Chronic Occupational Exposure to Chemical Mixtures Induces Genomic Instability in Paint Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Chemicals and Equipment
2.3. Single Cell Gel Electrophoresis (Comet) Assay
2.4. Buccal Micronucleus Cytome Assay (BMCyt Assay)
2.5. Biomarker Analysis for Occupational Exposure
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Groups
3.2. Comet Assay
3.3. Buccal Micronucleus Cytome Assay Results (BMCyt Assay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Vol. 98. In Painting, Firefighting and Shiftwork; IARC: Lyon, France, 2010; Available online: https://publications.iarc.who.int/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Painting-Firefighting-And-Shiftwork-2010 (accessed on 1 January 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Interaction Profile for: Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX); Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2004; Appendix B: Back-ground Information for Toluene. Available online: https://www.ncbi.nlm.nih.gov/books/NBK602458 (accessed on 1 January 2025).
- Vattanasit, U.; Sukchana, J.; Kongsanit, S.; Dumtip, P.; Sirimano, V.; Kongpran, J. Toluene and Heavy Metals in Small Automotive Refinishing Shops and Personal Protection of the Workers in Nakhon Si Thammarat, Thailand. J. Environ. Public Health 2021, 2021, 8875666. [Google Scholar] [CrossRef]
- Lertxundi, N.; Baccini, M.; Mirabelli, D. Toluene: Correlation between Occupational Exposure Limits and Biological Exposure Indices. Int. J. Environ. Res. Public Health 2023, 20, 2341. [Google Scholar]
- Zhou, B.; Wu, Q.; Fan, S.; Su, Z.; Lu, C.; Peng, J.; Zhang, N.; Jin, L.; Yu, D.; Zhang, J. Mediating Effect of Oxidative Stress on Blood Pressure Elevation in Workers Exposed to Low Concentrations of Benzene, Toluene, and Xylene (BTX). Sci. Rep. 2024, 14, 26139. [Google Scholar] [CrossRef]
- Maryiantari, E.S.; Keman, S. Effects of Acute Toluene Exposure on Oxidative Stress Parameters and Endothelial Markers in the Coronary Artery of Wistar Rats. F1000Research 2025, 14, 168. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Rodríguez-Gutiérrez, R.; Monreal-Robles, R.; González-González, J.G. Acute toluene intoxica-tion—clinical presentation, management and prognosis: A prospective observational study. BMC Emerg. Med. 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Meulenbelt, J.; de Groot, G.; Savelkoul, T.J. Two cases of acute toluene intoxication. Br. J. Ind. Med. 1990, 47, 417–420. [Google Scholar] [CrossRef]
- Van Hooste, W.L. Myoclonic seizure prior to diagnosis of chronic toxic encephalopathy: A case report. J. Med. Case Rep. 2017, 11, 36. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA Damage and Disease: Induction, Repair and Significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.; Lal, T.; Venugopal, V.; R, S.; Johnson, P. Effect of Volatile Organic Compounds on Pulmonary Functions Among Paint Industry Workers of Unorganized Sectors. Indian J. Occup. Environ. Med. 2024, 16, e58951. [Google Scholar] [CrossRef]
- Balkhyour, M.A.; Chakroun, R.; Faidi, F. Evaluation of Environmental and Biological Monitoring Methods for Toluene Exposure Assessment in Paint Industry. Saudi J. Biol. Sci. 2022, 30, 103538. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Sharaf, N.E.; Hasani, I.W.; Ragab, E.A.; Abdelhakim, H.K. Assessment of Thyroid Function and Oxidative Stress State in Foundry Workers Exposed to Lead. J. Health Pollut. 2020, 10, 200903. [Google Scholar] [CrossRef] [PubMed]
- American Conference of Governmental Industrial Hygienists (ACGIH). TLVs® and BEIs® Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices; ACGIH: Cincinnati, OH, USA, 2007; p. 104. [Google Scholar]
- Nakajima, T.; Wang, H.; Ito, Y.; Naito, H.; Wang, D.; Zhao, N.; Li, H.; Qiu, X.; Xia, L.; Chen, J.; et al. Exposure Reconstruction of Trichloroethylene among Patients with Occupational Trichloroethylene Hypersensitivity Syndrome. Ind. Health 2018, 56, 300–307. [Google Scholar] [CrossRef]
- Chang, F.K.; Mao, I.F.; Chen, M.L.; Cheng, S.F. Urinary 8-Hydroxydeoxyguanosine as a Biomarker of Oxidative DNA Damage in Workers Exposed to Ethylbenzene. Ann. Occup. Hyg. 2011, 55, 519–525. [Google Scholar] [CrossRef]
- Godderis, L.; De Boeck, M.; Haufroid, V.; Emmery, M.; Mateuca, R.; Gardinal, S.; Kirsch-Volders, M.; Veulemans, H.; Lison, D. Influence of Genetic Polymorphisms on Biomarkers of Exposure and Genotoxic Effects in Styrene-Exposed Workers. Environ. Mol. Mutagen. 2004, 44, 293–303. [Google Scholar] [CrossRef]
- Boyum, A. Isolation of Lymphocytes, Granulocytes and Macrophages. Scand. J. Immunol. 1976, 5 (Suppl. 5), 9–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Anlar, H.G.; Taner, G.; Bacanli, M.; Iritas, S.; Kurt, T.; Tutkun, E.; Yilmaz, O.H.; Basaran, N. Assessment of DNA Damage in Ceramic Workers. Mutagenesis 2018, 33, 97–104. [Google Scholar] [CrossRef]
- Kashyap, R.; Reddy, P. Micronuclei Assay of Exfoliated Oral Buccal Cells: Means to Assess the Nuclear Abnormalities in Different Diseases. J. Cancer Res. Ther. 2012, 8, 184–191. [Google Scholar] [CrossRef]
- Thomas, P.; Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. Buccal Micronucleus Cytome Assay. Nat. Protoc. 2009, 4, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Sasahara, T. A pilot study on the stability of toluene in blood from workers. J. Occup. Med. Toxicol. 2012, 7, 24. [Google Scholar]
- Xie, W.Q.; Gong, Y.X.; Yu, K.X. Utilizing two detectors in the measurement of trichloroacetic acid in human urine by reaction headspace gas chromatography. Biomed Chromatogr. 2022, 32, e4288. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Guidelines for Measuring Lead in Blood Using GFAAS; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Cassini, C.; Calloni, C.; Bortolini, G.; Garcia, S.C.; Dornelles, M.A.; Henriques, J.A.P. Occupational Risk Assessment of Oxidative Stress and Genotoxicity in Workers Exposed to Paints During a Working Week. Int. J. Occup. Med. Environ. Health 2011, 24, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Cetintepe, S.P.; Hazar, M.; Bilinmiş, I.; Dilsiz, S.A.; Basaran, N. Evaluation of Genotoxicity, Oxidative Stress and Immune Parameters of Auto-Paint Workers. Environ. Res. 2023, 237, 116970. [Google Scholar] [CrossRef]
- Burgaz, S.; Cakmak, G.; Erdem, O.; Yilmaz, M.; Karakaya, A.E. Micronuclei Frequencies in Exfoliated Nasal Mucosa Cells from Pathology and Anatomy Laboratory Workers Exposed to Formaldehyde. Neoplasma 2001, 48, 144–147. [Google Scholar]
- Cárdenas-Bustamante, O.; Varona-Uribe, M.; Patiño-Florez, R.I.; Groot-Restrepo, H.; Sicard-Suárez, D.; Tórres-Carvajal, M.M.; Pardo-Pardo, D. Exposición a Solventes Orgánicos y Efectos Genotóxicos en Trabajadores de Fábricas de Pinturas en Bogotá. Rev. Salud Pública 2007, 9, 275–288. [Google Scholar] [CrossRef]
- De Oliveira, H.M.; Dagostim, G.P.; da Silva, A.M.; Tavares, P.; da Rosa, L.A.Z.C.; de Andrade, V.M. Occupational Risk Assessment of Paint Industry Workers. Indian J. Occup. Environ. Med. 2011, 15, 52–58. [Google Scholar] [PubMed]
- Londoño-Velasco, E.; Martínez-Perafán, F.; Carvajal, S.; García-Vallejo, F.; Hoyos-Giraldo, L.S. Evaluation of Oxidative and Methylating DNA Damage in Painters Occupationally Exposed to Organic Solvents and Paints. Biomédica 2019, 39, 464–477. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Audi, C.; Baïz, N.; Maesano, C.N.; Ramousse, O.; Reboulleau, D.; Magnan, A.; Annesi-Maesano, I. Serum Cytokine Levels Related to Exposure to Volatile Organic Compounds and PM2.5 in Dwellings and Workplaces in French Farmers. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1363–1374. [Google Scholar] [CrossRef]
- García-Lestón, J.; Méndez, J.; Pásaro, E.; Laffon, B. Genotoxic effects of lead: An updated review. Environ. Int. 2010, 36, 623–636. [Google Scholar] [CrossRef]
- Grover, P.; Rekhadevi, P.V.; Danadevi, K.; Vuyyuri, S.B.; Mahboob, M.; Rahman, M.F. Genotoxicity evaluation in workers occupationally exposed to lead. Int. J. Hyg. Environ. Health 2010, 213, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.; Munari, C.C.; Sato, V.L.; Alves, J.M.; de Oliveira, P.F.; Mastrocola, D.F.; Martins, S.P.; Moraes, T.S.; de Oliveira, A.I.; Tozatti, M.G.; et al. Assessment of the Genotoxicity and Antigenotoxicity of (+)-Usnic Acid in V79 Cells and Swiss Mice by the Micronucleus and Comet Assays. Mutat. Res. 2013, 753, 101–106. [Google Scholar] [CrossRef]
- Dos Reis Filho, A.P.; Silveira, M.A.D.; Demarco, N.R.; D’Arce, L.P.G. Increased DNA Damage, Instability and Cytokinesis Defects in Occupationally Exposed Car Painters. Vivo 2019, 33, 1807–1811. [Google Scholar] [CrossRef]
- Fenech, M. The In Vitro Micronucleus Technique. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Çelik, M.; Gülcü, F.; Ozan, G.; Gürsu, M.F. Paraoxonase and Arylesterase Activity Levels in Workers Exposed to Organic Solvents. Turk. J. Biochem. 2005, 30, 194–199. [Google Scholar]
- Li, S.; Liao, X.; Ma, R.; Deng, N.; Wu, H.; Zhang, Z.; Chen, L.; Wang, Q.; Liao, Q.; Li, Q.; et al. Effects of co-exposure to benzene, toluene, and xylene, polymorphisms of microRNA genes, and their interactions on genetic damage in Chinese petrochemical workers. Toxics 2024, 12, 821. [Google Scholar] [CrossRef]
- Cheng, Y.; Kong, D.; Ci, M.; Guan, Y.; Luo, C.; Zhang, X.; Gao, F.; Li, M.; Deng, G. Oxidative Stress Effects of Multiple Pollutants in an Indoor Environment on Human Bronchial Epithelial Cells. Environ. Res. 2023, 234, 117672. [Google Scholar] [CrossRef] [PubMed]
- Mattia, V.; Scassellati-Sforzolini, G.; Nasciutti, A.; Manno, M.; Biagetti, C.; Magni, S. Oxidative Damage to DNA by Toluene in Human Lymphocytes. Mutat. Res. 1991, 262, 103–108. [Google Scholar]
- Salama, S.A.; Serrana, M.; Au, W.W. Biomonitoring Using Accessible Human Cells for Exposure and Health Risk Assessment. Mutat. Res. 1999, 436, 99–112. [Google Scholar] [CrossRef] [PubMed]
- De Los A Gutiérrez, M.; Palmieri, M.A.; Giuliani, D.S.; Colman Lerner, J.E.; Maglione, G.; Andrinolo, D.; Tasat, D.R. Monitoring Human Genotoxicity Risk Associated to Urban and Industrial Buenos Aires Air Pollution Exposure. Environ. Sci. Pollut. Res. 2020, 27, 13995–14006. [Google Scholar] [CrossRef]
- Kwon, J.W.; Park, H.W.; Kim, W.J.; Kim, M.G.; Lee, S.J. Exposure to Volatile Organic Compounds and Airway Inflammation. Environ. Health 2018, 17, 65. [Google Scholar] [CrossRef]
- Piña-Calva, A.; Madrigal-Bujaidar, E.; Fuentes, M.V.; Neria, P.; Pérez-Lucio, C.; Vélez-Zamora, N.M. Increased Frequency of Chromosomal Aberrations in Railroad Car Painters. Arch. Environ. Health 1991, 46, 335–339. [Google Scholar] [CrossRef]
- Testa, A.; Caporossi, L.; Rusciano, D. Cytogenetic Biomonitoring of Chromosomal Damage in Workers Exposed to Paint Vapors. J. Occup. Health 2005, 47, 423–429. [Google Scholar]
- Madhavi, D.; Devi, K.R.; Sowjanya, B.L. Increased Frequency of Chromosomal Aberrations in Industrial Painters Exposed to Lead-Based Paints. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 53–59. [Google Scholar] [CrossRef]
- Silva, J.D.; Santos-Mello, R. Cytogenetic Biomonitoring of Car Painters Exposed to Organic Solvents in Brazil. Braz. J. Genet. 1996, 19, 137–142. [Google Scholar]
- Pinto, D.; Ceballos, J.M.; García, G.; Guzmán, P.; Del Razo, L.M.; Vera, E.; Gonsebatt, M.E. Increased Cytogenetic Damage in Outdoor Painters. Mutat. Res. 2000, 467, 105–111. [Google Scholar] [CrossRef]
- Martino-Roth, M.G.; Viégas, J.; Roth, D.M. Occupational Genotoxicity Risk Evaluation through the Comet Assay and the Micronucleus Test. Genet. Mol. Res. 2003, 2, 410–417. [Google Scholar] [PubMed]
- Çelik, A.; Diler, S.B.; Eke, D. Assessment of Genetic Damage in Buccal Epithelium Cells of Painters: Micronucleus, Nuclear Changes, and Repair Index. DNA Cell Biol. 2010, 29, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Fonseca, G.; Fernandez, I. Analysis of Lymphocyte and Oral Mucosa Cell Micronuclei in Cuban Paint Industry Workers. Hereditas 1990, 113, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.M.; Brucker, N.; Charão, M.; Bulcão, R.; Freitas, F.; Baierle, M.; Nascimento, S.; Valentini, J.; Cassini, C.; Salvador, M.; et al. Evaluation of Genotoxicity and Oxidative Damage in Painters Exposed to Low Levels of Toluene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 746, 42–48. [Google Scholar] [CrossRef]
- Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Buresti, G.; Paci, E.; Pigini, D.; Gherardi, M.; Carbonari, D.; et al. Occupational Exposure in Industrial Painters: Sensitive and Noninvasive Biomarkers to Evaluate Early Cytotoxicity, Genotoxicity and Oxidative Stress. Int. J. Environ. Res. Public Health 2021, 18, 4645. [Google Scholar] [CrossRef] [PubMed]
| Factors | Controls (n = 80) | Workers (n = 80) | p |
|---|---|---|---|
| Age (years) a | 37.83 ± 8.16 | 39 ± 7.17 | non-significant * |
| 19–34 | 19 (23.75% **) | 17 (21.25% **) | non-significant *** |
| ≥35 | 61 (76.25% **) | 63 (78.75% **) | |
| Smokers | 28 (35% ***) | 45 (56.25% ***) | 0.006 *** |
| Non-smokers | 52 (65%*) | 35 (43.75% ***) | |
| Alcohol consumption | Non applicable | ||
| Yes | 0 (0% ***) | 0 (0% ***) | |
| No | 80 (100% ***) | 80 (100% ***) | |
| Duration of Exposure (years) | Non applicable | ||
| 1–17 | 0 (0% *) | 63 (78.75%) | |
| 18–35 | 0 (0% *) | 17 (21.25%) |
| Factors | Tail Intensity in Lymphocytes (%) | p ** | Tail Intensity in Whole Blood (%) | p ** | ||
|---|---|---|---|---|---|---|
| Controls (n = 80) | Workers (n = 80) | Controls (n = 80) a | Workers (n = 80) a | |||
| All groups | 2.69 ± 1.05 | 5.00 ± 0.50 * | <0.05 | 5.48 ± 2.41 | 7.65 ± 3.47 | <0.05 |
| Age | ||||||
| 19–34 | 2.14 ± 0.97 | 4.58 ± 4.22 | non-significant | 4.82 ± 2.41 | 8.75 ± 3.39 | non-significant |
| ≥35 | 2.89 ± 1.01 | 6.16 ± 1.93 | non-significant | 5.74 ± 2.04 | 7.36 ± 3.46 | non-significant |
| Smokers | 2.53 ± 1.18 | 5.91 ± 5.60 | non-significant | 5.01 ± 2.50 | 8.50 ± 3.70 | non-significant |
| Non-smokers | 2.78 ± 0.97 | 4.26 ± 4.52 | non-significant | 5.76 ± 2.34 | 6.98 ± 3.16 | non-significant |
| Duration of Exposure (years) | - | n/a | - | n/a | ||
| 1–17 | 5.19 ± 0.29 | 7.50 ± 3.58 | ||||
| 18–35 | 4.35 ± 4.26 | 8.24 ± 3.07 | ||||
| Factors | Buccal MN Frequencies (‰) | p * | |
|---|---|---|---|
| Controls (n = 80) a | Workers (n = 80) a | ||
| All groups | 2.36 ± 1.93 | 3.23 ± 2.71 | <0.05 |
| Age | |||
| 19–34 | 2.36 ± 2.17 | 2.24 ± 2.14 | non-significant |
| ≥35 | 2.37 ± 1.85 | 3.52 ± 4.01 | non-significant |
| Smokers | 2.64 ± 1.98 | 3.30 ± 4.08 | non-significant |
| Non-smokers | 2.23 ± 1.92 | 3.16 ± 3.20 | non-significant |
| Duration of Exposure (years) | |||
| 1–17 | - | 2.93 ± 1.41 | n/a |
| 18–35 | - | 4.38 ± 4.63 | n/a |
| Controls (n = 80) a | Workers (n = 80) a | p * | ||
|---|---|---|---|---|
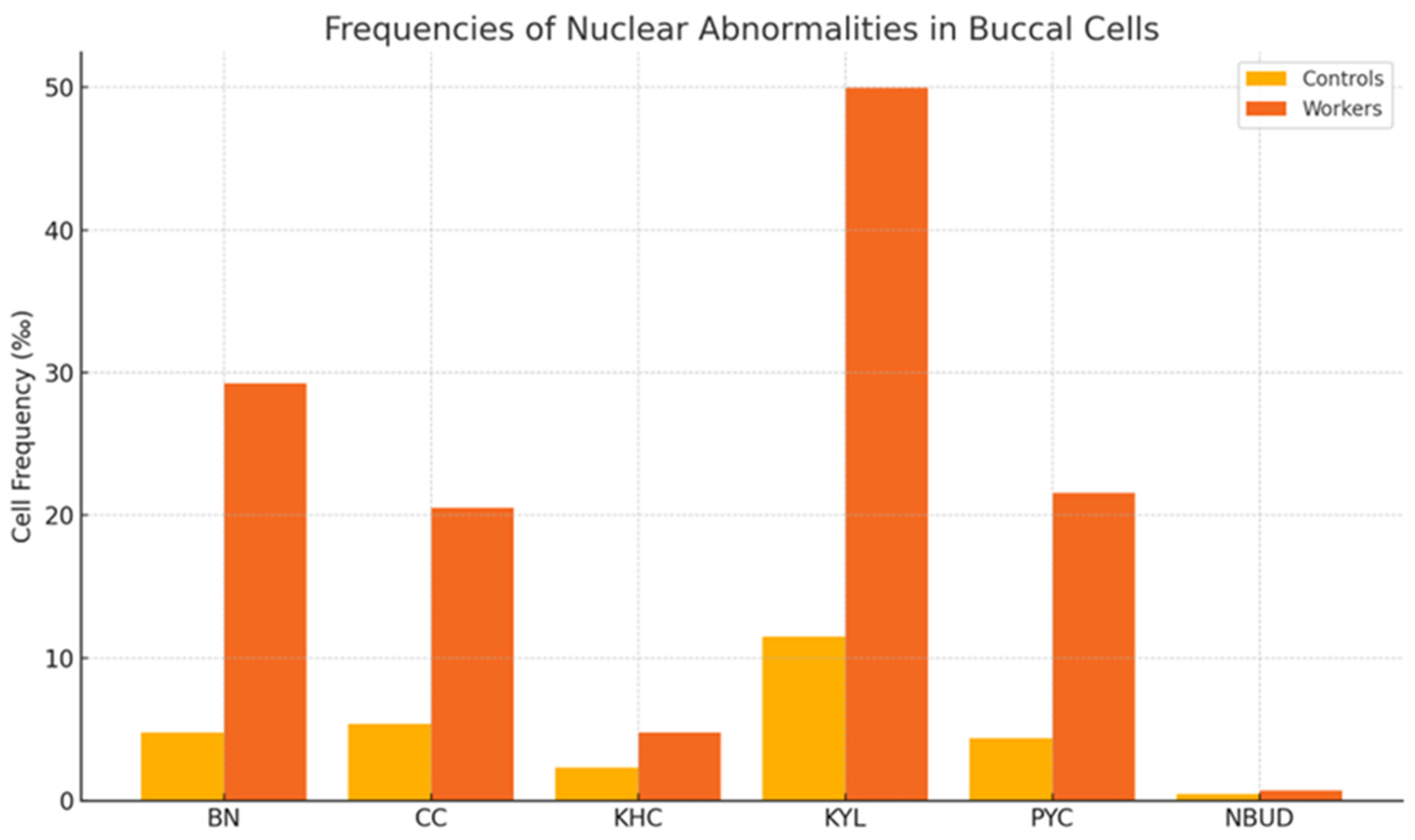
| Cell frequencies (‰) | BN | 4.80 ± 3.25 | 29.23 ± 10.32 | p < 0.01 |
| CC | 5.40 ± 3.63 | 20.55 ± 18.18 | p < 0.01 | |
| KHC | 2.33 ± 3.65 | 4.76 ± 1.84 | p < 0.05 | |
| KYL | 11.50 ± 11.10 | 49.99 ± 5.99 | p < 0.01 | |
| PYC | 4.38 ± 3.51 | 21.57 ± 4.06 | p < 0.05 | |
| NBUD | 0.48 ± 0.68 | 0.71 ± 0.42 | p < 0.05 | |
| Biomarker Analysis | TCA | 2.01 ± 1.30 | 8.62 ± 6.13 | p < 0.05 |
| H. ACID | 212.7 ± 78.38 | 1396.66 ± 791.71 | p < 0.01 | |
| PHENOL | 1.40 ± 0.31 | 10.94 ± 20.18 | p < 0.05 | |
| Pb | 1.16 ± 0.45 | 3.40 ± 3.72 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İritaş, S.B.; Bacanlı, M.G.; Taner, G.; Türksoy, V.A.; Tutkun, L.; Yilmaz, Ö.H.; Başaran, A.N. Chronic Occupational Exposure to Chemical Mixtures Induces Genomic Instability in Paint Workers. Toxics 2025, 13, 785. https://doi.org/10.3390/toxics13090785
İritaş SB, Bacanlı MG, Taner G, Türksoy VA, Tutkun L, Yilmaz ÖH, Başaran AN. Chronic Occupational Exposure to Chemical Mixtures Induces Genomic Instability in Paint Workers. Toxics. 2025; 13(9):785. https://doi.org/10.3390/toxics13090785
Chicago/Turabian Styleİritaş, Servet Birgin, Merve Güdül Bacanlı, Gökçe Taner, Vugar Ali Türksoy, Lütfiye Tutkun, Ömer Hınç Yilmaz, and Ayşe Nurşen Başaran. 2025. "Chronic Occupational Exposure to Chemical Mixtures Induces Genomic Instability in Paint Workers" Toxics 13, no. 9: 785. https://doi.org/10.3390/toxics13090785
APA Styleİritaş, S. B., Bacanlı, M. G., Taner, G., Türksoy, V. A., Tutkun, L., Yilmaz, Ö. H., & Başaran, A. N. (2025). Chronic Occupational Exposure to Chemical Mixtures Induces Genomic Instability in Paint Workers. Toxics, 13(9), 785. https://doi.org/10.3390/toxics13090785







