Toxicological Impacts of Polypropylene Nanoparticles Similar in Size to Nanoplastics in Plastic-Bottle Injections on Human Umbilical Vein Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Preparation of PP-NPs
2.3. Cell Viability
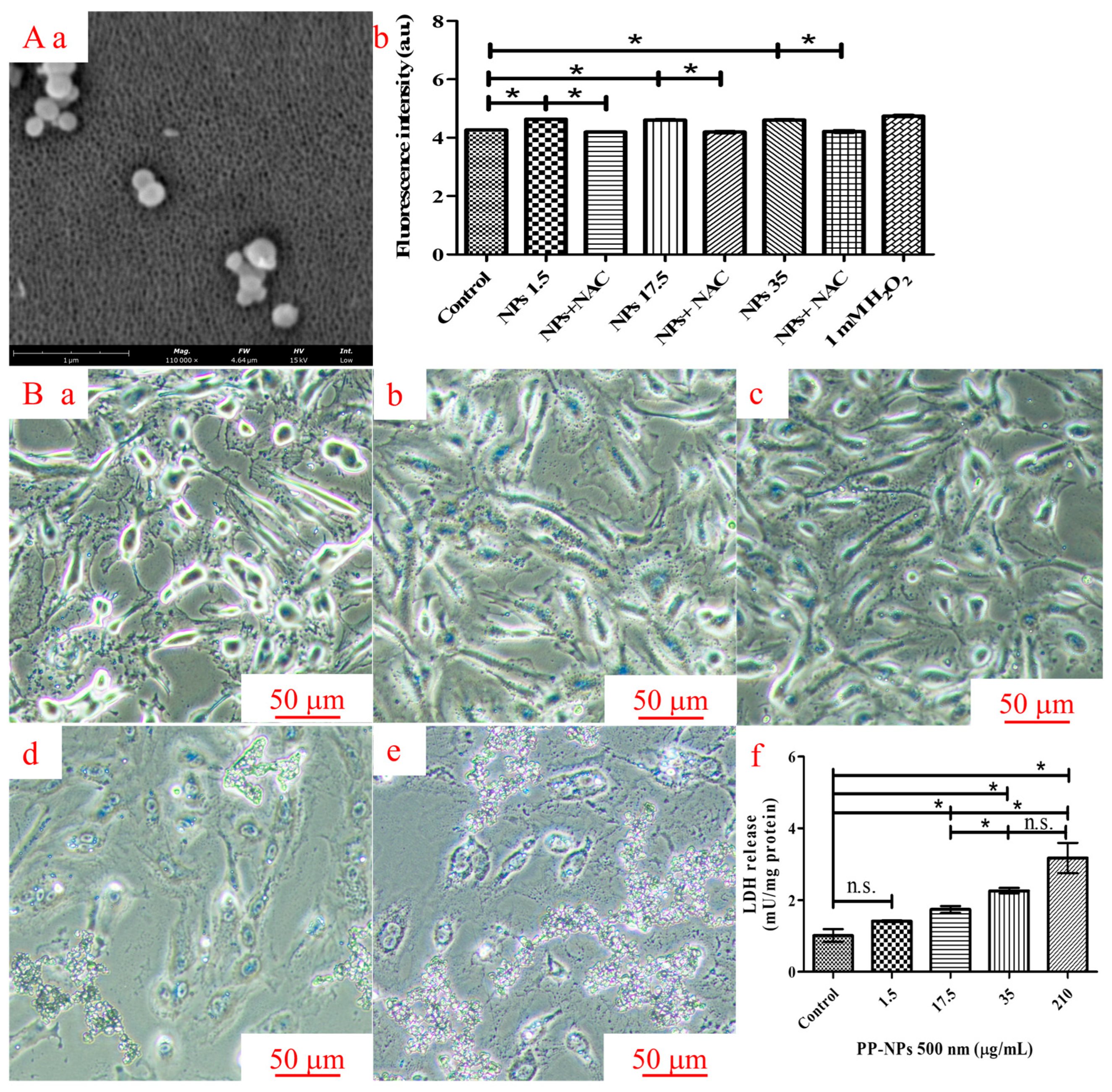
2.4. Detection of Reactive Oxygen Species (ROS)
2.5. Lactate Dehydrogenase (LDH) Activity Assay
2.6. Cytokine Test
2.7. Wound Healing Assay
2.8. Transwell Migration
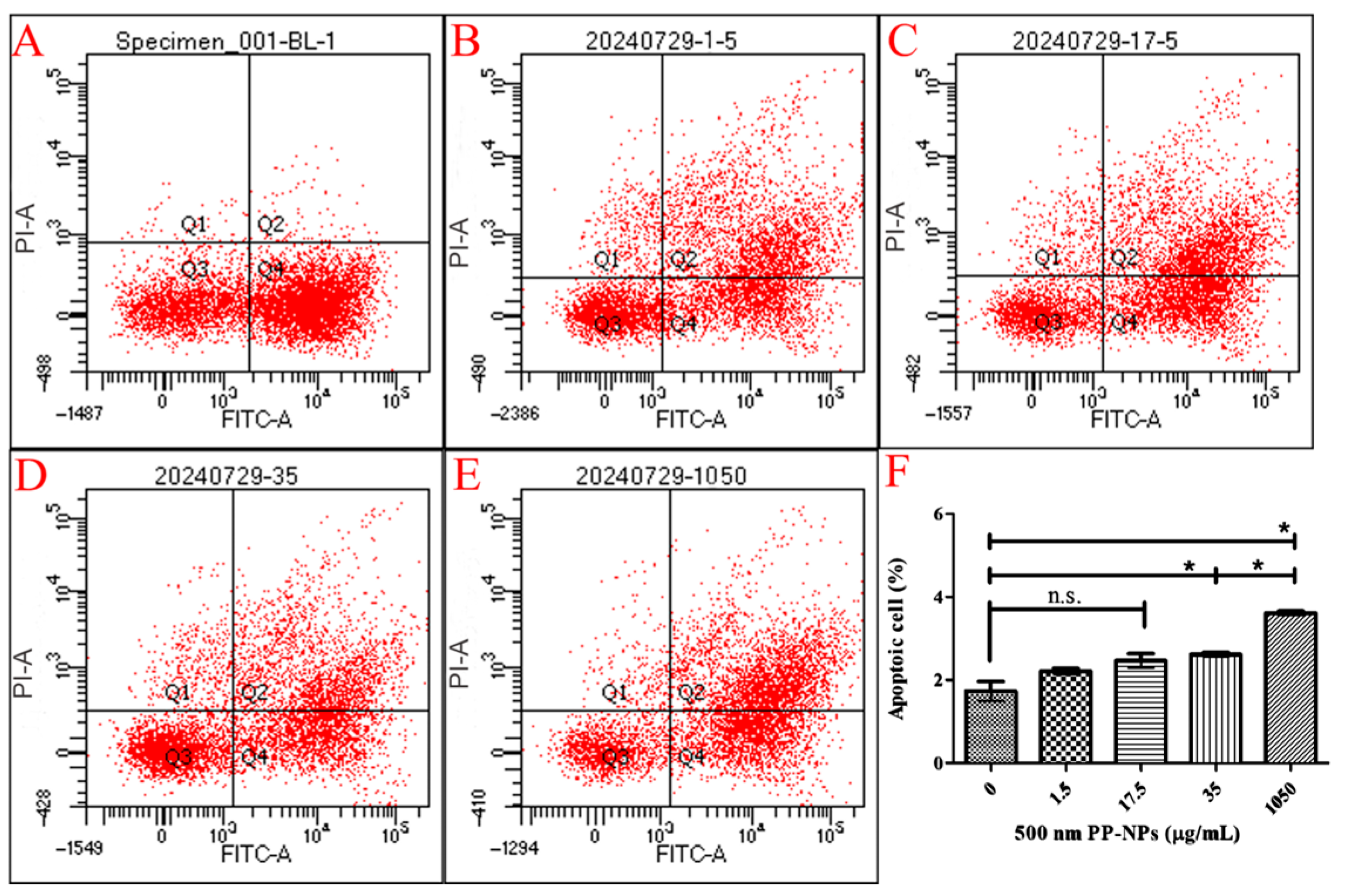
2.9. Annexin V-FITC and PI Staining
2.10. RNA Extraction and Real-Time PCR
2.11. Microtubule-Associated Protein 1 Light Chain 3 (LC3) Test (Immunofluorescence Staining)
2.12. Statistical Analysis
3. Results
3.1. PP-NPs Reduce the Viability of HUVECs in a Dose-Dependent Manner
3.2. Necrosis, Not Autophagy, Mediated PP-NP-Induced Decrease in Viability
3.3. PP-NPs Induced Oxidative Stress and Cell Membrane Damage in HUVECs
3.4. PP-NPs Inhibit the Migration of HUVECs
3.5. PP-NP-Induced Inflammation
3.6. PP-NPs Induce Changes in Cytokines in HUVECs
4. Discussion
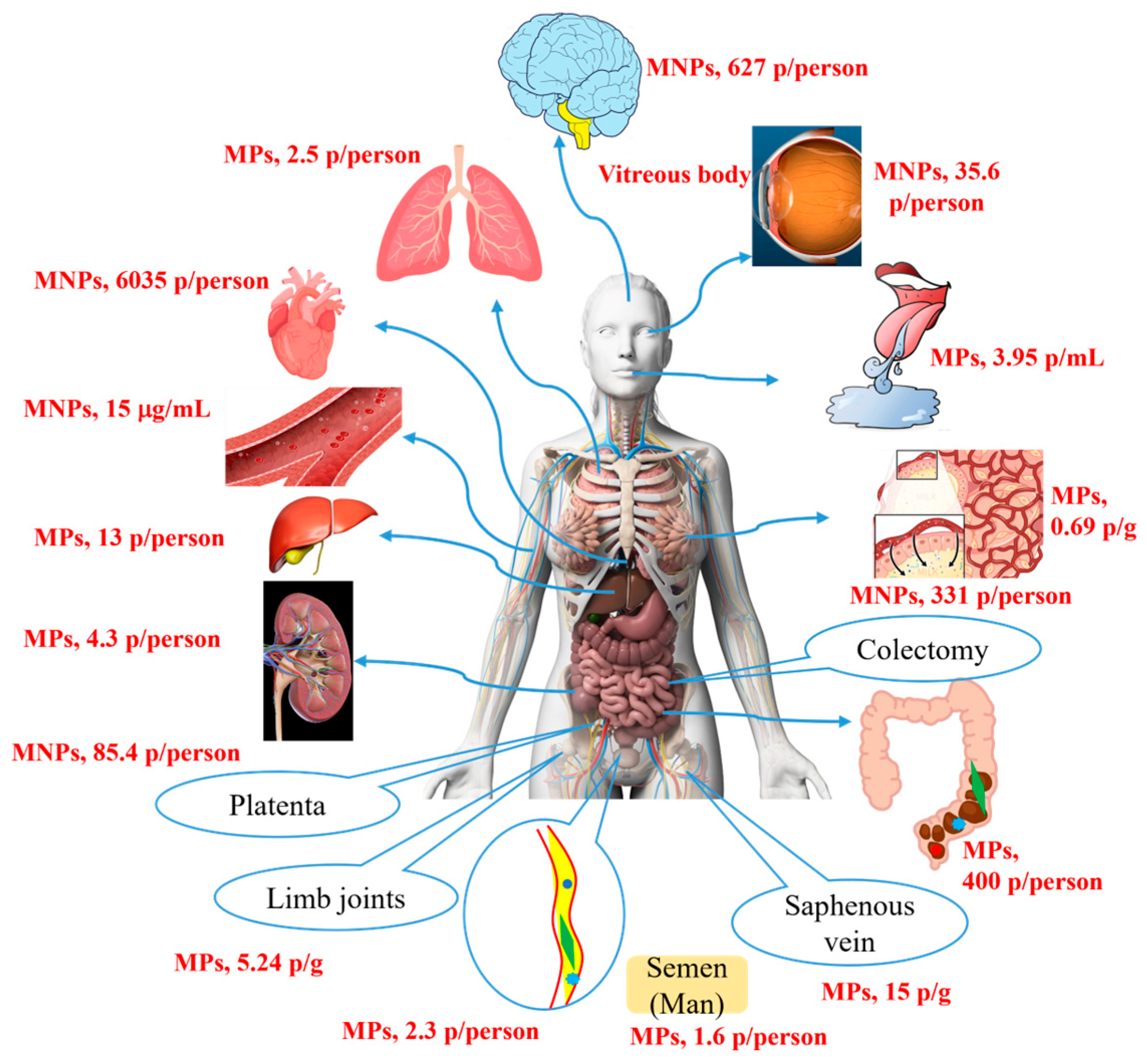
The Possible Metabolism of MNP Particles In Vivo
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Huang, D.; Chen, H.; Shen, M.; Tao, J.; Chen, S.; Yin, L.; Zhou, W.; Wang, X.; Xiao, R.; Li, R. Recent advances on the transport of microplastics/nanoplastics in abiotic and biotic compartments. J. Hazard. Mater. 2022, 438, 129515. [Google Scholar] [CrossRef] [PubMed]
- Abad López, A.P.; Trilleras, J.; Arana, V.A.; Garcia-Alzate, L.S.; Grande-Tovar, C.D. Atmospheric microplastics: Exposure, toxicity, and detrimental health effects. RSC Adv. 2023, 13, 7468–7489. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.G.; Wu, X.F.; Zhao, Z.G.; Yang, H.Y.; Sun, H.M. Identification and quantification of micronano-plastics in polypropylene-bottled injections. Heliyon 2024, 10, e35101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, L.G.; Wu, X.F.; Zhao, Z.G.; Lu, Y.; Sun, H.M. Impact of micronano plastics in daily life on human health: Toxicological evaluation from the perspective of normal tissue cells and organoids. Toxicol. Res. 2024, 13, tfae205. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Lu, D.; Luo, Q.; Chen, R.; Zhuansun, Y.; Jiang, J.; Wang, W.; Yang, X.; Zhang, L.; Liu, X.; Li, F.; et al. Chemical multifingerprinting of exogenous ultrafine particles in human serum and pleural effusion. Nat. Commun. 2020, 11, 2567. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, W.T.; Jin, K.Q.; Yan, J.; Qi, Y.T.; Huang, W.H.; Liu, Y.L. Real-time quantification of nanoplastics-induced oxidative stress in stretching alveolar cells. ACS Nano 2024, 18, 6176–6185. [Google Scholar] [CrossRef]
- Abafe, O.A.; Harrad, S.; Abdallah, M.A. Novel insights into the dermal bioaccessibility and human exposure to brominated flame retardant additives in microplastics. Environ. Sci. Technol. 2023, 57, 10554–10562. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. eBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Jenner, L.C.; Chapman, E.; Bennett, R.T.; Bolanle, I.O.; Loubani, M.; Sadofsky, L.; Hobkirk, J.; Palmer, T.M. Detection of microplastics in human saphenous vein tissue using μFTIR: A pilot study. PLoS ONE 2023, 18, e0280594. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Maimaiti, Z.; Fu, J.; Yang, F.; Li, Z.Y.; Shi, Y.; Hao, L.B.; Chen, J.Y.; Xu, C. Identification and analysis of microplastics in human lower limb joints. J. Hazard. Mater. 2024, 461, 132640. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of various microplastics in patients undergoing cardiac surgery. Environ. Sci. Technol. 2023, 57, 10911–10918. [Google Scholar] [CrossRef]
- Qin, X.; Cao, M.; Peng, T.; Shan, H.; Lian, W.; Yu, Y.; Shui, G.; Li, R. Features, potential invasion pathways, and reproductive health risks of microplastics detected in human uterus. Environ. Sci. Technol. 2024, 58, 10482–10493. [Google Scholar] [CrossRef]
- Jochum, M.; Garcia, M.; Hammerquist, A.; Howell, J.; Stanford, M.; Liu, R.; Olewine, M.; Hayek, E.E.; Phan, E.; Showalter, L.; et al. Elevated micro- and nanoplastics detected in preterm human placentae. Res. Sq. 2025, rs.3.rs-5903715. [Google Scholar] [CrossRef]
- Massardo, S.; Verzola, D.; Alberti, S.; Caboni, C.; Santostefano, M.; Eugenio Verrina, E.; Angeletti, A.; Lugani, F.; Ghiggeri, G.M.; Bruschi, M.; et al. MicroRaman spectroscopy detects the presence of microplastics in human urine and kidney tissue. Environ. Int. 2024, 184, 108444. [Google Scholar] [CrossRef] [PubMed]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and nanoplastics breach the blood–brain barrier (BBB): Biomolecular corona’s role revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, Y.; Zhang, L.; Ma, D.; Wen, K.; Cai, J.; Cai, Z.; Wang, C.; Chai, X.; Zhong, J.; et al. Revealing new insights: Two-center evidence of microplastics in human vitreous humor and their implications for ocular health. Sci. Total Environ. 2024, 921, 171109. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics 2022, 11, 40. [Google Scholar] [CrossRef]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman microspectroscopy evidence of microplastics in human semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef]
- Delaney, S.; Rodriguez, C.; Sarrett, S.M.; Dayts, E.J.; Zeglis, B.M.; Keinänen, O. Unraveling the in vivo fate of inhaled micro- and nanoplastics with PET imaging. Sci. Total Environ. 2023, 904, 166320. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Ji, Y.; Mei, R.; Chen, Y.; Zhang, Z.; Wang, X.; Chen, L. Polystyrene nanoplastics demonstrate high structural stability in vivo: A comparative study with silica nanoparticles via SERS tag labeling. Chemosphere 2022, 300, 134567. [Google Scholar] [CrossRef]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene micro and nano-particles induce metabolic rewiring in normal human colon cells: A risk factor for human health. Chemosphere 2022, 303 Pt 1, 134947. [Google Scholar] [CrossRef]
- Oddone, E.; Modonesi, C.; Gatta, G. Occupational exposures and colorectal cancers: A quantitative overview of epidemiological evidence. World J. Gastroenterol. 2014, 20, 12431–12444. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.H.; Cai, Z.; Zheng, C. Metabolomics reveal nanoplastic-induced mitochondrial damage in human liver and lung cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hua, T.; Sang, Q.A. Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef] [PubMed]
- Najahi, H.; Alessio, N.; Squillaro, T.; Conti, G.O.; Ferrante, M.; Di Bernardo, G.; Galderisi, U.; Messaoudi, I.; Minucci, S.; Banni, M. Environmental microplastics (EMPs) exposure alter the differentiation potential of mesenchymal stromal cells. Environ. Res. 2022, 214 Pt 4, 114088. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Kiran, S.; Li, Y.; Sang, Q.A. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids. J. Hazard. Mater. 2022, 435, 128884. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Meng, R.; Chen, G.; Lai, T.; Qing, R.; Hao, S.; Deng, J.; Wang, B. Distinct accumulation of nanoplastics in human intestinal organoids. Sci. Total Environ. 2022, 838 Pt 2, 155811. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806 Pt 1, 150328. [Google Scholar] [CrossRef]
- Amereh, F.; Amjadi, N.; Mohseni-Bandpei, A.; Isazadeh, S.; Mehrabi, Y.; Eslami, A.; Naeiji, Z.; Rafiee, M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ. Pollut. 2022, 314, 120174. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Limosani, R.V.; Oliviero, C.; Saeed, S.; Iulini, M.; Passoni, F.C.; Racchi, M.; Corsini, E. Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu. J. Xenobiot. 2025, 15, 13. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- Nikitakos, V.; Porfyris, A.D.; Beltsios, K.; Pfaendner, R.; Yecora, B.; Perez, A.; Brkić, F.; Miketa, F.; Papaspyrides, C.D. Closed-Loop Recycling of Poly(vinyl butyral) Interlayer Film via Restabilization Technology. Polymers 2025, 17, 317. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Tyc, H.J.; Kłodnicka, K.; Teresińska, B.; Karpiński, R.; Flieger, J.; Baj, J. Micro- and Nanoplastics as Disruptors of the Endocrine System-A Review of the Threats and Consequences Associated with Plastic Exposure. Int. J. Mol. Sci. 2025, 26, 6156. [Google Scholar] [CrossRef]
- Dasmahapatra, A.K.; Chatterjee, J.; Tchounwou, P.B. A systematic review of the effects of nanoplastics on fish. Front. Toxicol. 2025, 7, 1530209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Cui, F.; Kolehmainen, M.; Chen, J.; Zhang, L.; Zarei, I. Exploring the potential protective role of anthocyanins in mitigating micro/nanoplastic-induced reproductive toxicity: A steroid receptor perspective. J. Pharm. Anal. 2025, 15, 101148. [Google Scholar] [CrossRef] [PubMed]
- Poinsignon, L.; Lefrère, B.; Ben Azzouz, A.; Chissey, A.; Colombel, J.; Djelidi, R.; Ferecatu, I.; Fournier, T.; Beaudeux, J.L.; Lespes, G.; et al. Exposure of the human placental primary cells to nanoplastics induces cytotoxic effects, an inflammatory response and endocrine disruption. J. Hazard. Mater. 2025, 490, 137713. [Google Scholar] [CrossRef] [PubMed]
- van Boxel, J.; Khargi, R.R.J.; Nijmeijer, S.M.; Heinzelmann, M.T.; Pereira, D.D.C.; Lamoree, M.H.; van Duursen, M.B.M. Effects of polystyrene micro- and nanoplastics on androgen- and estrogen receptor activity and steroidogenesis in vitro. Toxicol. Vitr. 2024, 101, 105938. [Google Scholar] [CrossRef]
- Qu, J.; Wu, L.; Mou, L.; Liu, C. Polystyrene microplastics trigger testosterone decline via GPX1. Sci. Total Environ. 2024, 947, 174536. [Google Scholar] [CrossRef]
- Adhikari, M.; Biswas, C.; Mazumdar, P.; Sarkar, S.; Pramanick, K. Evaluating the potential of daily intake of polystyrene microplastics via drinking water in inducing PCOS and its ovarian fibrosis progression using female zebrafish. NanoImpact 2024, 34, 100507. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Gelb, H.; Schumacher, H.R.; Cuckler, J.; Ducheyne, P.; Baker, D.G. In vivo inflammatory response to polymethylmethacrylate particulate debris: Effect of size, morphology, and surface area. J. Orthop. Res. 1994, 12, 83–92. [Google Scholar] [CrossRef]
- Li, Z.; Van Dyk, A.K.; Fitzwater, S.J.; Fichthorn, K.A.; Milner, S.T. Atomistic Molecular Dynamics Simulations of Charged Latex Particle Surfaces in Aqueous Solution. Langmuir 2016, 32, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Seo, H.J.; Lee, J.Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part. Fibre Toxicol. 2023, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Amarakoon, D.; Wei, C.I.; Choi, K.Y.; Smolensky, D.; Lee, S.H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, X.; Wu, D.; Song, E.; Song, Y. Compromised autophagic effect of polystyrene nanoplastics mediated by protein corona was recovered after lysosomal degradation of corona. Environ. Sci. Technol. 2020, 54, 11485–11493. [Google Scholar] [CrossRef]
- Parsai, T.; Figueiredo, N.; Dalvi, V.; Martins, M.; Malik, A.; Kumar, A. Implication of microplastic toxicity on functioning of microalgae in aquatic system. Environ. Pollut. 2022, 308, 119626. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Chen, X.; Du, J.; Zhan, W.; Shao, B.; Jiang, H.; Chen, Z.; Wang, C. Polyene phosphatidylcholine promotes tibial fracture healing in rats by stimulating angiogenesis dominated by the VEGFA/VEGFR2 signaling pathway. Biochem. Biophys. Res. Commun. 2024, 719, 150100. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; Thomas, J.; Mukherjee, A.; Jenkins, D.F.; Chandrasekaran, N. Prospects on the nano-plastic particles internalization and induction of cellular response in human keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Sui, C.; Yang, C.; Li, D.; Liu, H.; Zhang, G.; Li, G.; Li, S.; Zhang, J.; et al. Exposure to polypropylene microplastics causes cardiomyocyte apoptosis through oxidative stress and activation of the MAPK-Nrf2 signaling pathway. Environ. Toxicol. 2024, 39, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zou, L.; Bao, M.; Feng, Q.; Xia, W.; Zhu, C. Toxicity of polystyrene nanoparticles for mouse ovary and cultured human granulosa cells. Ecotoxicol. Environ. Saf. 2023, 249, 114371. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, Z.; Lu, Z.; Yan, L.; Dong, X.; Dai, Z.; Sun, R.; Hong, P.; Zhou, C.; Li, C. Differences in toxicity induced by the various polymer types of nanoplastics on HepG2 cells. Sci. Total Environ. 2024, 918, 170664. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qu, L.; Dai, S.; Li, Y.; Wang, H.; Feng, Y.; Chen, X.; Jiang, L.; Guo, M.; Li, J.; et al. Structural insight into the molecular mechanism of p53-mediated mitochondrial apoptosis. Nat. Commun. 2021, 12, 2280. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions as regulators of tissue remodelling. Curr. Opin. Cell Biol. 2016, 42, 94–101. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Chattopadhyay, R.; Dyukova, E.; Singh, N.K.; Ohba, M.; Mobley, J.A.; Rao, G.N. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C ε-mediated zona occludens-1 phosphorylation at threonine 770/772. J. Biol. Chem. 2014, 289, 3148–3163. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Lin, Y.; Yin, F.; Pan, C.; Hou, J.; Wu, T.; Xia, W.; Zuo, R.; Cao, B.; Jiang, C.; et al. Effects of astragaloside IV on glucocorticoid-induced avascular necrosis of the femoral head via regulating Akt-related pathways. Cell Prolif. 2023, 56, e13485. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, D.; Yang, Y.; Jiang, Y.; Wang, J.; Li, J.; Zhang, Y.; Xie, R.; Hong, X. 17β estradiol activates autophagy and attenuates homocysteine mediated inflammation in endothelial cells through PI3K AKT MTOR signaling. Sci. Rep. 2025, 15, 24270. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tan, X.; Cheng, G. SPTBN1 overexpression ameliorates atherosclerosis by inhibiting oxidative stress and inflammation via regulating the TRIM37/TRAF2/NF-κB pathway. Eur. J. Med. Res. 2025, 30, 781. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef]
- Boratkó, A.; Gergely, P.; Csortos, C. RACK1 is involved in endothelial barrier regulation via its two novel interacting partners. Cell Commun. Signal. 2013, 11, 2. [Google Scholar] [CrossRef]
- Xu, H.; He, T.Q.; Chen, S.Y.; Shi, R.R.; Xu, J.; Xing, Y.R.; Shi, D.; Liu, Y.Q.; He, B.S.; Gu, J.H. Isoquercitrin mitigates intestinal ischemia-reperfusion injury by regulating intestinal flora and inhibiting NLRP3 inflammasome activation. Redox Biol. 2025, 86, 103803. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Limosani, R.V.; Fagiani, F.; Oliviero, C.; Colombo, G.; Cari, L.; Gentili, M.; Lusenti, E.; Rosati, L.; et al. Disruption of Epithelial Barrier Integrity via Altered GILZ/c-Rel/RACK1 Signaling in Inflammatory Bowel Disease. J. Crohn’s Colitis 2025, 19, jjae191. [Google Scholar] [CrossRef]
- Sultan, M.; Cai, Z.X.; Bao, L.; Duan, J.J.; Liu, Y.Y.; Yang, G.; Pei, D.S. Trophic transfer induced gut inflammation, dysbiosis, and inflammatory pathways in zebrafish via Artemia franciscana: A differential analysis of nanoplastic toxicity. J. Hazard. Mater. 2024, 480, 136030. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano- and microplastics: A comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Li, Y.; Sun, Z.; Chen, Y. Recent advances on transport and transformation mechanism of nanoplastics in lung cells. Sci. Total Environ. 2024, 952, 175881. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Li, T.; He, H.; Xu, X.; Zhang, C.; Lu, X.; Wang, Y.; Cao, J.; Lu, Y.; Liu, Y.; et al. Analysis of biodistribution and in vivo toxicity of varying sized polystyrene micro and nanoplastics in mice. Int. J. Nanomed. 2024, 19, 7617–7630. [Google Scholar] [CrossRef] [PubMed]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the human body: Exposure, detection, and risk of carcinogenesis: A state-of-the-art review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Banares, M.A.; Fernandez, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Zhu, Q.; Zhang, Y. Tissue accumulation of microplastics and toxic effects: Widespread health risks of microplastics exposure. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]





| Cell or Organoid Types | Characteristics of Particles | Toxicological Effects | Ref. | ||
|---|---|---|---|---|---|
| Type | Size (Φ, nm) | Conc. (μg/mL) | |||
| CCD-18Co | PS | 500 | 5 | Basal metabolic rewiring, cancerization | [28] |
| L02 | PS | 80 | 12.5 | Mitochondrial damage, metabolic disorders | [30] |
| HEK293 | PS | 1000 | 5 | Decrease proliferation, glucose metabolism, and antioxidant stress ability | [31] |
| BMMSCs & AMSCs | PET | <1000 | 10 | Decrease in the number of proliferating cells, and loss of the ability to differentiate | [32] |
| hiPSC (Forebrain cortex spheroids) | PS | 1000 10,000 | 5 | Neuroroxicity | [34] |
| hiPSC (Intestinal organs) | PS | 50 | 10 | Apoptosis and inflammation | [35] |
| Embryonic stem cells (Hepatoid organs) | PS | 1000 | 0.25 | Metabolic dysfunction and lipid accumulation | [36] |
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| GAPDH | CTCTGACTTCAACAGCGACA | AAATGAGCTTGACAAAGTGG |
| β-actin | CCCTGGAGAAGAGCTACGAG | TCCATGCCCAGGAAGGAAG |
| TNF-α | TGTTGTAGCAAACCCTCAAG | TTGAAGAGGACCTGGGAG |
| Bcl-2 | TTGTGGCCTTCTTTGAGTTC | TTATCCTGGATCCAGGTGTG |
| ZO-1 | ACATACATTCTAAGGGAGC | CTCGGTTTGGTGGTCTG |
| Caspase-3 | GACTCTGGAATATTCCCTGGACAACA | AGGTTTGCTGCATCGACATCTG |
| p53 | AGAGCTGAATGAGGCCTTGGAA | GAGTCAGGCCCTTCTGTCTTGAAC |
| Bax | CATGGGCTGGACATTGGACT | AAAGTAGGAGAGGAGGCCGT |
| IL-2 | GTTCTCCTTGCCTAGTGTGGATGG | CCAACAGAGATAACCACGGCTTCC |
| IL-6 | GCGCCTTCGGTCCAGTTG | CTCCTTTCTCAGGGCTGAG |
| NLRP3 | GGACCTCAGTGACAATTCTC | ACAATCTCCGAATGTTACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, Z.-L.; Liang, C.-G.; Yang, H.-Y.; Wu, X.-F.; Sun, H.-M. Toxicological Impacts of Polypropylene Nanoparticles Similar in Size to Nanoplastics in Plastic-Bottle Injections on Human Umbilical Vein Endothelial Cells. Toxics 2025, 13, 802. https://doi.org/10.3390/toxics13090802
Wang J, Chen Z-L, Liang C-G, Yang H-Y, Wu X-F, Sun H-M. Toxicological Impacts of Polypropylene Nanoparticles Similar in Size to Nanoplastics in Plastic-Bottle Injections on Human Umbilical Vein Endothelial Cells. Toxics. 2025; 13(9):802. https://doi.org/10.3390/toxics13090802
Chicago/Turabian StyleWang, Jie, Zhong-Lan Chen, Cheng-Gang Liang, Hui-Ying Yang, Xian-Fu Wu, and Hui-Min Sun. 2025. "Toxicological Impacts of Polypropylene Nanoparticles Similar in Size to Nanoplastics in Plastic-Bottle Injections on Human Umbilical Vein Endothelial Cells" Toxics 13, no. 9: 802. https://doi.org/10.3390/toxics13090802
APA StyleWang, J., Chen, Z.-L., Liang, C.-G., Yang, H.-Y., Wu, X.-F., & Sun, H.-M. (2025). Toxicological Impacts of Polypropylene Nanoparticles Similar in Size to Nanoplastics in Plastic-Bottle Injections on Human Umbilical Vein Endothelial Cells. Toxics, 13(9), 802. https://doi.org/10.3390/toxics13090802








