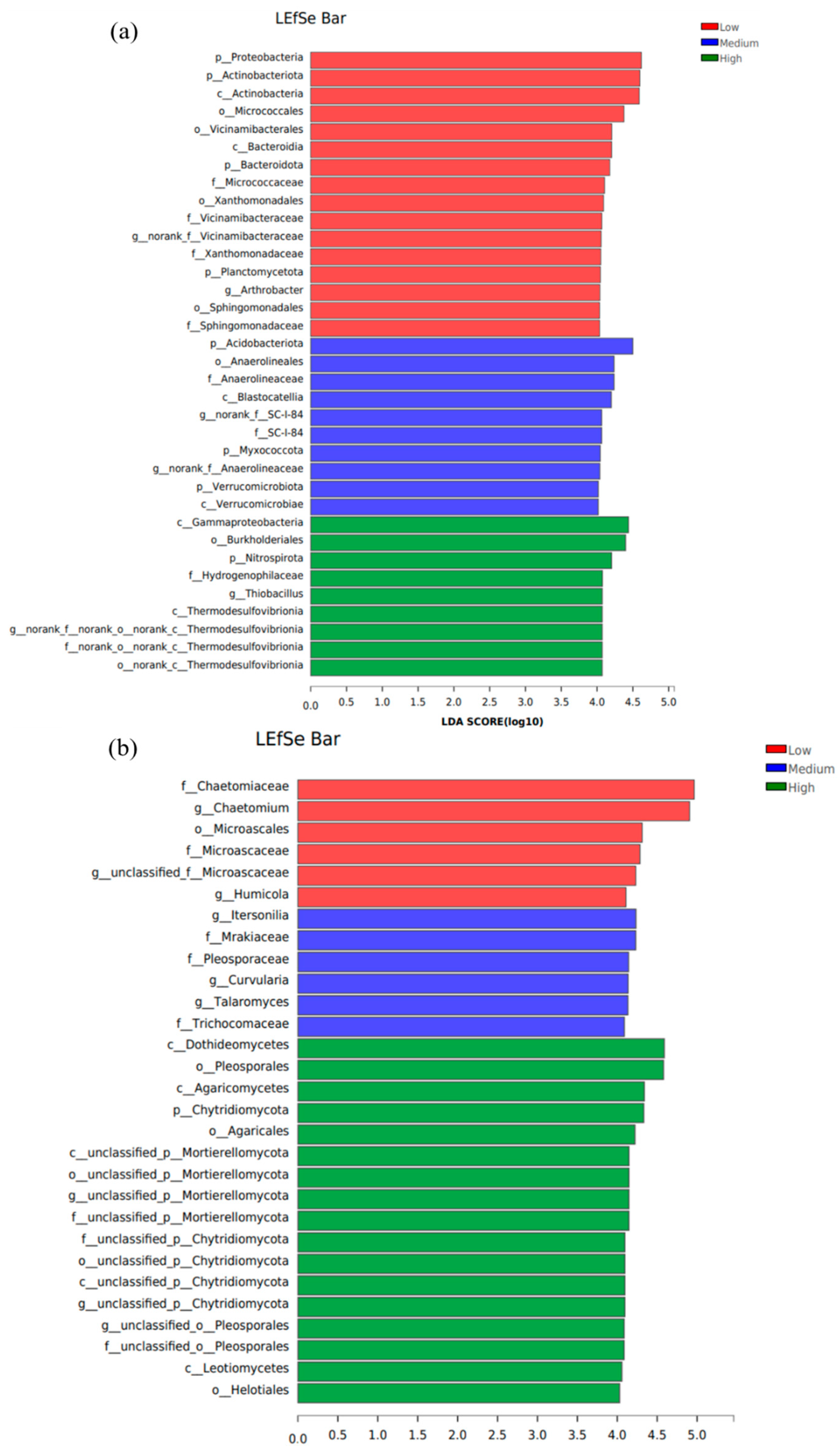
3.5. Linear Discriminant Analysis Effect Size (Lefse)
3.6. Relationship Between Soil Properties and Soil Bacterial and Fungal Community Structures
3.7. Core Operational Taxonomic Units in Bacterial and Fungal Communities from Soils with Heavy Metal Pollution
4. Discussion
4.1. Relationship Between Heavy Metal Pollution Levels and Basic Physicochemical Properties of Soil
4.2. Relationship Between Heavy Metal Pollution Levels and Bacterial Communities
4.3. Relationship Between Environmental Factors and Bacterial Communities
4.4. Effects of Environmental Factors and Heavy Metal Pollution Levels on Fungal Community Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef]
- Kemmitt, S.J.; Wright, D.; Goulding, K.W.T.; Jones, D.L. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol. Biochem. 2006, 38, 898–911. [Google Scholar] [CrossRef]
- Shi, S.H.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.S.; Yang, F.; Wang, Z.C.; Tian, C.J. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xu, Y.; Xu, Y.M.; Wang, L.; Liang, X.F.; Li, Y. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environ. Pollut. 2016, 208, 739–746. [Google Scholar] [CrossRef]
- Hou, D.Y.; O’connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.Z. Diversity and Co-occurrence Patterns of Soil Bacterial and Fungal Communities in Seven Intercropping Systems. Front. Microbiol. 2018, 9, 1521. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, A.F. Effects of Passivating Agents on the Availability of Cd and Pb and Microbial Community Function in a Contaminated Acidic Soil. Bull. Environ. Contam. Toxicol. 2019, 103, 98–105. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.M.; Wu, H.P.; Hua, S.S.; Liu, J.Y.; Yuan, Y.J.; Xiao, H.B.; Deng, L.J.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–790. [Google Scholar] [CrossRef]
- Sheik, C.S.; Mitchell, T.W.; Rizvi, F.Z.; Rehman, Y.; Faisal, M.; Hasnain, S.; Mclnerney, M.J.; Krumholz, L.R. Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS ONE 2012, 7, e40059. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jiang, K.; Zhou, J.W.; Liu, J.; Wu, B.D. Responses of soil N-fixing bacterial communities to redroot pigweed (Amaranthus retroflexus L.) invasion under Cu and Cd heavy metal soil pollution. Agric. Ecosyst. Environ. 2018, 267, 15–22. [Google Scholar] [CrossRef]
- Kenarova, A.; Radeva, G.; Traykov, I.; Boteva, S. Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotoxicol. Environ. Saf. 2014, 100, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Mi, N.N.; Hao, W.Y.; Zhou, Z.X.; Li, L.C.; Wang, F.Y.; Gai, J.P. Effects of amendments and indigenous microorganisms on the growth and Cd and Pb uptake of coriander (Coriandrum sativum L.) in heavy metal-contaminated soil. Toxics 2022, 10, 408. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Walker, T.W.; Adams, A.F.R. Studies on soil organic matter: I. Influence of phosphorus content of parent materials on accumulations of carbon, nitrogen, sulfur, and organic phosphorus in grassland soils. Soil Sci. 1958, 85, 307–318. [Google Scholar] [CrossRef]
- Chen, H.B.; Yang, X.; Wang, H.L.; Sarkar, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.L.; et al. Animal carcass and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, R.D.; Pesti, G.M.; Foster, E.H. A comparison of nitrogen values obtained utilizing the Kjeldahl nitrogen and Dumas combustion methodologies (Leco CNS 2000) on samples typical of an animal nutrition analytical laboratory. Anim. Feed. Sci. Technol. 1998, 73, 21–28. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- BAOSD. Soil and Agrochemistry Analysis; Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Bei, S.K.; Zhang, Y.L.; Li, T.T.; Christie, P.; Li, X.L.; Zhang, J.L. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric. Ecosyst. Environ. 2018, 260, 58–69. [Google Scholar] [CrossRef]
- Zheng, G.D.; Wang, X.K.; Chen, T.B.; Yang, J.; Yang, J.X.; Liu, J.W.; Shi, X.X. Passivation of lead and cadmium and increase of the nutrient content during sewage sludge composting by phosphate amendments. Environ. Environ. Res. 2020, 185, 109431. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L.Q.; Rhue, R.D.; Reve, W. Selectivities of potassium-calcium and potassium-lead exchange in two tropical soils. Soil Sci. Soc. Am. J. 2003, 67, 1707–1714. [Google Scholar] [CrossRef]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Domingo, J.W.S. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, R.; Kowalska, J.; Gasiorek, M.; Zadrozny, P.; Józefowska, A.; Zaleski, T.; Kepka, W.; Tymczuk, M.; Orlowska, K. Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere 2017, 168, 839–850. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolastico, C.; Ruiz-Fernandez, J.; Masaguer, A.; Moliner, A. Bioavailability and extraction of heavy metals from contaminated soil by Atriplex halimus. Environ. Exp. Bot. 2013, 88, 53–59. [Google Scholar] [CrossRef]
- Choudhury, S.G.; Sivastava, S.; Singh, R.; Chaudhari, S.K.; Sharma, D.K.; Singh, S.K.; Sarkar, D. Tillage and residue management effects on soil aggregation, organic carbon dynamics and yield attribute in rice-wheat cropping system under reclaimed sodic soil (vol 136, pg 76, 2014). Soil Tillage Res. 2014, 141, 62. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.X.; Qin, J.H. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef]
- Guo, H.H.; Nasir, M.; Lv, J.L.; Dai, Y.C.; Gao, J.K. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Aqil, F.; Ahmad, Q. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561. [Google Scholar] [CrossRef]
- Shen, L.; Li, Z.-H.; Zeng, W.-M.; Yu, R.-L.; Wu, X.-L.; Li, J.-K.; Wang, S.-K. Microbial Communities in Soils of Qingshuitang Industrial District in Zhuzhou. Environ. Sci. 2018, 39, 5151–5162. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Huang, J.; Lu, J.; Sun, Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Sinkko, H.; Lukkari, K.; Sihvonen, L.M.; Sivonen, K.; Leivuori, M.; Rantanen, M.; Paulin, L.; Lyra, C. Bacteria Contribute to Sediment Nutrient Release and Reflect Progressed Eutrophication-Driven Hypoxia in an Organic-Rich Continental Sea. Public Libr. Sci. One 2013, 8, e67061. [Google Scholar] [CrossRef]
- Karelova, E.; Harichova, J.; Stojnev, T.; Pangallo, D.; Ferianc, P. The isolation of heavy-metal resistant culturable bacteria and resistance determinants from a heavy-metal-contaminated site. Biologia 2011, 66, 18–26. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, C.; Li, D. Types and species composition of biological soil crust in metal tailings ponds. Acta Hydrobiol. Sin. 2020, 44, 622–630. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.L.; Xing, Y.; Deng, S.Q.; Guo, L.M.; Liang, Y.T.; Zhang, D.Y. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef]
- Gao, T.P.; Wan, Z.D.; Liu, X.X.; Fu, J.W.; Chang, G.H.; Sun, H.L.; Li, H.J.; Shen, Y.Y.; Liu, Y.B.; Fang, X.W. Effects of heavy metals on bacterial community structure in the rhizosphere of Salsola collina and bulk soil in the Jinchuan mining area. Geomicrobiol. J. 2021, 38, 620–630. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.C.; He, Z.; Chen, X.M.; E, G.J.; Han, Y.Y.; Wang, Y.X. Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res. 2016, 101, 393–401. [Google Scholar] [CrossRef]
- Mehrani, M.J.; Sobotka, D.; Kowal, P.; Ciesielski, S.; Makinia, J. The occurrence and role of Nitrospira in nitrogen removal systems. Bioresour. Technol. 2020, 303, 122936. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Breznak, J.A.; Schmidt, T.M. Isolation and characterization of soil bacteria that define Teniglobus gen. nov., in the phylum Acidobacteria. Appl. Environ. Microbiol. 2007, 73, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Morse, J.L.; Berthrong, S.T.; Bernhardt, E.S.; Jackson, R.B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 2007, 88, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; Abu Al-Soud, W.; Sorensen, S.J.; He, Z.L.; White, D.; Sinclair, A.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef]
- Fernandez, N.; Sierra-Alvarez, R.; Field, J.A.; Amils, R.; Sanz, J.L. Microbial community dynamics in a chemolithotrophic denitrification reactor inoculated with methanogenic granular sludge. Chemosphere 2008, 70, 462–474. [Google Scholar] [CrossRef]
- Lin, Y.B.; Ye, Y.M.; Hu, Y.M.; Shi, H.K. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef]
- Xu, Y.L.; Seshadri, B.; Sarkar, B.; Wang, H.L.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.D.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef]
- Jiang, B.H.; Zhang, B.; Li, L.; Zhao, Y.; Shi, Y.; Jiang, Q.; Jia, L.P. Analysis of microbial community structure and diversity in surrounding rock soil of different waste dump sites in fushun western opencast mine. Chemosphere 2021, 269, 128777. [Google Scholar] [CrossRef]
- Kuang, J.L.; Huang, L.N.; Chen, L.X.; Hua, Z.S.; Li, S.J.; Hu, M.; Li, J.T.; Shu, W.S. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013, 7, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Sui, Y.Y.; Yu, Z.H.; Shi, Y.; Chu, H.Y.; Jin, J.; Liu, X.B.; Wang, G.H. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Chodak, M.; Golebiewski, M.; Morawska-Ploskonka, J.; Kuduk, K.; Niklinska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef]
- Luo, J.P.; Liu, Y.Y.; Tao, Q.; Hou, Q.; Wu, K.R.; Song, Y.C.; Liu, Y.K.; Guo, X.Y.; Li, J.X.; Hashmi, M.L.U.R.; et al. Successive phytoextraction alters ammonia oxidation and associated microbial communities in heavy metal contaminated agricultural soils. Sci. Total Environ. 2019, 664, 616–625. [Google Scholar] [CrossRef]
- Li, X.Q.; Meng, D.L.; Li, J.; Yin, H.Q.; Liu, H.W.; Liu, X.D.; Cheng, C.; Xiao, Y.H.; Liu, Z.H.; Yan, M.L. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, E.; Ahari, A.B.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Mohammad, A.; Mittra, B. Effects of inoculation with stress-adapted arbuscular mycorrhizal fungus Glomus deserticola on growth of Solanum melogena L. and Sorghum sudanese Staph. seedlings under salinity and heavy metal stress conditions. Arch. Agron. Soil Sci. 2013, 59, 173–183. [Google Scholar] [CrossRef]
- Ma, A.Z.; Zhuang, X.L.; Wu, J.M.; Cui, M.M.; Lv, D.; Liu, C.Z.; Zhuang, G.Q. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. Public Libr. Sci. One 2013, 8, e66146. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, C.B.; Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L. Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ. Microbiol. 2007, 9, 1306–1316. [Google Scholar] [CrossRef]
- Rajapaksha, R. Heavy metal tolerance of culturable bacteria and fungi in a long-term cultivated tropical ultisol. Eur. J. Soil Biol. 2011, 47, 9–15. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wu, X.H.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Iskandar, N.L.; Zainudin, N.; Tan, S.G. Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J. Environ. Sci. 2011, 23, 824–830. [Google Scholar] [CrossRef] [PubMed]





| Pollutant Categories | 5.5 < pH ≤ 6.5 | 6.5 < pH ≤ 7.5 | |
|---|---|---|---|
| Risk screening concentration(mg/kg) | Cd | 0.3 | 0.3 |
| Pb | 90 | 120 | |
| Zn | 200 | 250 | |
| Risk control concentration(mg/kg) | Cd | 2.0 | 3.0 |
| Pb | 500 | 700 | |
| Zn | / | / |
| Contamination Level | * Low | Medium | High |
|---|---|---|---|
| pH | 6.42 ± 0.06 a | 6.59 ± 0.19 a | 6.29 ± 0.18 a |
| EC (μs/cm) | 591 ± 270 a | 128 ± 12 b | 204 ± 24 b |
| TP (g/kg) | 0.96 ± 0.17 a | 0.39 ± 0.01 b | 0.38 ± 0.02 b |
| AP (mg/kg) | 55.8 ± 13.3 a | 18.2 ± 1.4 b | 15.8 ± 1.2 b |
| AK (mg/kg) | 322 ± 34 a | 89.2 ± 10.0 b | 125 ± 24 b |
| TN (g/kg) | 2.99 ± 0.19 a | 2.08 ± 0.06 c | 2.44 ± 0.09 b |
| SOC (g/kg) | 69.4 ± 9.3 a | 54.4 ± 2.0 b | 59.7 ± 2.3 ab |
| TCd (mg/kg) | 0.02 ± 0.01 c | 3.12 ± 0.22 b | 8.58 ± 1.29 a |
| TZn (mg/kg) | 1014 ± 457 a | 457 ± 27 b | 628 ± 185 ab |
| TPb (mg/kg) | 21.7 ± 12.0 b | 122 ± 7 a | 98.6 ± 18.0 a |
| ACd (mg/kg) | 0.004 ± 0.001 c | 1.48 ± 0.34 b | 3.40 ± 0.59 a |
| AZn (mg/kg) | 4.91 ± 1.61 b | 8.79 ± 0.55 b | 15.9 ± 2.0 a |
| APb(mg/kg) | 8.32 ± 2.01 b | 23.5 ± 2.8 a | 25.0 ± 2.2 a |
| Df | F | R2 | p | ||
|---|---|---|---|---|---|
| Contamination level | Low–High | 1 | 3.93 | 0.247 | 0.003 |
| Low–Medium | 1 | 5.10 | 0.317 | 0.001 | |
| Medium–High | 1 | 2.78 | 0.156 | 0.003 | |
| Df | F | R2 | p | ||
|---|---|---|---|---|---|
| Contamination level | Low–High | 1 | 1.92 | 0.138 | 0.022 |
| Low–Medium | 1 | 2.38 | 0.178 | 0.006 | |
| Medium–High | 1 | 1.75 | 0.104 | 0.063 | |
| CCA1 | CCA2 | r2 | p | |
|---|---|---|---|---|
| pH | −0.2222 | −0.9750 | 0.203 | 0.125 |
| EC | −0.9996 | 0.0293 | 0.163 | 0.156 |
| AP | −0.9981 | −0.0618 | 0.701 | 0.001 |
| TN | −0.9999 | −0.0157 | 0.310 | 0.035 |
| SOM | −0.9508 | 0.3097 | 0.586 | 0.015 |
| TCd | 0.9079 | 0.4192 | 0.191 | 0.115 |
| TZn | −0.6510 | −0.7591 | 0.162 | 0.171 |
| TPb | 0.6865 | −0.7271 | 0.281 | 0.040 |
| ACd | 0.6980 | 0.7161 | 0.557 | 0.001 |
| APb | 0.9827 | 0.1852 | 0.453 | 0.001 |
| CCA1 | CCA2 | r2 | p | |
|---|---|---|---|---|
| pH | 0.0738 | 0.9973 | 0.357 | 0.021 |
| EC | 0.9995 | −0.0319 | 0.356 | 0.090 |
| AP | 0.9903 | 0.1392 | 0.698 | 0.002 |
| TN | 0.9967 | 0.0810 | 0.328 | 0.024 |
| SOM | 0.9960 | 0.0889 | 0.695 | 0.001 |
| TCd | −0.9967 | −0.0815 | 0.098 | 0.350 |
| TZn | 0.0566 | 0.9984 | 0.056 | 0.398 |
| TPb | −0.5977 | 0.8017 | 0.487 | 0.002 |
| ACd | −0.6357 | −0.7719 | 0.267 | 0.048 |
| APb | −0.9591 | −0.2831 | 0.308 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Q.; Wu, Y.; Cai, H.; Xu, Z.; Zhao, Y.; Guan, R.; Fan, X.; Guo, J. Effects of Long-Term Heavy Metal Pollution on Microbial Community Structure in Soil. Toxics 2025, 13, 806. https://doi.org/10.3390/toxics13090806
Mi Q, Wu Y, Cai H, Xu Z, Zhao Y, Guan R, Fan X, Guo J. Effects of Long-Term Heavy Metal Pollution on Microbial Community Structure in Soil. Toxics. 2025; 13(9):806. https://doi.org/10.3390/toxics13090806
Chicago/Turabian StyleMi, Qiannuo, Yan Wu, Huaisen Cai, Zuben Xu, Yue Zhao, Ronghao Guan, Xin Fan, and Jianhua Guo. 2025. "Effects of Long-Term Heavy Metal Pollution on Microbial Community Structure in Soil" Toxics 13, no. 9: 806. https://doi.org/10.3390/toxics13090806
APA StyleMi, Q., Wu, Y., Cai, H., Xu, Z., Zhao, Y., Guan, R., Fan, X., & Guo, J. (2025). Effects of Long-Term Heavy Metal Pollution on Microbial Community Structure in Soil. Toxics, 13(9), 806. https://doi.org/10.3390/toxics13090806







