Ametryn and Clomazone Disrupt Mitochondrial Bioenergetics in Rat Liver: Evidence for Inhibition of Complexes I and II and ATP Synthase
Highlights
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Isolation and Disruption of Rat Liver Mitochondria
2.5. Mitochondrial Respiration Assay
2.6. Estimation of Mitochondrial Membrane Potential (Δψ)
2.7. ATP Quantification
2.8. Determination of Enzymatic Activities
2.9. Statistical Analysis
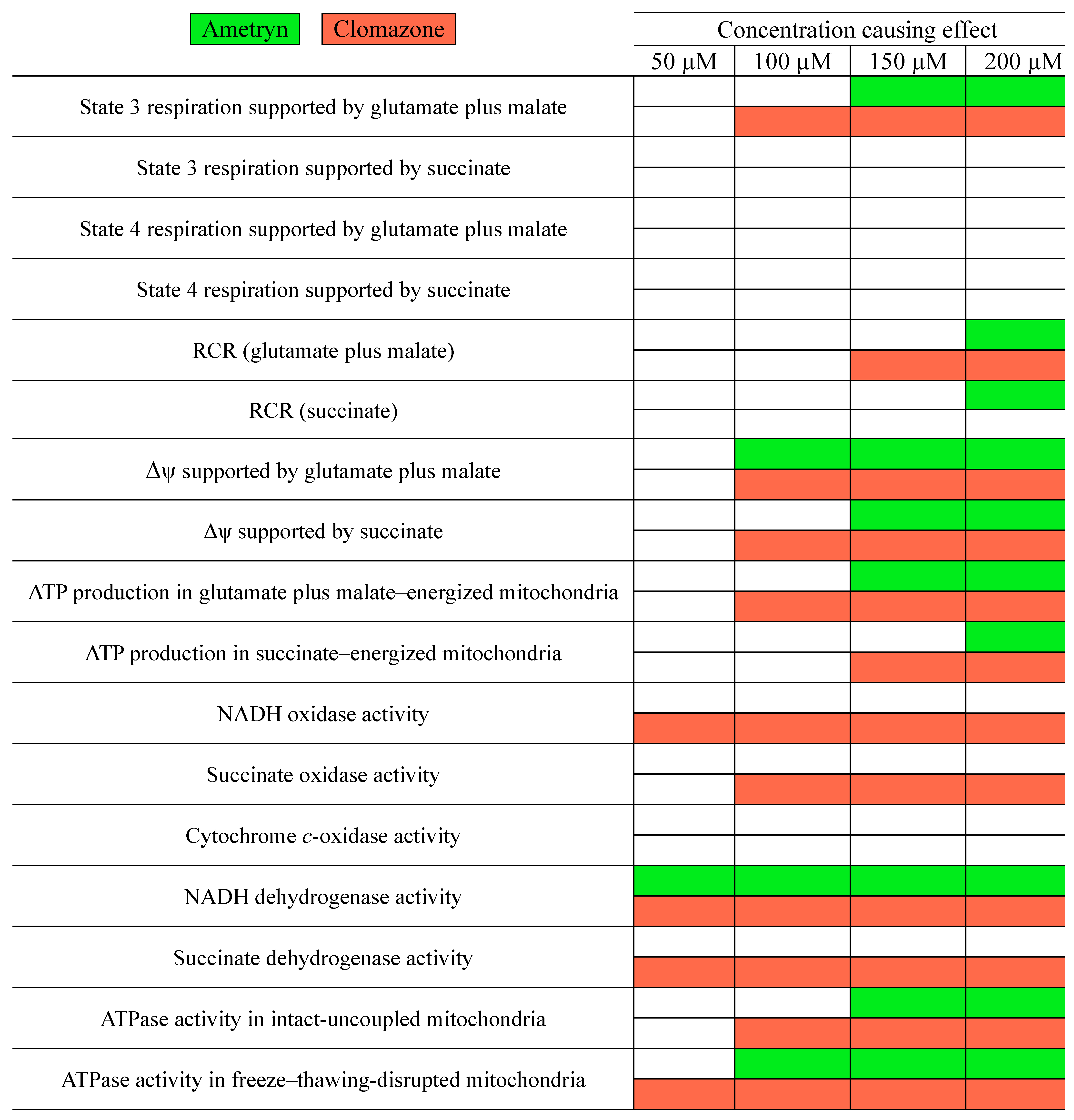
3. Results
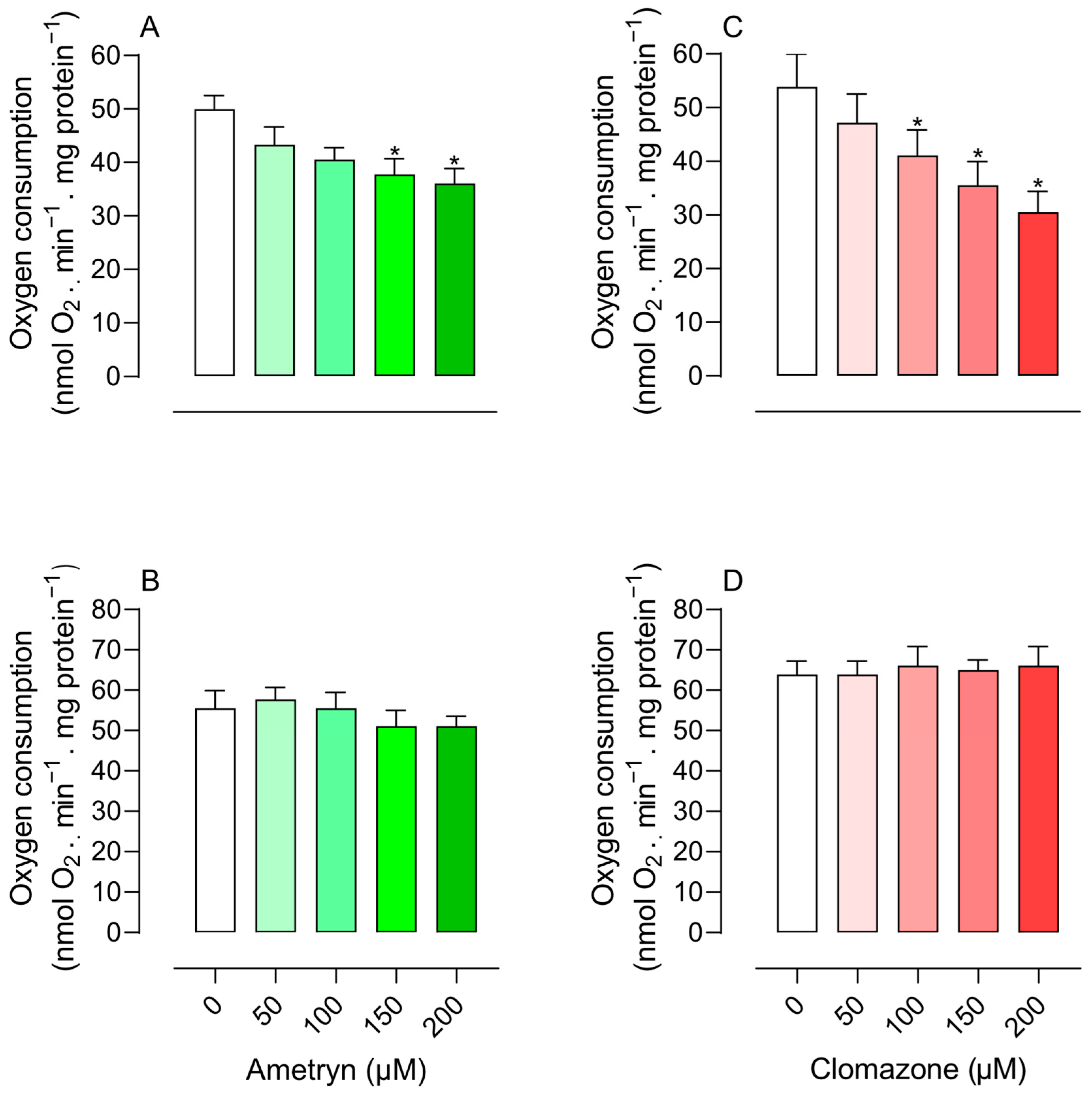
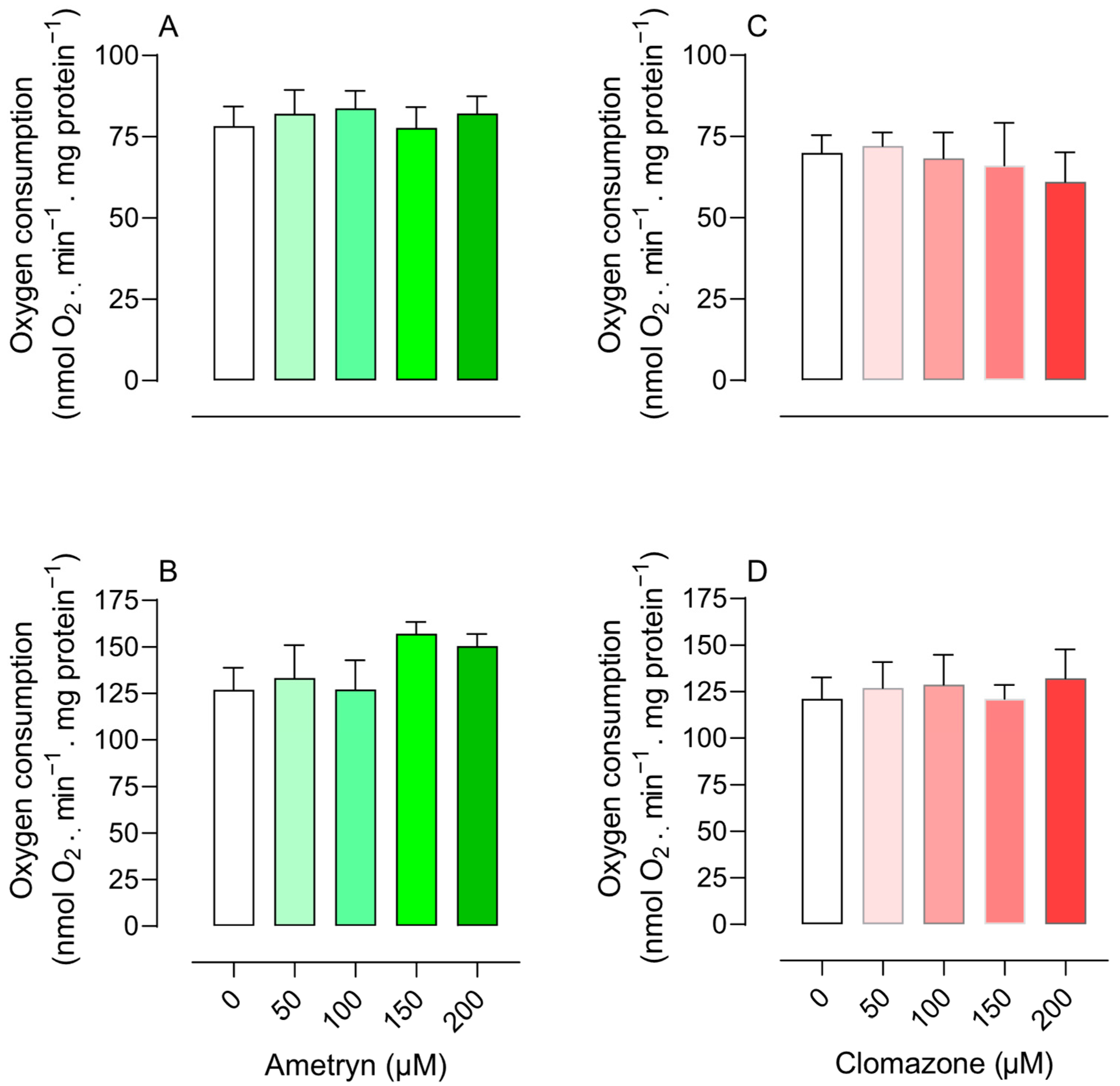
3.1. Effects of AMT and CLZ on Mitochondrial Respiration
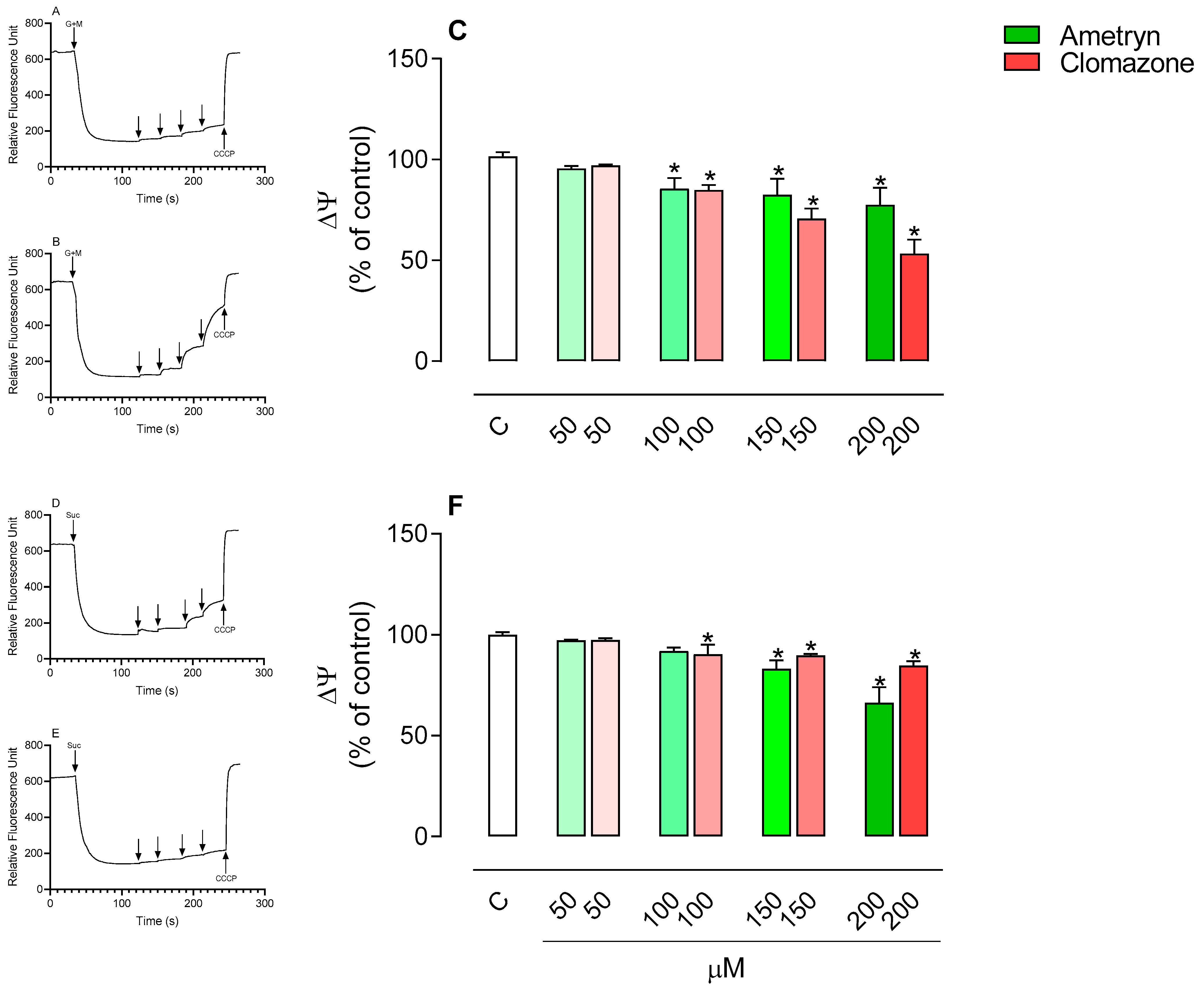
3.2. Effect of AMT and CLZ on Mitochondrial Membrane Potential (Δψ)
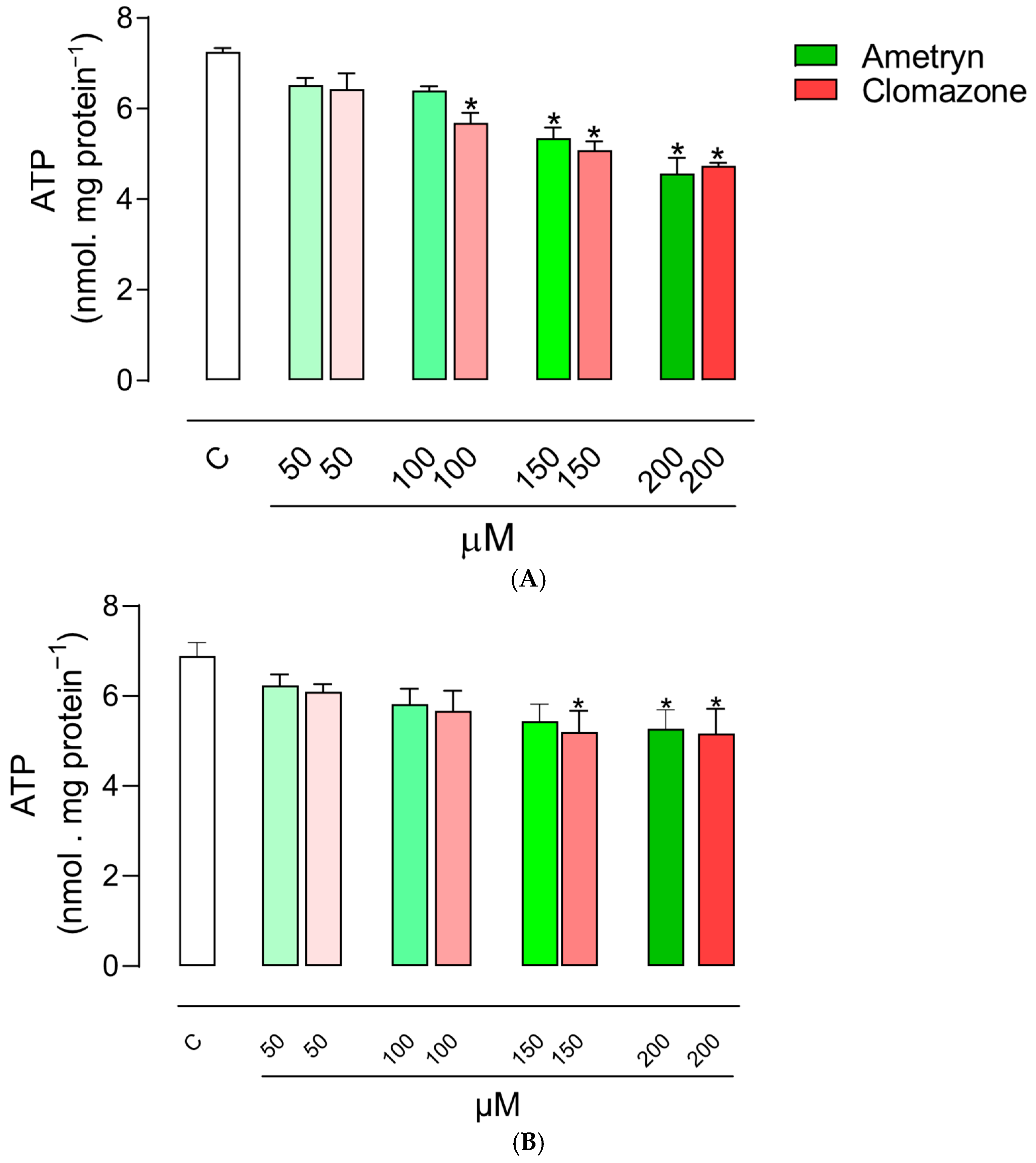
3.3. Effect of AMT and CLZ on Mitochondrial ATP Levels
3.4. Influence of AMT and CLZ on Mitochondrial Enzymatic Activities
3.5. Effects of AMT and CLZ on FoF1-ATPase Activity
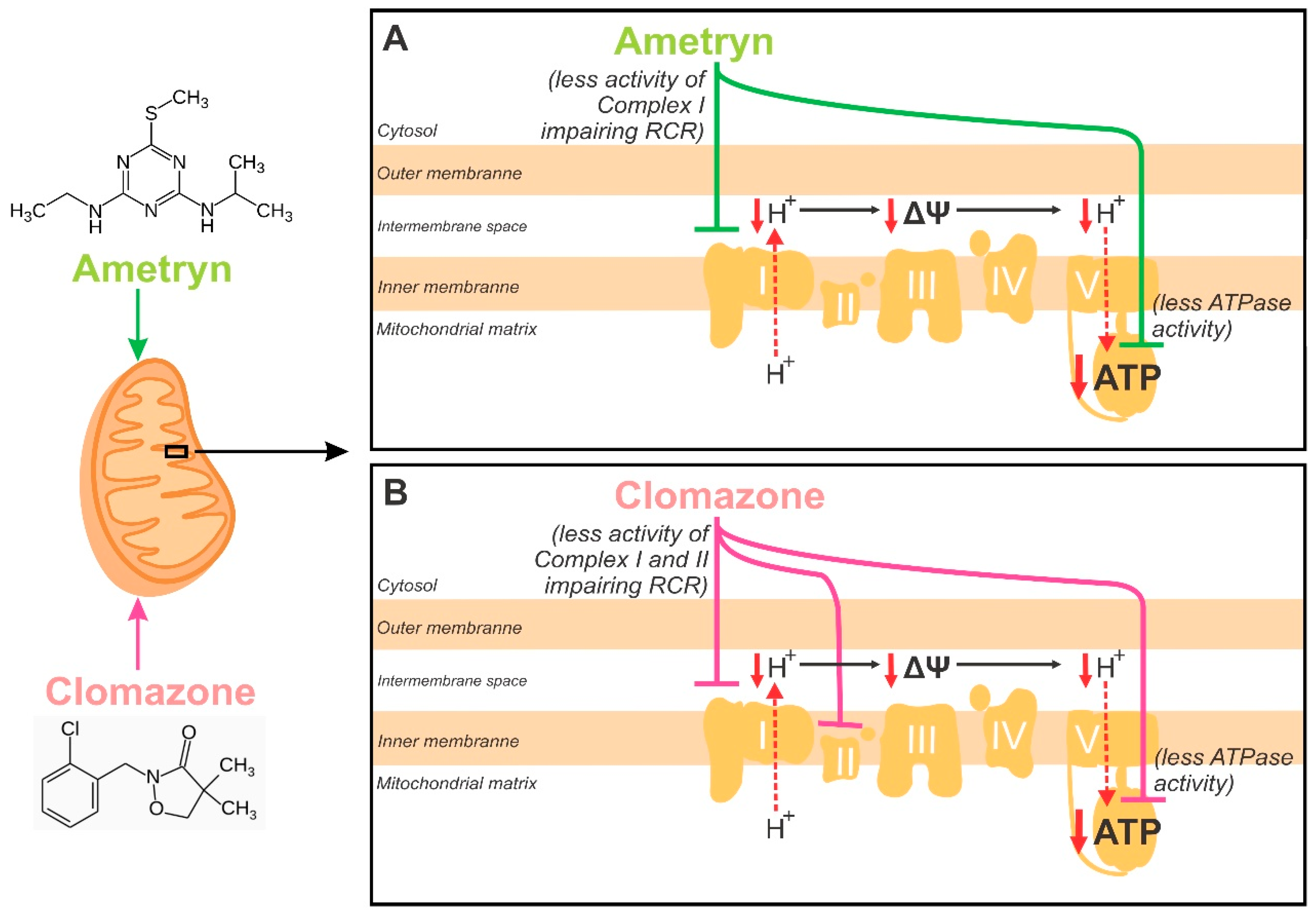
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duke, S.O. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Msibi, S.S.; Su, L.J.; Chen, C.-Y.; Chang, C.-P.; Chen, C.-J.; Wu, K.-Y.; Chiang, S.-Y. Impacts of agricultural pesticide contamination: An integrated risk assessment of rural communities of Eswatini. Toxics 2023, 11, 770. [Google Scholar] [CrossRef]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Pescara, I.C.; Albuquerque, A.F.; Umbuzeiro, G.A.; Jardim, W.F. Ten Years-Snapshot of the Occurrence of Emerging Contaminants in Drinking, Surface and Ground Waters and Wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Acayaba, R.D.; de Albuquerque, A.F.; Ribessi, R.L.; Umbuzeiro, G.A.; Montagner, C.C. Occurrence of pesticides in waters from the largest sugar cane plantation region in the world. Environ. Sci. Pollut. Res. 2021, 28, 9824–9835. [Google Scholar] [CrossRef]
- Silva, M.C.S.C.; Silva, H.C.M.P.; Teixeira, K.P.S.B.; Bedor, D.C.G.; Leal, L.B.; Santana, D.P. Investigation into the occurrence of herbicide residues in the rivers of the Acaú-Goiana extractive reserve. Rev. Ibero-Am. Ciênc. Ambient. 2020, 11, 360–366. [Google Scholar] [CrossRef]
- Echeverría-Sáenz, S.; Spínola-Parallada, M.; Soto, A.C. Pesticides burden in neotropical rivers: Costa Rica as a case study. Molecules 2021, 26, 7235–7254. [Google Scholar] [CrossRef] [PubMed]
- US EPA—United States Environmental Protection Agency. Ametryn: Preliminary Human Health Risk Assessment for Registration Review; Office of Chemical Safety and Pollution Prevention, Health Effects Division: Washington, DC, USA, 2017. Available online: https://downloads.regulations.gov/EPA-HQ-OPP-2013-0249-0022/content.pdf (accessed on 8 August 2025).
- Li, Q.X.; Hwang, E.-C.; Guo, F. Occurrence of herbicides and their degradates in Hawaii’s groundwater. Bull. Environ. Contam. Toxicol. 2001, 66, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Lu, Z.-X.; Lin, M.-Z. Ametryn residues and its degradation dynamics in pineapple and soil. Chin. J. Eco-Agric. 2006, 14, 138–140. Available online: https://www.ecoagri.ac.cn/en/article/id/2006241 (accessed on 8 August 2025).
- Programa de Análise de Resíduos de Agrotóxicos em Alimentos (PARA). Relatório dos Resultados das Análises de Amostras Monitoradas no Ciclo 2023; Brasília. 2024. Available online: https://www.gov.br/anvisa/pt-br/assuntos/agrotoxicos/programa-de-analise-de-residuos-em-alimentos/arquivos/relatorio-2013-para-2023 (accessed on 8 August 2025).
- Wang, Y.-J.; Huang, H.-L.; Jiang, L.-J.; Zhuo, H.-H.; Liu, H.-Z. Determination of ametryn residue in four plant-derived foods by gas chromatography coupled with solid phase extraction. Food Sci. 2010, 31, 182–185. [Google Scholar]
- Code of Federal Regulations (CFR). Title 40, §180.258—Ametryn; Tolerances for Residues; U.S. Government Publishing Office: Washington, DC, USA, 2024. Available online: https://www.ecfr.gov/current/title-40/part-180/section-180.258 (accessed on 8 August 2025).
- National Health and Medical Research Council (NHMRC). Australian Drinking Water Guidelines; NHMRC: Canberra, Australia, 2022. Available online: https://www.lachlan.nsw.gov.au/files/assets/public/v/1/council/australian-drinking-water-standards-2011.pdf (accessed on 8 August 2025).
- Gunasekara, A.S.; Dela Cruz, I.D.; Curtis, M.J.; Claassen, V.P.T.; Jeerdema, R.S. The behavior of clomazone in the soil environment. Pest Manag. Sci. 2009, 65, 711–716. [Google Scholar] [CrossRef]
- Wang, H.; Ren, W.; Xu, Y.; Wang, X.; Ma, J.; Sun, Y.; Hu, W.; Chen, S.; Dai, S.; Song, J.; et al. Long-term herbicide residues affect soil multifunctionality and the soil microbial community. Ecotoxicol. Environ. Saf. 2024, 283, 116783. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, E.; Zanella, R.; Avila, L.A.; Camargo, E.R.; Machado, S.L.O.; Macedo, V.R.M. Rice herbicide monitoring in two Brazilian rivers during the rice growing season. Sci. Agric. 2007, 64, 131–137. [Google Scholar] [CrossRef]
- Brum, A.; Dotta, G.; Roumbedakis, K.; Gonçalves, E.L.T.; Garcia, L.P.; Garcia, P.; Scussel, V.M.; Martins, M.L. Hematological and histopathological changes in silver catfish Rhamdia quelen (Siluriformes) exposed to clomazone herbicide in the Madre River, Santa Catarina State, Southern Brazil. J. Environ. Sci. Health B 2013, 49, 169–175. [Google Scholar] [CrossRef]
- Fournie, M.L.; Castillo, L.E.; Ramírez, F.; Moraga, G.; Ruepert, C. Evaluación preliminar del área agrícola y su influencia sobre la calidad del agua en el Golfo Dulce, Costa Rica. Rev. Cienc. Ambient. 2018, 53, 92–102. [Google Scholar] [CrossRef]
- Guarda, P.M.; Pontes, A.M.S.; Domiciano, R.S.; Gualberto, L.S.; Mendes, D.B.; Guarda, E.A.; Silva, J.E.C. Assessment of ecological risk and environmental behavior of pesticides in environmental compartments of the Formoso River in Tocantins, Brazil. Arch. Environ. Contam. Toxicol. 2020, 79, 524–536. [Google Scholar] [CrossRef]
- Seben, D.; Toebe, M.; Wastowski, A.D.; Hofstatter, K.; Volpatto, F.; Zanella, R.; Prestes, O.D.; Golombieski, J.I. Water quality variables and emerging environmental contaminant in water for human consumption in Rio Grande do Sul, Brazil. Environ. Chall. 2021, 5, 100266–100276. [Google Scholar] [CrossRef]
- Spurgeon, D.; Besien, T.; Armenise, E.; Hutt, L.; Kieboom, N.; Wilkinson, H. Worst-Case Ranking of Organic Substances Detected in Groundwater and Surface Waters in England; Final Version; UK Centre for Ecology and Hydrology, Environment Agency: Wallingford, UK, 2021; Available online: https://nora.nerc.ac.uk/id/eprint/531239/1/N531239CR.pdf (accessed on 11 April 2025).
- Liu, H.; Li, R.; Hu, W.; Jian, L.; Huang, B.; Fan, Y.; Zhao, Y.; Zhang, H.; Khan, K.S. Multi-medium residues and ecological risk of herbicides in a typical agricultural watershed of the Mollisols region, Northeast China. Sci. Total Environ. 2024, 937, 173507–173518. [Google Scholar] [CrossRef]
- Health Canada. Proposed Maximum Residue Limit PMRL2024-xx: Clomazone; Pest Management Regulatory Agency: Ottawa, ON, Canada, 2024; Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/consultations/proposed-maximum-residue-limit/2024/clomazone/document.html (accessed on 11 August 2025).
- EFSA (European Food Safety Authority); Álvarez, F.; Arena, M.; Auteri, D.; Batista Leite, S.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; et al. Peer review of the pesticide risk assessment of the active substance clomazone. EFSA J. 2025, 23, e9206. [Google Scholar] [CrossRef]
- University of Hertfordshire. Clomazone (Ref: FMC 57020); Pesticide Properties DataBase (PPDB), Agriculture & Environment Research Unit (AERU), University of Hertfordshire. 2023. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/168.htm (accessed on 11 August 2025).
- Ohno, Y.; Miyajima, A.; Sunouchi, M. Alternative methods for mechanistic studies in toxicology: Screening of hepatotoxicity of pesticides using freshly isolated and primary cultured hepatocytes and non-liver-derived cells, SIRC cells. Toxicol. Lett. 1998, 102–103, 569–573. [Google Scholar] [CrossRef]
- EPA—United States Environmental Protection Agency. Registration Review: Preliminary Human Health Risk Assessment for Ametryn. 2018. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2013-0249-0022 (accessed on 12 December 2024).
- Crestani, M.; Menezes, C.; Glusczak, L.; Miron, D.S.; Spanevell, R.; Silveira, A.; Gonçalves, F.F.; Zanella, R.; Loro, V.L. Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 2007, 67, 2305–2311. [Google Scholar] [CrossRef]
- Cattaneo, R.; Moraes, B.S.; Loro, V.L.; Pretto, A.; Menezes, C.; Sartori, G.M.S.; Clasen, B.; Avila, L.A.; Marchesan, E.; Zanella, R. Tissue biochemical alterations of Cyprinus carpio exposed to commercial herbicide containing clomazone under rice-field conditions. Arch. Environ. Contam. Toxicol. 2012, 62, 97–106. [Google Scholar] [CrossRef]
- Fagundes, M.Z.; Gonçalves, M.A.; Soares, M.P.; Martins, M.L.; Zanella, R.; Riet-Correa, F.; Anjos, B.L. Clinicopathological and toxicological aspects of poisoning by the clomazone herbicide in sheep. Small Rumin. Res. 2015, 124, 120–126. [Google Scholar] [CrossRef][Green Version]
- Oliveira, C.R.; Fraceto, L.F.; Rizzi, G.M.; Salla, R.F.; Abdalla, F.C.; Costa, M.J.; Silva-Zacarin, E.C. Hepatic effects of the clomazone herbicide in both its free form and associated with chitosan-alginate nanoparticles in bullfrog tadpoles. Chemosphere 2016, 149, 304–313. [Google Scholar] [CrossRef]
- Sevior, D.K.; Pelkonen, O.; Ahokas, J.T. Hepatocytes: The powerhouse of biotransformation. Int. J. Biochem. Cell Biol. 2012, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.I.; Luo, J.; Vanderpuye, C.M.; Brizendine, P.; Muddasani, P.; Bolatimi, O.; Heinig, S.A.; Ekuban, F.A.; Siddiqui, H.; Ekuban, A.; et al. Environmental Pollutants, Occupational Exposures, and Liver Disease. Semin. Liver Dis. 2025, 45, 148–166. [Google Scholar] [CrossRef]
- Tolman, K.G.; Dalpiaz, A.S. Occupational and Environmental Hepatotoxicity. In Drug-Induced Liver Disease, 3rd ed.; Kaplowitz, N., DeLeve, L.D., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 659–675. [Google Scholar] [CrossRef]
- VoPham, T.; Bertrand, K.A.; Hart, J.E.; Laden, F.; Brooks, M.M.; Yuan, J.M.; Talbott, E.O.; Ruddell, D.; Chang, C.H.; Weissfeld, J.L. Pesticide exposure and liver cancer: A review. Cancer Causes Control 2017, 28, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Jellali, R.; Jacques, S.; Essaouiba, A.; Gilard, F.; Letourneur, F.; Gakière, B.; Legallais, C.; Leclerc, E. Investigation of steatosis profiles induced by pesticides using liver organ-on-chip model and omics analysis. Food Chem. Toxicol. 2021, 152, 112155. [Google Scholar] [CrossRef]
- Ramachandran, A.; Visschers, R.G.J.; Duan, L.; Akakpo, J.Y.; Jaeschke, H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: Current understanding and future perspectives. J. Clin. Transl. Res. 2018, 4, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Vinken, M. Mitochondria as the target of hepatotoxicity and drug-induced liver injury: Molecular mechanisms and detection methods. Int. J. Mol. Sci. 2022, 23, 3315. [Google Scholar] [CrossRef]
- Dykens, J.A.; Jamieson, J.D.; Marroquin, L.D.; Nadanaciva, S.; Xu, J.J.; Dunn, M.C.; Smith, A.R.; Will, Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone and buspirone. Toxicol. Sci. 2008, 103, 335–345. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Meyer, J.N. Mitochondria as a target of organophosphate and carbamate pesticides: Revisiting common mechanisms of action with new approach methodologies. Reprod. Toxicol. 2019, 89, 83–92. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Chen, P.; Yao, L.; Yuan, M.; Wang, Z.; Zhang, Q.; Jiang, Y.; Li, L. Mitochondrial dysfunction: A promising therapeutic target for liver diseases. Genes Dis. 2024, 11, 101115. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhou, W.; Qiao, Q.; Liang, H.; Li, G.; Wang, J.; Cai, F. The interactive effects of cytoskeleton disruption and mitochondria dysfunction lead to reproductive toxicity induced by microcystin-LR. PLoS ONE 2013, 8, e53949. [Google Scholar] [CrossRef]
- Santos, T.; Cancian, G.; Neodini, D.N.R.; Mano, D.R.S.; Capucho, C.; Predes, F.S.; Barbieri, R.; Oliveira, C.A.; Pigoso, A.A.; Dolder, H.; et al. Toxicological evaluation of ametryn effects in Wistar rats. Exp. Toxicol. Pathol. 2015, 67, 525–532. [Google Scholar] [CrossRef]
- Moura, M.A.M.; Oliveira, R.; Jonsson, C.M.; Domingues, I.; Soares, A.M.V.M.; Nogueira, A.J.A. The sugarcane herbicide ametryn induces oxidative stress and developmental abnormalities in zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2018, 25, 13416–13425. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.; Menezes, C.; Duarte, M.M.; Leitemperger, J.; Lópes, T.; Loro, V.L. Oxidative stress biomarkers and acetylcholinesterase activity in human erythrocytes exposed to clomazone (in vitro). Interdiscip. Toxicol. 2011, 4, 149–153. [Google Scholar] [CrossRef]
- Menezes, C.C.; Loro, V.L.; Fonseca, M.B.; Cattaneo, R.; Pretto, A.; Miron, D.S.; Santi, A. Oxidative parameters of Rhamdia quelen in response to commercial herbicide containing clomazone and recovery pattern. Pestic. Biochem. Physiol. 2011, 100, 145–150. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Machado, I.F.; Palmeira, C.M.; Rolo, A.P. Determination of oxidative phosphorylation complexes activities. In Mitochondrial Regulation; Palmeira, C.M., Rolo, A.P., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2310, pp. 15–28. [Google Scholar] [CrossRef]
- Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985, 54, 1015–1069. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem. Anal. 1974, 22, 123–175. [Google Scholar] [CrossRef]
- Rich, P.R.; Maréchal, A. The mitochondrial respiratory chain. Essays Biochem. 2010, 47, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Huo, X.; Zhai, Y.; Wang, A.; Xu, J.; Su, D.; Bartlam, M.; Rao, Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 2005, 121, 1043–1057. [Google Scholar] [CrossRef]
- Thompson, O.C.; Truelove, B.; Davis, D.E. Effects of triazines on energy relations of mitochondria and chloroplasts. Weed Sci. 1974, 22, 164–166. [Google Scholar] [CrossRef]
- Timossi, P.; Alves, P. Effects of clomazone drift, sprayed alone or in mixture with ametryn, on the productive characteristics of Hamlin orange. Planta Daninha 2001, 19, 295–304. [Google Scholar] [CrossRef]
- Esqueda, V.A.; Altamirano, L.; Hernández, Y.; López, A. Evaluation of the mixture ametryn + clomazone on sugarcane. Agron. Mesoam. 2006, 12, 161–167. [Google Scholar] [CrossRef]
- Costa, N.V.; Gibbert, A.M.; Ferreira, S.D.; Canavessi, H.; Salvalaggio, A.C. Strategies of chemical management for weed control in cassava. Rev. Ceres 2020, 67, 240–246. [Google Scholar] [CrossRef]
- Armas, E.D.; Monteiro, R.T.R.; Antunes, P.M.; Santos, M.A.P.F.; Camargo, P.B.; Abakerli, R.B. Diagnóstico espaço-temporal da ocorrência de herbicidas nas águas superficiais e sedimentos do Rio Corumbataí e principais afluentes. Química Nova 2007, 30, 1119–1127. [Google Scholar] [CrossRef]
- Santos, E.A.; Correia, N.M.; Silva, J.R.M.; Velini, E.D.; Passos, A.B.R.J.; Durigan, J.C. Herbicide detection in groundwater in Córrego Rico-SP watershed. Planta Daninha 2015, 33, 147–155. [Google Scholar] [CrossRef]
- CETESB—Companhia Ambiental do Estado do São Paulo. Diagnóstico da Contaminação de Águas Superficiais, Subterrâneas e Sedimentos por Agrotóxicos; CETESB: São Paulo, Brasil, 2021; 150p. Available online: https://cetesb.sp.gov.br/wp-content/uploads/2021/10/Diagnostico-da-Contaminacao-de-Aguas-Superficiais-Subterraneas-e-Sedimentos-por-Agrotoxicos_.pdf (accessed on 22 March 2025).
- Li, R.; Hu, W.; Liu, H.; Huang, B.; Jia, Z.; Liu, F.; Zhao, Y.; Khan, K.S. Occurrence, distribution and ecological risk assessment of herbicide residues in cropland soils from the Mollisols region of Northeast China. J. Hazard. Mater. 2024, 465, 133054. [Google Scholar] [CrossRef]
- Pedersen, P.L.; Greenawalt, J.W.; Reynafarje, B.; Hullihen, J.; Decker, G.L.; Soper, J.W.; Bustamante, E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 1978; pp. 411–481. [Google Scholar]
- Cain, K.; Skilleter, D.N. Preparation and use of mitochondria in toxicological research. In Biochemical Toxicology; Snell, K., Mullock, B., Eds.; IRL Press: Oxford, UK, 1987; pp. 217–254. [Google Scholar]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, A.; Azzone, G.F. Safranine as membrane potential probe in rat liver mitochondria. Arch. Biochem. Biophys. 1980, 201, 255–265. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Hackenbrock, C.R. Continuous measurement and rapid kinetics of ATP synthesis in rat liver mitochondria, mitoplasts and inner membrane vesicles determined by firefly-luciferase luminescence. Eur. J. Biochem. 1976, 67, 1–10. [Google Scholar] [CrossRef]
- Bracht, A.; Ishii-Iwamoto, E.L.; Salgueiro-Pagadigorria, C.L. Estudo do metabolismo energético em mitocôndrias isoladas de tecido animal. In Métodos de Laboratório em Bioquímica; Bracht, A., Ishii-Iwamoto, E.L., Eds.; Manole: Barueri, Brazil, 2003; pp. 227–247. [Google Scholar]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Takayasu, T.; Sano, Y.; Nagai, T.; Saito, K.; Chiba, T.; Yoshida, K.; Ameno, K. A fatal poisoning with the herbicide Gesapax: Ametryn and atrazine determination in human tissues. J. Anal. Toxicol. 2010, 34, 287–291. [Google Scholar] [CrossRef]
- Miranda, C.A.; Peixoto, P.V.L.; Viriato, C.; Aggio, L.; Pereira, L.C.; Mingatto, F.E. Toxicity of ametryn and clomazone in zebrafish: Environmentally relevant concentrations induce developmental and enzymatic alterations. Environ. Toxicol. Pharmacol. 2025, 116, 104721. [Google Scholar] [CrossRef]
- Yamano, T.; Morita, S. Effects of pesticides on isolated rat hepatocytes, mitochondria, and microsomes. Arch. Environ. Contam. Toxicol. 1993, 25, 271–278. [Google Scholar] [CrossRef]
- Binukumar, B.K.; Bal, A.; Kandimalla, R.; Sunkaria, A.; Gill, K.D. Mitochondrial energy metabolism impairment and liver dysfunction following chronic exposure to dichlorvos. Toxicology 2010, 270, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Q.; Xu, C.; Shao, W.; Zhang, C.; Liu, H.; Jiang, Z.; Gu, A. Organochloride pesticides impaired mitochondrial function in hepatocytes and aggravated disorders of fatty acid metabolism. Sci. Rep. 2017, 7, 46339. [Google Scholar] [CrossRef] [PubMed]
- Bizerra, P.F.V.; Guimarães, A.R.J.S.; Maioli, M.A.; Mingatto, F.E. Imidacloprid affects rat liver mitochondrial bioenergetics by inhibiting FoF1-ATP synthase activity. J. Toxicol. Environ. Health A 2018, 81, 229–239. [Google Scholar] [CrossRef]
- Miranda, C.A.; Guimarães, A.R.J.S.; Bizerra, P.F.V.; Mingatto, F.E. Diazinon impairs bioenergetics and induces membrane permeability transition on mitochondria isolated from rat liver. J. Toxicol. Environ. Health A 2020, 83, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Mirkhamidova, P.; Abduraxmonova, M.; Nishanbayev, S.; Mukhamedov, G. Oxidative phosphorylation of rat liver mitochondria with intoxication of haloxyfop-r-methyl and indoxacarb pesticides. BIO Web Conf. 2024, 130, 06008. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef]
- Boelsterli, U.A. Disruption of mitochondrial function and mitochondria mediated toxicity. In Mechanistic Toxicology: The Molecular Basis of How Chemicals Disrupt Biological Targets, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 357–389. [Google Scholar]
- Althaher, A.R.; Alwahsh, M. An overview of ATP synthase, inhibitors, and their toxicity. Heliyon 2023, 9, e22459. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Wallace, K.B. Depletion of adenine nucleotide translocator protein in heart mitochondria from doxorubicin-treated rats. Relevance for mitochondrial dysfunction. Toxicology 2006, 220, 160–168. [Google Scholar] [CrossRef]
- Hase, Y.; Tatsuno, M.; Nishi, T.; Kataoka, K.; Kabe, Y.; Yamaguchi, Y.; Ozawa, N.; Natori, M.; Handa, H.; Watanabe, H. Atrazine binds to F1F0-ATP synthase and inhibits mitochondrial function in sperm. Biochem. Biophys. Res. Commun. 2008, 366, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, S.Y.; Song, I.C.; Chung, M.H.; Jang, H.C.; Park, K.S.; Lee, K.U.; Pak, Y.K.; Lee, H.K. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS ONE 2009, 4, e5186. [Google Scholar] [CrossRef]
- Karadayian, A.G.; Paez, B.; Bustamante, J.; Lores-Arnaiz, S.; Czerniczyniec, A. Mitochondrial dysfunction due to in vitro exposure to atrazine and its metabolite in striatum. J. Biochem. Mol. Toxicol. 2023, 37, e23232. [Google Scholar] [CrossRef]
- Fidelis, J.P.S.; Miranda, C.A.; Nicodemo, D.; Mingatto, F.E. Clomazone herbicide impairs bioenergetics in mitochondria isolated from the thorax of honey bees (Apis mellifera L.). Braz. J. Anim. Environ. Res. 2024, 7, e70709. [Google Scholar] [CrossRef]
- Cestonaro, L.V.; da Silva, A.C.G.; Garcia, S.C.; Valadares, M.C.; Arbo, M.D. Mitochondrial impairment related to the immunotoxicity of the herbicides clomazone, glyphosate and sulfentrazone in THP-1 cells. Toxicol. Res. 2024, 13, tfae005. [Google Scholar] [CrossRef]
- Yamano, T.; Morita, S. Effects of pesticides on isolated rat hepatocytes, mitochondria, and microsomes II. Arch. Environ. Contam. Toxicol. 1995, 28, 1–7. [Google Scholar] [CrossRef]
- Nave, O.P. Modification of semi-analytical method applied system of ODE. Mod. Appl. Sci. 2020, 14, 75. [Google Scholar] [CrossRef]
- Msibi, S.S.; Chen, C.-Y.; Chang, C.-P.; Chen, C.-J.; Chiang, S.-Y.; Wu, K.-Y. High pesticide inhalation exposure from multiple spraying sources amongst applicators in Eswatini, Southern Africa. Pest Manag. Sci. 2021, 77, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, Y.E.; Steiniche, T.; Xia, C.; Romanak, K.; Ogwang, J.; Mutegeki, R.; Wasserman, M.; Venier, M. Tracking toxic chemical exposure in Uganda: Insights from silicone wristbands. Environ. Res. 2025, 277, 121522. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Ferguson, S.J. The respiratory chain. In Bioenergetics, 4th ed.; Academic Press: Oxford, UK, 2013; pp. 39–62. [Google Scholar]



| Malate Plus Glutamate | Succinate | |
|---|---|---|
| RCR | RCR | |
| Ametryn (µM) | ||
| 0 | 6.25 ± 0.45 | 4.68 ± 0.15 |
| 50 | 5.76 ± 0.48 | 3.83 ± 0.27 |
| 100 | 5.21 ± 0.39 | 3.85 ± 0.33 |
| 150 | 5.03 ± 0.26 | 3.68 ± 0.53 |
| 200 | 4.48 ± 0.30 * | 3.09 ± 0.19 * |
|
Clomazone (µM)
0 | 7.31 ± 0.31 | 4.91 ± 0.31 |
| 50 | 6.55 ± 0.24 | 4.70 ± 0.24 |
| 100 | 6.09 ± 0.45 | 4.73 ± 0.25 |
| 150 | 5.59 ± 0.54 * | 4.94 ± 0.32 |
| 200 | 5.05 ± 0.18 * | 4.11 ± 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Silva, H.P.; Ortiz, C.; Miranda, C.A.; Veiga Bizerra, P.F.; Palmeira, C.M.; Mingatto, F.E. Ametryn and Clomazone Disrupt Mitochondrial Bioenergetics in Rat Liver: Evidence for Inhibition of Complexes I and II and ATP Synthase. Toxics 2025, 13, 784. https://doi.org/10.3390/toxics13090784
dos Santos Silva HP, Ortiz C, Miranda CA, Veiga Bizerra PF, Palmeira CM, Mingatto FE. Ametryn and Clomazone Disrupt Mitochondrial Bioenergetics in Rat Liver: Evidence for Inhibition of Complexes I and II and ATP Synthase. Toxics. 2025; 13(9):784. https://doi.org/10.3390/toxics13090784
Chicago/Turabian Styledos Santos Silva, Heberth Paulo, Camila Ortiz, Camila Araújo Miranda, Paulo Francisco Veiga Bizerra, Carlos Manuel Palmeira, and Fábio Erminio Mingatto. 2025. "Ametryn and Clomazone Disrupt Mitochondrial Bioenergetics in Rat Liver: Evidence for Inhibition of Complexes I and II and ATP Synthase" Toxics 13, no. 9: 784. https://doi.org/10.3390/toxics13090784
APA Styledos Santos Silva, H. P., Ortiz, C., Miranda, C. A., Veiga Bizerra, P. F., Palmeira, C. M., & Mingatto, F. E. (2025). Ametryn and Clomazone Disrupt Mitochondrial Bioenergetics in Rat Liver: Evidence for Inhibition of Complexes I and II and ATP Synthase. Toxics, 13(9), 784. https://doi.org/10.3390/toxics13090784








